Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
Better Clinical Results in Rheumatoid Arthritis Patients Treated Under a Multidisciplinary Care Model When Compared with a National Rheumatoid Arthritis Registry
Authors Santos-Moreno P , Rodríguez-Vargas GS, Martínez S, Ibatá L, Villarreal-Peralta L, Aza-Cañon A, Rivero M, Rodriguez P , Rojas-Villarraga A
Received 8 August 2022
Accepted for publication 4 November 2022
Published 18 November 2022 Volume 2022:14 Pages 269—280
DOI https://doi.org/10.2147/OARRR.S385423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Chuan-Ju Liu
Pedro Santos-Moreno,1 Gabriel-Santiago Rodríguez-Vargas,1 Susan Martínez,2 Linda Ibatá,2 Laura Villarreal-Peralta,1 Anggie Aza-Cañon,1 Manuel Rivero,1 Pedro Rodriguez,1 Adriana Rojas-Villarraga3
1Rheumatology Department, Biomab IPS, Bogotá, Colombia; 2Epidemiology Department, Epithink Health Consulting, Bogotá, Colombia; 3Research Institute, Fundación Universitaria de Ciencias de la Salud–FUCS, Bogotá, Colombia
Correspondence: Pedro Santos-Moreno, Rheumatology Department, Biomab IPS, Calle 48 # 13-86, Bogotá, Colombia, Tel +57 320 8094232, Email [email protected]
Purpose: To describe clinical characteristics and effectiveness of health care in patients with rheumatoid arthritis (RA) as part of a multidisciplinary care model (MCM) in a specialized rheumatology center, compared with the results of a national registry of RA (NARRA) as evidence of real-world management.
Patients and Methods: We conducted a real-world study (July 1, 2018 to June 30, 2019) based on an analysis of electronic health records of a cohort of RA patients managed with the “Treat-to-Target” strategy in a specialized rheumatology center in Colombia with an MCM, compared with the NARRA that includes different models of usual care.
Results: We have analyzed 7053 subjects with RA treated at a specialized rheumatology center and 81,492 patients from the NARRA. Cohorts were similar in their baseline characteristics, with women in predominance and diagnosis age close to 50 years. At the time of diagnosis, a higher proportion of clinical diagnostic test use and rheumatology consultation access was observed in the specialized rheumatology center than in the national registry (4– 6 per year versus three or less). In addition, higher proportions of patients in remission and low disease activity were reported for the specialized rheumatology center, with a > 40% amount of data lost in the national registry. Pharmacological management was similar regarding the analgesic use. In the specialized center, Certolizumab was more frequently used than in the NARRA registry; also, there were significant differences in methotrexate, leflunomide, and sulfasalazine use, being higher in the specialized rheumatology center.
Conclusion: The MCM of a specialized center in RA can guarantee comprehensive care, with better access to all the services required to manage the disease. It ensures specialist management and evidence-based care that facilitates the achievement of therapeutic objectives. In addition, better patient records and follow-ups are available to evaluate health outcomes.
Keywords: rheumatoid arthritis, therapeutics, multidisciplinary healthcare, real-world data
Introduction
Rheumatoid arthritis (RA) is a chronic, disabling autoimmune disease. It is estimated that up to 1% of the world population is affected by RA, with increased morbidity and mortality.1,2 This disease imposes a significant economic impact due to treatment costs and its effect on the work capacity of those who suffer it.3 It also generates higher out-of-pocket costs and consumes a considerable portion of the resources of the health system.4 This situation has led to the creation of different RA registries as primary sources of epidemiological data to monitor and characterize populations with specific risks, use of health services, and plan for care costs.5–9
Chronic diseases such as RA have required comprehensive care strategies that ensure adequate coverage of patients’ needs.10 One of these strategies is the creation of multidisciplinary care models (MCM) called also Centers of Excellence (CoE), whose objective is to obtain high-quality health results from the appropriate and minimum use of resources.11
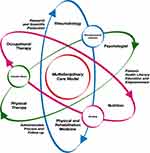
Although the country does not have a standardized model of care for the population with RA, unmet needs have encouraged the start of specialized healthcare centers CoE type. These centers are oriented to optimize results through an MCM, ensuring timely access to the services required to manage the disease.11 Comprehensive care model strategies MCM type for RA include 1) designing an educational program to involve the patient as part of the care process; 2) driving efforts to prevent complications or avoiding disability, reducing costs; 3) regular interdisciplinary care to determine disease progression and impact using a comprehensive care by a multidisciplinary team which includes rheumatologists, psychologists, nutritionists, physical and rehabilitation medicine, occupational therapists, physiotherapists, nurses, and pharmaceutical chemists by periodical appointments. Consultations are provided monthly in case of high or moderate disease activity and every three months in case of low disease activity or remission; 4) assessing compliance with pharmacological and non-pharmacological treatment to verify patient’s adherence to the medication. Also, taking into account the treat to target (T2T)12,13 strategy, which depending on the disease activity, patient must have more frequently rheumatologist’s appointments. Measures of disease activity must be obtained and documented regularly, as frequently as monthly for patients with high/moderate disease activity or less frequently (such as every 3–6 months) for patients in sustained low disease activity or remission. This strategy is recommended to manage patients with RA by international and national guidelines14,15 and 5) implementing risk management strategies that must be cost-effective in achieving therapeutic goals. Figure 1.
In Colombia, the health system that operates under a public and private insurance model serves the population with RA under a comprehensive Healthcare Policy,16 requiring follow-up and monitoring of risk management of patients with RA through a national registry. Furthermore, the Colombian Ministry of Health has designated this task to the National Registry of Rheumatoid Arthritis (NARRA),17 a non-governmental technical body of the Health System which was created by resolution 3974 of 2009 and established RA as a high-cost disease. This non-government entity performs the data analysis requested through resolution 1393 of 2015,18 in order to detect the disease early, to avoid the progression and death that impact on the Colombian RA population, thus forcing all entities that provide care services to patients with RA to provide the information that is requested. The institutions that provide the information collected by NARRA, in general, have a healthcare model without a multidisciplinary team, only based on the rheumatologist attention, so data obtained at NARRA are heterogeneous because it came from different institutional registries.18 Based on these data, the most relevant information related to the care of patients with RA is published annually, and this information is considered real-world evidence. It is necessary to clarify that previously,10 an article with some similar characteristics had already been published by our group, but unlike the previous one, many more patients are included in this paper; in the previous publication, the emphasis was on showing how the multidisciplinary care model of our specialized rheumatology center works, while in the current article, the emphasis is more on the clinical results and is also described in more detail the frequencies of use of conventional and biological drugs; in the current article, the frequency of rheumatology consultations and its relationship with the clinical outcome (DAS28) is more detailed, which for us was very important to show, since this demonstrates the effectiveness of multidisciplinary care models beside conventional care alone in rheumatology.
Under such context, the aim of this study is to describe the characteristics of care in patients with RA within the framework of an MCM of a specialized CoE in rheumatology, compared with national reports as evidence of the real-world data.
Materials and Methods
Design
We conducted a retrospective cohort study comparing clinical characteristics and effectiveness of health care obtained from an MCM model of a specialized CoE to manage RA in Colombia versus the NARRA data. In the present study, only the prevalent clinical, diagnostic and therapeutic data from CoE vs NARRA were analyzed and incident data were not included.
The specialized CoE in rheumatology database included data from institutional electronic medical records. The type of care followed by the patient under this model has been described in the introduction section. All the specialties involved in the MCM are detailed in Figure 1. The data published by the NARRA correspond to all patients seen in the Colombian health system and reported by care providers and insurers. Therefore, the medical records of patients with RA are sources of information, which is updated and validated periodically. This analysis is for the period between July 1, 2018 to June 30, 2019, for both cohorts. Data from 2020 to 2021 were not included because information from the pandemic period could be biased and do not reflect typical conditions of patient care.
Data Capture
The population included were adults (≥18 years) with a confirmed clinical diagnosis of RA defined by a rheumatologist, according to the 2010 ACR/EULAR classification criteria.19 In patients diagnosed before 2010, patients were classified according to the 1987 ACR/EULAR criteria.20 Patients from the specialized CoE in rheumatology were treated under the institutional “Treat to Target” (T2T) management strategy. Thus, the population of the national registry was treated within the health system with different models of care that cannot be identified based on the available published data.
The information collected included sociodemographic and clinical variables such as sex, age at diagnosis, duration of the disease, comorbidities, the current clinical condition of the disease, RA laboratory test [C-Protein Reactive (CPR), erythrocyte sedimentation rate (ESR), rheumatoid factor, and anti-citrulline antibodies], conventional radiography (foot and hand x-rays). Disease activity was measured using the disease activity score with 28-joint counts (DAS28) [interpreted as high (DAS28 >5.1), moderate (DAS28 ≤5.1–≥3.2), low (DAS28 <3.2–≥2.6), and remission (DAS-28216 <2.6)]. Physical disability was assessed routinely at least 2–3 times per year in the institution using the Health Assessment Questionnaire (HAQ), and NARRA requests it at least once a year. DAS28 was measured in the last six months before June 30, 2019. Information about pharmacological treatment was also obtained.
Statistical Analysis
Data were presented as frequencies and percentages and quantitative data as mean and standard deviations (SD) or median and interquartile range (IQR), according to the distribution.
For the comparative analysis between the institutional and the national registry, the pearson’s chi-square test or Fisher’s exact test were used for categorical variables. Normality data was checked by Kolmogorov–Smirnov test. For numeric variables, statistical significance was not calculated because the data for median difference calculation were not available. A p-value <0.05 was considered to indicate statistical significance for all tests. Statistical analysis was performed using STATA software release 16, College Station, Texas, USA.
Ethics Approval and Informed Consent
This study was conducted following the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee on Human Beings – Hospital de San José, Bogota, Colombia (Record 0317–2021, June 1st 2021). On the other hand, according to Resolution 1393 of 2015 from the Ministry of Health of Colombia, for whole private/public healthcare providers and insurances/payers companies is mandatory to report the data of patients with RA diagnosis to NARRA, which includes 89 clinical and administrative variables; all patients had previously signed informed consent for data use; the database from specialized in rheumatology center was anonymized to protect the confidentiality and privacy of patients.
Results
From July 1, 2018 to June 30, 2019, 7053 patients were treated at the specialized CoE in rheumatology using an MCM approach, and during the same period, the national registry of RA reported 81,492 patients. Baseline characteristics of patients are described in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of the Study Cohorts |
Concerning the demographic characteristics of the patients, females prevailed in both cohorts. The age of patients at diagnosis was about 50 years in both groups. Hypertension and osteoporosis were the most frequent comorbidities reported in both cohorts.
Clinical Tests (Laboratory and Radiographic) at the Time of Diagnosis
Of the total records, 4.20% (n = 303) of the cases treated in the specialized center and 5.86% (n = 4.766) in the national registry were incident cases. In this subgroup, patients from the specialized CoE in rheumatology had a higher proportion of clinical tests performed at the time of diagnosis than the national registry (Table 2).
 |
Table 2 Initial Tests in Incident Cases of Both Cohorts |
Clinical Characterization of Patients with RA
Table 3, reports the clinical characteristics of the patients. In the specialized CoE, 70% of patients received between 4 and 6 rheumatology visits per year, while the national registry reports three or fewer visits per year. A 56% remission status was achieved at the specialized center, while at the national level, remission was 31.1%, p-value <0.001. Similarly, significant differences were reported in the proportion of patients with low disease activity, favoring the specialized CoE. However, a substantial proportion of cases in the national registry do not have information related to the measurement of DAS28 (44.81%), p-value <0.001. Table 3 describes the main findings of the clinical variables. It is important to note the high proportion of missing data (33.7% to 80.5%) in laboratory tests and hand and foot x-ray in the national registry.
 |
Table 3 Clinical RA Characteristics of the Population in Both Cohorts |
Pharmacological Treatment in the Management of Rheumatoid Arthritis
Regarding pharmacological treatment, pain management was mainly based on non-opioid analgesics such as acetaminophen and dipyrone. In addition, the specialized CoE prescribed corticosteroids with more frequency. Significant differences (Table 4) were found about conventional drug-modifying antirheumatic-drugs (csDMARDs) due to the increased prescription of methotrexate, leflunomide, and sulfasalazine in the specialized CoE. In the specialized CoE, there were more patients receiving Certolizumab in comparison to the national registry (p-value= 0.042).
 |
Table 4 Frequency of Use of Medications in Study Cohorts |
Discussion
The specialized CoEs in RA based on an MCM model have three pillars: target population, continuous improvement, and healthcare quality. They improve access to treatment and achievement of disease remission, improve patients’ quality of life, reduce long-term disability risks, and reduce care costs by proposing the financial sustainability of health systems.10,11,21 This study compared the outcomes of a specialized CoE, which operates under a comprehensive care model based on the T2T strategy, with national RA registry data. The findings show greater access to rheumatologist care, laboratory studies, and radiographic images necessary for diagnosing and following the disease in the specialized CoE. These results are similar to other reports that have identified fewer barriers to access to specialized health care in patients with this type of care compared to conventional programs.10,11,22
In the centers specialized in RA, the rheumatologist is the leading physician who coordinates the patient’s comprehensive care, performs the follow-up, and assesses response to treatment.23 Timely rheumatological care allows the linking of new patients to specialized care models. As an outcome, these individuals have fewer complications and receive earlier and more effective treatments that decrease the progression of the disease and the need for surgical interventions for joint damage.24–27
Ensuring attention by the rheumatologist is also related to reducing gaps in inequity. For example, a study conducted in Canada showed 30% less likelihood of being diagnosed in rural populations promptly because of the lack of this specialty.28 The reduced number of consultations in the national registry may reflect the growing burden of RA on the supply of rheumatology services and the geographic gaps that limit care. Another advantage that specialized centers have is the availability of rheumatologists, greatly needed in Colombia as it has 0.4 specialists per 100,000 inhabitants, compared with countries such as Canada23 or Spain29 with two rheumatologists per 100,000 inhabitants.
The management of RA in Colombia aims to achieve a therapeutic target of remission according to the clinical practice guideline published in 2014.15 In addition, the Colombian evidence-based RA consensus4 proposes remission as a goal (measured by DAS28) in 30% of the treatment population. However, international guidelines30,31 recommend a minimum initial treatment goal of low disease activity over a remission goal since remission may not be attainable in a high percentage of patients and should be reevaluated periodically by individualizing cases.30
Studies have identified some factors associated with no remission, which may be potentially modifiable. These include the patient’s adverse perception of risks caused by medication, the low adherence to treatments, and the suboptimal communication between physicians and patients regarding the benefits of strict disease control activities in RA.32 In addition, there are other factors related to early detection of RA, since lower disease activity at the first visit is a strong clinical predictor of achieving remission and low disease activity.33 Therefore, specialized centers must keep up with the strategies necessary to ensure quality care over time.10,11,22
This analysis showed a higher percentage of patients with RA who achieved remission in the specialized CoE. In this regard, the high rate of patients who do not report evaluation of disease activity status using the DAS28 scale in the national registry is striking. Methods of manual data extraction15 could explain this absence of data, barriers in the availability of laboratories required (CRP or ESR), or lack of awareness among health professionals in their measurement. Specialized centers have among their advantages the availability of an information system that allows the capture of critical variables and standardized protocols for using validated composite measures such as DAS28. This feature allows analysis of the cohort behavior to evaluate the treatment in place, make better therapeutic decisions, and check research hypotheses in response to the needs of the patients.34
Additionally, specialized CoE performed more clinical tests because they adhere to updated clinical practice guidelines. Specialized CoE has also shown outstanding management by integrating multidisciplinary models into the care of patients with RA, which also guarantees the management of comorbidities.24,25 The cohorts analyzed and the literature34 show arterial hypertension and osteoporosis as the most frequent comorbidities related to the conditions of the disease.
According to Kvien,24 multidisciplinary care models must support their evidence practices, which allows the identification and prioritization of the most effective interventions in the RA population. A higher proportion of conventional therapy drug use is evident in the specialized center cohort, with no significant differences with the national registry regarding the use of biological DMARDs (except for Certolizumab). This finding suggests a rational use of pharmacological interventions in specialized CoEs by optimizing resources with more accurate patient selection for more advanced therapies.
The study limitations are inherent to the retrospective nature of the research and the inclusion of the data from the specialized center in the national registry—a mandatory report containing information from all institutions providing RA services in the country. Therefore, it was not possible to have homogenous data nor to separate the data of the specialized center from those of the national registry. Another limitation is that data cannot be extrapolated to other institutions, and it is necessary to compare the results of different centers using the same MCM model. However, the results described provide an approximation to the expected results of our multidisciplinary care model, including comprehensive equipment accessibility, periodic monitoring, and T2T strategy-based management. Moreover, there was a higher proportion of missing data at least 44% regarding rheumatology consultations in the national registry; maybe this happens because not all the institutions have the same attention model. However, the availability of comparable data, including information from clinical records in this study, is the main strength. We highlight the need for accurate and completeness of the data in real-life conditions for better analysis on the impact of the attention models.
Conclusion
The multidisciplinary care model of specialized Centers of Excellence in RA can guarantee comprehensive care, with better access to all the services required to manage the disease through a multidisciplinary team (led by the rheumatologist). This study shows how this model impacts the maintenance of remission and low disease activity when compared with other models of healthcare attention. It ensures specialist management and evidence-based care that facilitates the achievement of therapeutic goals. Specialized centers have more clinical care expertise, better patient records, and follow-up strategies to evaluate care outcomes. In fact, these types of models have begun to be introduced at the Latin American level, as shown in the REAL-PANLAR project.35 Future research should aim to assess the effectiveness of these centers as an option to ensure quality care, with the assessment of the disease status and progression of RA and other disease management and user satisfaction indicators in these types of models. Also, it will be important to assess the impact through an economic analysis on the disease’s progression in future research.
Data Sharing Statement
The authors declare that the database and other study materials are available for review at any time. All files, databases and other documents related to the study are available on a computer in our research office and with access only to the team of researchers. In any case, please contact PSM.
Acknowledgments
The authors would like to thank Biomab IPS for the administrative data. To Michael Cabrera-González who help in the data collection and Fernando Rodriguez-Florido for figure conception and design.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Pedro Santos-Moreno reports personal fees, non-financial support from AbbVie, grants, personal fees, non-financial support from Janssen, grants, personal fees, non-financial support from Pfizer, grants, personal fees, non-financial support from Novartis, grants, personal fees, non-financial support from Biopas – UCB, personal fees, non-financial support from Roche, personal fees, non-financial support from Bristol, personal fees, non-financial support from Sanofi, personal fees, non-financial support from Lilly, personal fees, non-financial support from Tecnofarma, outside the submitted work. The other authors declare that they have no known competing commercial, financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
References
1. Fazal SA, Khan M, Nishi SE, et al. A clinical update and global economic burden of rheumatoid arthritis. Endocr Metab Immune Disord Drug Targets. 2018;18(2):98–109. doi:10.2174/1871530317666171114122417
2. van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32(2):174–187. doi:10.1016/J.BERH.2018.10.005
3. Hsieh PH, Wu O, Geue C, McIntosh E, McInnes IB, Siebert S. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis. 2020;79(6):S771–S777. doi:10.1136/ANNRHEUMDIS-2019-216243
4. Cuenta de Alto Costo. Fondo Colombiano de Enfermedades de Alto Costo Consenso Basado En La Evidencia: indicadores de Gestión Del Riesgo En Pacientes Con Artritis Reumatoide En Colombia; 2018. Available from: https://cuentadealtocosto.org/site/publicaciones/consenso-basado-en-evidencia-indicadores-de-gestion-del-riesgo-en-pacientes-con-artritis-reumatoide-en-colombia/.
5. Bridges SL, Hughes LB, Mikuls TR, et al. Early rheumatoid arthritis in African-Americans: the CLEAR registry. Clin Exp Rheumatol. 2003;21(SUPPL. 31):S138–S145.
6. Kremer J. The Corrona US registry of rheumatic and autoimmune diseases. Clin Exp Rheumatol. 2016;34(Supp101):S96–S99.
7. Shadick NA, Gerlanc NM, Frits ML, et al. The longitudinal effect of biologic use on patient outcomes (disease activity, function, and disease severity) within a rheumatoid arthritis registry. Clin Rheumatol. 2019;38(11):3081–3092. doi:10.1007/S10067-019-04649-4
8. Yamanaka H, Tanaka E, Nakajima A, et al. A large observational cohort study of rheumatoid arthritis, IORRA: providing context for today’s treatment options. Mod Rheumatol. 2020;30(1):1–6. doi:10.1080/14397595.2019.1660028
9. Curtis JR, Jain A, Askling J, et al. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin Arthritis Rheum. 2010;40(1):2–14.e1. doi:10.1016/J.SEMARTHRIT.2010.03.003
10. Santos-Moreno P, Castillo P, Villareal L, Pineda C, Sandoval H, Valencia O. Clinical outcomes of patients with rheumatoid arthritis treated in a disease management program: real-world results. Open Access Rheumatol Res Rev. 2020;12:249–256. doi:10.2147/OARRR.S270700
11. Santos-Moreno P, Castañeda O, Garro B, Flores D, Sánchez G, Castro C. From the model of integral attention to the creation of centers of excellence in rheumatoid arthritis. Clin Rheumatol. 2015;34(Suppl1):71–77. doi:10.1007/S10067-015-3017-8
12. Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–637. doi:10.1136/ARD.2009.123919
13. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15. doi:10.1136/ANNRHEUMDIS-2015-207524
14. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):S685–S699. doi:10.1136/ANNRHEUMDIS-2019-216655
15. Ministry of health and social protection. Clinical Practice Guideline for Early Detection, Diagnosis and Treatment of Rheumatoid Arthritis; 2014. Available from: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/CA/gpc-tratamiento-artritis-reumatoide-completa.pdf.
16. Ministry of health and social protection. Política de Atención Integral en Salud Comprehensive Health Care Policy; 2016. Available from: https://www.minsalud.gov.co/Paginas/politica-integral-de-atencion-en-salud.aspx.
17. Ministry of health and social protection. Resolution 123 of 2015; 2015. Available from: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/resolucion-0123-de-2015.pdf.
18. Ministry of health and social protection. Resolución 1393 de 2015 Resolution 1393 of 2015; 2015. Available from: https://cuentadealtocosto.org/site/wp-content/uploads/2019/10/Resolucion-1393-de-2015-ARTRITIS.pdf.
19. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/ART.27584
20. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/ART.1780310302
21. Santos-Moreno P, Alvis-Zakzuk NJ, Villarreal-Peralta L, Carrasquilla-Sotomayor M, de la Hoz-Restrepo F, Alvis-Guzmán N. Centers of excellence implementation for treating rheumatoid arthritis in Colombia: a cost-analysis. Clinicoecon Outcomes Res. 2021;13:583–591. doi:10.2147/CEOR.S308024
22. Santos-Moreno P, Alvis-Zakzuk NJ, Villarreal-Peralta L, Carrasquilla-Sotomayor M, Paternina-Caicedo A, Alvis-Guzmán N. A comprehensive care program achieves high remission rates in rheumatoid arthritis in a middle-income setting. experience of a center of excellence in Colombia. Rheumatol Int. 2018;38(3):499–505. doi:10.1007/S00296-017-3903-2
23. Widdifield J, Paterson JM, Bernatsky S, et al. Access to rheumatologists among patients with newly diagnosed rheumatoid arthritis in a Canadian universal public healthcare system. BMJ Open. 2014;4:1. doi:10.1136/BMJOPEN-2013-003888
24. Kvien TK, Balsa A, Betteridge N, et al. Considerations for improving quality of care of patients with rheumatoid arthritis and associated comorbidities. RMD Open. 2020;6:2. doi:10.1136/RMDOPEN-2020-001211
25. Barber CEH, Then KL, Bohm V, et al. Development of a patient-centered quality measurement framework for measuring, monitoring, and optimizing rheumatoid arthritis care in Canada. J Rheumatol. 2021;48(3):326–334. doi:10.3899/JRHEUM.200688
26. Meisters R, Putrik P, Ramiro S, et al. EULAR/eumusc.net standards of care for rheumatoid arthritis: cross-sectional analyses of importance, level of implementation and care gaps experienced by patients and rheumatologists across 35 European countries. Ann Rheum Dis. 2020;79(11):1423–1431. doi:10.1136/ANNRHEUMDIS-2020-217520
27. Taylor PC, Law ST. When the first visit to the rheumatologist is established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33(5):101479. doi:10.1016/J.BERH.2019.101479
28. Barnabe C. Disparities in rheumatoid arthritis care and health service solutions to equity. Rheum Dis Clin North Am. 2020;46(4):685–692. doi:10.1016/J.RDC.2020.07.005
29. Sanchez-Piedra C, Yoldi B, Valero M, Andreu JL, Díaz-González F, Gómez-Reino JJ. Status of rheumatology in Spain in 2017: 2.0 rheumatologists per 100,000 population. Reumatol Clin. 2018;14(5):311–312. doi:10.1016/J.REUMA.2017.10.003
30. Benham H, Rutherford M, Kirby S, et al. Treat-to-target in rheumatoid arthritis: evaluating the patient perspective using the patient opinion real-time anonymous liaison system: the RA T2T PORTAL study. Int J Rheum Dis. 2019;22(5):874–879. doi:10.1111/1756-185X.13514
31. Nagy G, Roodenrijs NMT, Welsing PMJ, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80(1):31–35. doi:10.1136/ANNRHEUMDIS-2020-217344
32. Owensby JK, Chen L, O’Beirne R, et al. Patient and rheumatologist perspectives regarding challenges to achieving optimal disease control in rheumatoid arthritis. Arthritis Care Res. 2020;72(7):933–941. doi:10.1002/ACR.23907
33. Gamboa-Cárdenas RV, Ugarte-Gil MF, Loreto M, et al. Clinical predictors of remission and low disease activity in Latin American early rheumatoid arthritis: data from the GLADAR cohort. Clin Rheumatol. 2019;38(10):2737–2746. doi:10.1007/S10067-019-04618-X
34. Belmonte Serrano MÁ. [Is the DAS28 score the most adequate method to estimate activity in rheumatoid arthritis? Clinimetric considerations and simulations scenarios]. Reumatol Clin. 2008;4(5):183–190. Spanish. doi:10.1016/S1699-258X(08
35. Santos-Moreno P, Caballero-Uribe CV, Cardiel MH, et al. A consensus position paper from REAL-PANLAR group about the methodological approach for the accreditation process of centers of excellence in rheumatoid arthritis in Latin America. J Clin Rheumatol. 2019;25(1):54–58. doi:10.1097/RHU.0000000000000773
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.