Back to Journals » Patient Preference and Adherence » Volume 14
BetaEval Global: Prospective, Multinational, Observational Cohort Study of Patients Using BETACONNECT®
Authors Patti F, Martínez Ginés ML, Norenberg C, Duarte Caron F
Received 17 January 2020
Accepted for publication 26 March 2020
Published 28 April 2020 Volume 2020:14 Pages 771—779
DOI https://doi.org/10.2147/PPA.S245955
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Francesco Patti,1 María Luisa Martínez Ginés,2 Christiane Norenberg,3 Fernando Duarte Caron4
1Department of Medical and Surgical Sciences and Advanced Technologies, GF Ingrassia; Multiple Sclerosis Center, University of Catania, Catania, Italy; 2Department of Neurology, Hospital General Universitario Gregorio Marañon, Madrid, Spain; 3Bayer AG, Wuppertal, Germany; 4Bayer, Whippany, New Jersey, NY, USA
Correspondence: Francesco Patti
Multiple Sclerosis Center, University of Catania, Piazza Università, 2, Catania 95131, Italy
Email [email protected]
Purpose: Patients with multiple sclerosis (MS) would benefit from continued long-term treatment with disease-modifying therapies, and autoinjectors may help improve patients’ satisfaction with therapy, thereby increasing adherence rates. BETACONNECT® is an autoinjector for interferon beta-1b designed to improve the injection experience for patients. The BetaEval Global study assessed medication intake in patients using BETACONNECT to further investigate the value of this autoinjector.
Patients and Methods: The BetaEval Global study was a prospective, non-interventional cohort study across multiple European countries in patients with relapsing-remitting MS (RRMS) or clinically isolated syndrome (CIS) who were starting interferon beta-1b treatment. The decision to administer interferon beta-1b was made independently of the study. Patients were assessed at the initial visits and planned follow-up visits at Weeks 4, 12, and 24. The primary outcome variable was compliance with therapy based on the medication possession ratio (MPR). Injections were automatically recorded by the BETACONNECT device or, in some instances, self-reported by the patients. This allowed for a complete dataset that could be used in the calculation of the MPR.
Results: Four hundred ninety-eight patients were enrolled and completed 93.9%, 95.2%, and 95.4% of prescribed injections at Weeks 4, 12, and 24, respectively. Similarly, 76.4% (n=318), 76.6% (n=297), and 81.1% (n=284) of patients completed at least 80% of their prescribed injections. Median scores assessing patient satisfaction with the autoinjector were consistently high across the study.
Conclusion: Overall, the results from BetaEval Global demonstrated that in this cohort of patients with RRMS or CIS on interferon beta-1b, use of the BETACONNECT autoinjector was associated with high rates of compliance, adherence, and patient satisfaction.
Keywords: multiple sclerosis, interferon beta-1b, autoinjector, adherence, disease-modifying therapy
Introduction
Multiple sclerosis (MS) is a chronic degenerative neurologic disorder in which patients usually require treatment over years or decades to minimize relapses and limit disability. Consequently, disease-modifying therapies (DMTs) should be most effective when patients remain on treatment with no or only minimal interruptions. A 2003 report from the World Health Organization (WHO), which defines adherence as the extent to which a person’s behavior, such as following medication regimen, dietary and lifestyle changes, corresponds with the recommendations of health care providers, states that:
increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.1
However, many studies have found insufficient rates of adherence to DMTs among patients with MS.2–6 Regardless of disease and factors such as disease state or prognosis, 30% to 50% of patients did not adhere to appropriate use of prescription medications.5 This poor adherence, in turn, can be associated with worse disease outcomes, including a higher risk of relapses, higher risk of disease progression, lower quality of life, and higher health care resource utilization.2,4,5,7-10
Understanding the factors that contribute to poor adherence may help physicians and patients to counter these negative influences, improve medication intake behaviors, and potentially improve outcomes. Many factors have been associated with poor adherence, including age, disease duration, psychologic factors, ease of injections, overall patient satisfaction with therapy, perceived lack of efficacy, adverse events (AEs), disease-related factors, depression, fatigue, motor impairments, and cognitive deficits.2,11-14 It has been reported that injectable DMTs for regular self-administration have slightly higher adherence (68%) than orally administered DMTs (63%).15 The use of autoinjectors to administer injectable DMTs has been associated with improved adherence by, among other factors, reducing the rate at which injection site reactions occur.11,16
BETACONNECT® is a fully electronic autoinjector for interferon beta-1b (Betaferon®/Betaseron®; Bayer AG; Berlin, Germany) that incorporates a number of features to facilitate the injection process, including a four-phase injection technology and individual control of injection speed, depth, dwell time, and needle retraction rate.17 The autoinjector also provides visual and auditory indications at the end of the dose. Furthermore, operation of the device has been made quieter to reduce anxiety around injection. BETACONNECT has also been designed with a shape that should be easier to handle for patients with motor impairments affecting their arms and hands. Lastly, BETACONNECT has communication capabilities that include capture of data on injections that can help patients remain adherent with prescribed therapy. Specifically, the device offers the capability to record injection time and data, injection status (OK, user interrupted, failed), insertion depth, injection speed, and the volume of the injected dose. These data are synchronized using a patient’s personal computer, tablet, or smartphone via the myBETAapp® to allow for personalization of the injection to suit the patient’s individual preferences.
The potential benefits of BETACONNECT have been investigated in three studies to date. Two user surveys found preference for the novel device over the participants’ previous injection methods.18,19 In addition, a recent prospective, observational study of patients in Germany also found high levels of patient satisfaction with therapy and high adherence rates among BETACONNECT users.17 The goal of the present study was to further investigate the benefits of this autoinjector by evaluating medication intake among patients using BETACONNECT to administer interferon beta-1b in a multinational study across European countries.
Patients and Methods
The BetaEval Global study was a prospective, non-interventional cohort study conducted in 77 centers in 11 European countries (Austria, Belgium, Bosnia and Herzegovina, Croatia, Czech Republic, France, Greece, Hungary, Italy, Spain, and Switzerland). Patients with relapsing-remitting MS (RRMS) or clinically isolated syndrome (CIS) who were starting or currently on treatment with interferon beta-1b were enrolled. The decision to start interferon beta-1b was made independently from enrollment in the study. Assessments were conducted at the initial visits with follow-up visits planned for Weeks 4, 12, and 24.
The primary outcome variable was compliance with therapy based on the medication possession ratio (MPR). An indirect measure of compliance, MPR is beneficial for identifying patients at risk for treatment failure and providing data on medication refilling patterns.20 Per protocol, injections were to be automatically recorded by the BETACONNECT device, and case report forms allowed for additional injection information entry if needed, allowing for a complete dataset that could be used in the calculation of the MPR. The number of recorded injections was compared with the number of expected injections using the following formula: (number of treatment days during the observation period/expected number of treatment days during the observation period) x 100. Based on the standard definition of adherence used in studies of medication intake behavior,20 patients were considered adherent if they injected ≥80% of the expected interferon beta-1b dosages up to the respective follow-up visit over the course of the study.
Secondary outcome variables included persistence of interferon beta-1b treatment, satisfaction with and evaluation of the BETACONNECT autoinjector, evaluation of injection site pain, and analgesic use prior to injection of interferon beta-1b, which were recorded by a patient questionnaire. Persistence of interferon beta-1b treatment through BETACONNECT usage was measured as the time in days from initial usage to termination of usage or final visit, whichever came first. Additional secondary outcome assessments included the Functional Assessment of Multiple Sclerosis (FAMS) for health-related quality of life, Hospital Anxiety and Depression Scale (HADS) and Center for Epidemiologic Studies Depression Scale (CES-D) for symptoms of anxiety and depression, Fatigue Scale for Motor and Cognitive functions (FSMC) for fatigue, and Symbol Digit Modalities Test (SDMT) for cognitive function. Local skin reactions and injection-related specifics such as injection date, time, and speed (all recorded automatically by the BETACONNECT) were also assessed. Satisfaction with BETACONNECT and the patient’s previous method of injection was assessed using questionnaires at the initial visit and at each individual visit. Questionnaires asked patients to rank their satisfaction on a scale from 0 (lowest) to 10 (highest).
This study was reviewed and approved by the relevant ethics review committees, with approval by the coordinating centers’ ethics committees on June 25, 2014 (Table S1). Study sites were requested to obtain a written informed consent from each patient prior to inclusion in the study.
Experimental Design and Statistics
Statistical analyses were exploratory and of a descriptive nature. Continuous variables were described by sample statistics (ie, mean, standard deviation, median, and interquartile range [IQR]). The description of categorical variables was done with frequency tables displaying the actual number of patients in a category as well as percentages. The number of patients with missing data was presented as a separate category. For data over time, the respective change from baseline was analyzed with Wilcoxon’s signed-rank test for continuous data and McNemar's test or Cochran’s Q test for categorical data. The primary analysis was at 24 weeks. Additional analyses were also performed at 4 and 12 weeks.
Multivariate logistic regression models were used to identify potential predictors for compliance and adherence at the final visit. The following covariates were factored into the models: age; sex; baseline Expanded Disability Status Scale (EDSS) score; baseline CES-D score; presence of concomitant diseases of special interest (fatigue, anxiety, or depression); use of concomitant medications; education level; employment status; baseline FAMS total score; baseline FSMC; frequency of myBETAapp use; satisfaction with myBETAapp; baseline HADS score; MS duration at the initial visit; participation in the BETAPLUS® patient support program; previous treatment; pain intensity with previous injection method; baseline SDMT score; and previous injection method type.
Results
Patient Disposition
Between October 2014 and July 2016, BetaEval Global enrolled 500 patients, but 2 patients were excluded due to having insufficient information to assess compliance. The full analysis set included 498 patients (Table 1), with a median (IQR) age of 44 (35–52) years. Median (IQR) disease duration was 76.2 (30.5–144.1) months for patients with RRMS and 22.7 (8.9–81.4) for patients with CIS. Median (IQR) EDSS was 1.5 (1–3) at baseline and 315 patients (63%) had an EDSS <3 (EDSS missing from 51 patients [10.2%]). At study entry, 485 patients (97%) had already been treated with interferon beta-1b, of whom 18.1% had used the BETACONNECT autoinjector before the start of the study. Approximately 45.2% of patients participated in the BETAPLUS patient support program. A total of 15 (3%) patients had no prior history of receiving interferon beta-1b treatment. Among these patients, 12 (2.4%) had never received MS treatment while 3 (0.6%) patients received different forms of treatment other than interferon beta-1b.
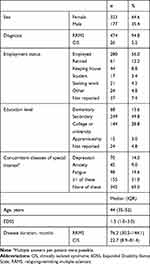 |
Table 1 Baseline Characteristics (N=500) |
A history of concomitant diseases of special interest (depression, anxiety, and/or fatigue) was reported by 155 patients (31%). During the course of the study, 56% of patients received concomitant treatment (33.6% took 1–2 medications, 15.8% took 3–5 medications, 5.6% took 6–10 medications, and 1% took >10 medications).
After 24 weeks, 405 patients (81.4%) completed the study. The main reasons for study discontinuation were switching to another method of Betaferon application (25.8%; switching to either the BETAJECT liteTM or BETAComfortTM autoinjector or opting for manual Betaferon injection), withdrawal of patient consent (22.6%), becoming lost to follow-up (17.2%), and adverse events (9.7%). Approximately 96.3% of patients who completed the study (and 26.9% of dropouts) still used BETACONNECT at their final visit.
Compliance and Adherence
The median percentage of injections completed using BETACONNECT remained stable between 93.9% and 95.4% at all visits in patients who completed the study (Figure 1A). Among patients with non-missing compliance data at Weeks 4, 12, and 24, 76.4% (n=318), 76.6% (n=297), and 81.1% (n=284), respectively, qualified as adherent (ie, had completed ≥80% of planned injections using BETACONNECT) (Figure 1B). According to sensitivity analyses, adherence rates were similar when automated recordings were incomplete. Patients who were never on MS treatment (12 [2.4%]) and those who were on treatment other than interferon beta-1b (3 [0.6%]) demonstrated no difference in adherence at final visit compared with patients with history of prior Betaferon treatment (OR 1.578; 95% CI: 0.469, 5.31 and OR 1.578, 95% CI: 0.142, 17.516, respectively).
Median (IQR) persistence with BETACONNECT use was 181 days (170–197) in patients who completed the study. Among those study completers, 91.9% still used BETACONNECT at Weeks 4 and 12 and 96.3% still used the autoinjector at Week 24.
Multivariate logistic regression models indicated that better compliance and adherence were associated with no presence of symptoms of concomitant diseases of special interest (fatigue, depression, or anxiety), and shorter MS duration at initial visit with a trend for association with higher satisfaction with the myBETAapp (Table 2).
 |
Table 2 Logistic Regression Results for Compliance and Adherence |
Satisfaction with BETACONNECT and Other Secondary Endpoints
Among patients being treated with interferon beta-1b at the start of the study (n=483), 450 (90.4%) completed the satisfaction survey at baseline. Sixty-six of these patients (14.7%) were already BETACONNECT users at study start. Prior experience with BETACONNECT did not affect patient-reported ratings of satisfaction with the device. Median satisfaction scores for BETACONNECT were either 8 or 9 (out of a possible 10) at all time points for both patients with and without prior BETACONNECT experience (Table 3). Median satisfaction with the previous injection method was 8 for patients with and without BETACONNECT experience.
 |
Table 3 Satisfaction with the Patient’s Previous Injection Method and BETACONNECT® Across Visits |
Median scores on the FAMS questionnaire indicated no major changes during the course of the study (Table 4). Median HADS scores for anxiety and depression were stable across the study period and below the threshold considered necessary for the presence of clinically relevant depression or anxiety (a score of 8). Consistent with the findings from the HADS, median CES-D scores were also below the threshold to suggest depressive symptoms (a score of 16). Median FSMC scores indicated a level of motor and cognitive fatigue below the threshold considered clinically relevant in the trial population (a score <43). Median scores on the SDMT remained similar during the period of the study.
 |
Table 4 Secondary Outcome Measures |
Discussion
BetaEval Global showed high rates of compliance and adherence among patients with RRMS or CIS using BETACONNECT to administer interferon beta-1b. The BETACONNECT injection data confirmed the high rate of compliance over the entire observation period, with a median of >90% of injections completed. Rates of compliance and adherence were associated with several patient- and disease-related factors but not education or employment level. This high rate of adherence comes along with high satisfaction scores reported during the study, suggesting that patients may perform their injections more regularly when the injection experience is less onerous.
The rate of patients classified as adherent in this study compares favorably with previous studies of patients on interferon beta-1b who were not utilizing BETACONNECT. A systematic review of published studies found adherence rates of 85.2% to 49.0%, although the methods to classify a patient as adherent varied across studies, with lower adherence in the retrospective studies.21 Only approximately 50% of patients were considered adherent when a similar definition to the current study was used. Overall this finding suggests that the use of an autoinjector can improve adherence to interferon beta-1b therapy.
Interpreting self-reported adherence data from questionnaire-based studies compared to claims-based data poses the risk of over-estimation due to patient recall bias and social desirability.3,22 The use of patient self-reported data is the most unreliable yet commonly practiced approach in assessing adherence, and gives rise to difficulties when comparing adherence among DMTs.23 In comparison to our study, one retrospective cohort study estimated adherence during 720 days of follow-up using the German Institute for Drug Use Evaluation (DAPI) pharmacy claims-based data from 2002 to 2009 by identifying both new and renewed prescriptions from 2001 to 2005. This study provided comparative adherence data on Avonex®, Betaferon, Rebif®, and Copaxone®. It was reported that Avonex had slightly higher adherence and persistence data (34.2% and 42.8%, respectively) than Betaferon (33.4% and 40.6%), followed by Rebif (31.7% and 39.2%) and Copaxone (29.8% and 37.0%) throughout this 2-year follow-up period.3 While it is important to note that the study was performed prior to the release of BETACONNECT and RebiSmart® autoinjectors, this study demonstrated that patients were slightly more adherent to Betaferon than other DMTs, such as Rebif and Copaxone. Future investigations comparing patient adherence to DMTs administered with the different auto-injectable devices available could provide valuable information toward increasing overall adherence in MS patients.
With the completion of the BetaEval Global study, the patient experience with BETACONNECT has now been investigated in 5 different studies to date, each using different methodologies. In one study, 1365 BETACONNECT users in Germany responded to a 13-question survey that assessed their experience and satisfaction with the autoinjector.19 The majority of patients in the study reported that they found the device to be helpful or very helpful in supporting their interferon beta-1b therapy. Among the individual features of the device, the optical/acoustic signals at the end of the injection and adjustable injection depth/speed were considered to be particularly important. A second survey was conducted among 118 BETACONNECT users in Germany to assess patient satisfaction and preference for the autoinjector.17 Ninety percent of survey respondents in this case reported that they preferred BETACONNECT over their previous injection method, typically because of its ease of use and reduced pain/irritation at the injection site. Patients also reported that the ability to adjust injection speed and depth, the overall quietness of the injection, and the ease of handling the device improved their experience with the autoinjector relative to their last injection method.
There are many factors that contribute to patient satisfaction and adherence to treatment regimen. Patient education through provision of easy to understand treatment information, and on identifying and managing adverse events can have a positive impact on patient adherence.24 Forgetting to administer their injections has been reported to be the most common reason for nonadherence.2,25,26 We speculate that the distinguishing technology and features of BETACONNECT may prevent these factors from playing a more significant role in patient nonadherence by conveniently providing reminders for upcoming injection administrations, or contributing to a more pleasurable experience with the lower levels of noise emitted by the device.
To date, there is a lack of data directly comparing the various autoinjectors available for MS. However, one study involved structured interviews of 90 BETACONNECT-naïve patients in the United States who were given a demonstration of the features of the device.18 Prior to using the autoinjector, 96% of patients reported that an injection process with minimal difficulty was considered to be an important attribute for any autoinjector. After experiencing the device, 83% of participants reported that they preferred BETACONNECT over their current injection method, most often because of the ease of injection, user-friendly functions, and the quietness of the device. Another study by Limmroth et al reported that 82% of patients using BETACONNECT were satisfied with the autoinjector device compared with RebiSmart (67%) and ExtaviPro® (60%), with key differentiators of BETACONNECT being its unique features such as next injection reminders and lower noise levels when operating the device.27
The BETACONNECT user experience was also investigated in a prospective, observational cohort study that enrolled 143 patients in Germany and followed them for 24 weeks.28 Based on injection data collected by the device throughout the study, patients completed 86.3% of injections at the first assessment and 92.9% of injections at Week 24. Over 81.1% of patients completed ≥80% of their injections at 4 weeks and 80.5% did so at the end of the study period. Approximately 75% of the patients in this study were still using BETACONNECT to perform injections at Week 24 and satisfaction with BETACONNECT remained high throughout the study.
The results of these 4 studies are consistent with the findings of BetaEval Global. Overall, patients consistently reported high rates of satisfaction with the device. Furthermore, high percentages of patients completed their prescribed injections.
Limitations of this study include the relatively short duration of follow-up, the lack of head-to-head comparisons with other autoinjectors, and the differences in medication availability during the study period of October 2014 through July 2016 in comparison with current DMT options based on the 2018 European Committee for Treatment and Research in Multiple Sclerosis/European Academy of Neurology (ECTRIMS/EAN) Guideline on pharmacologic treatment for MS patients.29 All patients were followed in European countries where cladribine, generic glatiramer acetate, and ocrelizumab were not approved at the time.30–32 A longer follow-up period would be needed to determine if patients maintained their high rates of compliance and adherence over the long term, which is the most desirable outcome of a switch to an autoinjector since patients’ preference of DMT use has been demonstrated to change over time.33 In addition, direct comparisons with other autoinjectors could be beneficial to determine the relative benefits of the unique features of BETACONNECT.
Furthermore, it is possible that the adherence rate in the current study is higher than what would be expected in clinical practice given that patients enrolled in a clinical trial may be more highly motivated to remain adherent than those in regular clinical practice.14
In this cohort of patients with RRMS or CIS being treated with interferon beta-1b, use of the BETACONNECT autoinjector was associated with high rates of compliance, adherence, and patient satisfaction. Ongoing studies will attempt to gain a better understanding of medication intake behavior over the long term and its relationship to outcomes. Additional research will also explore the potential benefits of improved compliance through the use of the autoinjector on disease outcomes.
Acknowledgments
This study was sponsored by Bayer AG. The authors wish to thank all of the patients and investigators in the BetaEval Global study for their contribution to improving the care of patients with MS. Eva-Maria Wicklein, Bayer AG, reviewed the manuscript and Jong Jun Shin, MD, of VMLY&R Health, provided medical writing assistance that was funded by Bayer. The contents of this paper were presented at ECTRIMS 2017 (Paris, FR) as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in Multiple Sclerosis Journal: [https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/199827/francesco.patti.betaeval.global.prospective.non-interventional.multinational.html].
Disclosure
F Patti served as an advisor for Almirall, Bayer, Biogen, Celgene, Merck, Novartis, Sanofi Genzyme, Roche, Reload Onlus, FISM and Teva, and received grants for speaking activities from the same companies. ML Martínez Ginés has received compensation for consulting services and speaking fees from Merck, Biogen, Novartis, Sanofi Genzyme, Almirall, Bayer, Roche, and Teva. C Norenberg is a salaried employee of Bayer AG. F Duarte Caron is a salaried employee of Bayer Healthcare. The authors report no other conflicts of interest in this work.
References
1. Sabaté E. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003.
2. Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18(1):69–77. doi:10.1111/j.1468-1331.2010.03110.x
3. Hansen K, Schüssel K, Kieble M, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One. 2015;10(7):e0133279. doi:10.1371/journal.pone.0133279
4. Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30(2):89–100. doi:10.2165/11533330-000000000-00000
5. Tremlett H, Van der Mei I, Pittas F, et al. Adherence to the immunomodulatory drugs for multiple sclerosis: contrasting factors affect stopping drug and missing doses. Pharmacoepidemiol Drug Saf. 2008;17(6):565–576. doi:10.1002/pds.1593
6. Rio J, Porcel J, Téllez N, et al. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Mult Scler. 2005;11(3):306–309. doi:10.1191/1352458505ms1173oa
7. Al-Sabbagh A, Bennett D, Kozma C, Dickson M, Meletiche D. Medication gaps in disease-modifying drug therapy for multiple sclerosis are associated with an increased risk of relapse: findings from a national managed care database. J Neurol. 2008;255(suppl 2):S79.
8. Ivanova JI, Bergman RE, Birnbaum HG, Phillips AL, Stewart M, Meletiche DM. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis in the US. J Med Econ. 2012;15(3):601–609. doi:10.3111/13696998.2012.667027
9. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251–260. doi:10.2147/CEOR.S130334
10. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi:10.1056/NEJMra050100
11. Lugaresi A, Florio C, Brescia-Morra V, et al. Patient adherence to and tolerability of self-administered interferon β-1a using an electronic autoinjection device: a multicentre, open-label, Phase IV study. BMC Neurol. 2012;12(1):7. doi:10.1186/1471-2377-12-7
12. Meyniel C, Spelman T, Jokubaitis VG, et al. Country, sex, EDSS change and therapy choice independently predict treatment discontinuation in multiple sclerosis and clinically isolated syndrome. PLoS One. 2012;7(6):e38661. doi:10.1371/journal.pone.0038661
13. Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256(4):568–576. doi:10.1007/s00415-009-0096-y
14. Lugaresi A, Rottoli MR, Patti F. Fostering adherence to injectable disease-modifying therapies in multiple sclerosis. Expert Rev Neurother. 2014;14(9):1029–1042. doi:10.1586/14737175.2014.945523
15. Morillo Verdugo R, Ramírez Herráiz E, Fernández-Del Olmo R, Roig Bonet M, Valdivia García M. Adherence to disease-modifying treatments in patients with multiple sclerosis in Spain. Patient Prefer Adherence. 2019;13:261–272. doi:10.2147/PPA.S187983
16. Pozzilli C, Schweikert B, Ecari U, Oentrich W, Bugge JP. Quality of life and depression in multiple sclerosis patients: longitudinal results of the BetaPlus study. J Neurol. 2012;259(11):2319–2328. doi:10.1007/s00415-012-6492-8
17. Ziemssen T, Sylvester L, Rametta M, Ross AP. Patient satisfaction with the new interferon beta-1b autoinjector BETACONNECTTM. Neurol Ther. 2015;4(2):125–136. doi:10.1007/s40120-015-0036-y
18. Barone DA, Singer BA, Merkov L, Rametta M, Suarez G. Survey of US patients with multiple sclerosis: comparison of the new electronic interferon beta-1b autoinjector (BETACONNECTTM) with mechanical autoinjectors. Neurol Ther. 2016;5(2):155–167. doi:10.1007/s40120-016-0047-3
19. Weller I, Saake A, Schreiner T, Vogelreuter J, Petroff N. Patient satisfaction with the BETACONNECTTM autoinjector for interferon beta-1b. Patient Prefer Adherence. 2015;9:951–959. doi:10.2147/PPA.S85917
20. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi:10.1155/2015/217047
21. Menzin J, Caon C, Nichols C, White LA, Friedman M, Pill MW. Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis. J Manag Care Pharm. 2013;19(1 Suppl A):S24–40. doi:10.18553/jmcp.2013.19.s1.S24
22. Cox D, Stone J. Managing self-injection difficulties in patients with relapsing-remitting multiple sclerosis. J Neurosci Nurs. 2006;38(3):167–171. doi:10.1097/01376517-200606000-00005
23. Klauer T, Zettl UK. Compliance, adherence, and the treatment of multiple sclerosis. J Neurol. 2008;255(suppl 6):87–92. doi:10.1007/s00415-008-6016-8
24. Arroyo E, González M, Grau C, et al. Proposals to improve adherence to immunomodulatory therapies in patients with multiple sclerosis. Neurología. 2010;25(9):544–551. doi:10.1016/j.nrl.2010.07.004
25. de Seze J, Borgel F, Brudon F. Patient perceptions of multiple sclerosis and its treatment. Patient Prefer Adherence. 2012;6:263–273. doi:10.2147/PPA.S27038
26. Conway D, Cecilia Vieira M, Thompson NR, Parker KN, Meng X, Fox R. Patient-reported disease-modifying therapy adherence in the clinic: a reliable metric? Mult Scler J Exp Transl Clin. 2018;4(2):2055217318777894.
27. Limmroth V, Reischl J, Mann B, et al. Autoinjector preference among patients with multiple sclerosis: results from a national survey. Patient Prefer Adherence. 2017;11:1325–1334. doi:10.2147/PPA.S137741
28. Kleiter I, Lang M, Jeske J, Norenberg C, Stollfuss B, Schürks M. Adherence, satisfaction and functional health status among patients with multiple sclerosis using the BETACONNECT® autoinjector: a prospective observational cohort study. BMC Neurol. 2017;17(1):174. doi:10.1186/s12883-017-0953-8
29. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96–120. doi:10.1177/1352458517751049
30. European Medicines Agency. European public assessment reports: background and context. Available from: https://www.ema.europa.eu/.
31. COPAXONE® is approved for treatment of patients with a first clinical event suggestive of multiple sclerosis [press release]. Jerusalem, Israel: BusinessWire; 2009. Available from: https://www.businesswire.com/news/home/20090204005697/en/COPAXONE%C2%AE-Approved-Treatment-Patients-Clinical-Event-Suggestive.
32. Mylan, in partnership with Synthon, receives marketing authorization approval in Europe for first generic for Copaxone® 40 mg/mL [press release]. Hertfordshire, England: Mylan N.V. PRNewswire; 2017. Available from: http://newsroom.mylan.com/2017-10-05-Mylan-in-Partnership-with-Synthon-Receives-Marketing-Authorization-Approval-in-Europe-for-First-Generic-for-Copaxone-R-40-mg-mL.
33. Ribes García S, Gómez-Pajares F, Albelda Puig C, García Herrera JL, Casanova Estruch B. Description of the characteristics of multiple sclerosis patients in the region of Valencia (Spain) who requested treatment with disease-modifying drugs during the 2005–2014 Decade. Eur Neurol. 2016;75(5–6):274–281. doi:10.1159/000446580
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

