Back to Journals » Infection and Drug Resistance » Volume 14
Beta-Lactamase Gene Expression Level of Hospital-Acquired CRAB Isolated from Children in Picu
Authors Xu X, Xu C, Salisu RB, Xu W
Received 2 June 2021
Accepted for publication 24 July 2021
Published 16 August 2021 Volume 2021:14 Pages 3195—3205
DOI https://doi.org/10.2147/IDR.S322604
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiao Xu,* Caifang Xu,* Rabiu Bilya Salisu, Wei Xu
Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Xu
Department of Pediatrics, Shengjing Hospital of China Medical University, No. 36 Sanhao Street, Heping District, Shenyang, Liaoning Province, People’s Republic of China
Email [email protected]
Purpose: Acinetobacter baumannii is a major cause of hospital-acquired infections. Studies showed that carbapenem resistance was related to mortality. Carbapenem resistance depends on expression of β-lactamase in adults. The present study explores the relationship between β-lactamase gene expression and carbapenem resistance and outcomes in children with A. baumannii infections.
Patients and Methods: We gathered clinical data of 131 children diagnosed with hospital-associated A. baumannii infections from the pediatrics unit of Shengjing Hospital of China Medical University. We obtained 131 isolates of A. baumannii, determined the minimal inhibitory concentrations (MICs) for common antibiotics, and measured carbapenemase-encoding genes expression using real-time PCR.
Results: We isolated 131 strains, 89 of which were carbapenem-resistant (MIC ≥ 8 μg/mL), and 42 carbapenem-sensitive strains. Univariate analysis identified statistically significant differences between the carbapenem-resistant group and the carbapenem-sensitive group for in-hospital days before infection, previous deep vein catheterization, previous urinary catheterization, previous treatment with a carbapenem (meropenem/imipenem), and expression of oxa-51 and oxa-23. Logistic regression analysis of factors associated with carbapenem-resistant A. baumannii infections found significant associations with oxa-23 expression (hazard ratio [HR] 0.005, confidence interval [CI] 95% 0– 0.153, P = 0.002) and previous carbapenem treatment (HR 0.031 CI 95% 0.1– 0.959, P = 0.042). Of 131 patients, 27 died within 30 days. Cox regression analysis of factors associated with 30-day mortality from A.baumannii infections showed that cephalosporin combined with sulbactam (HR 0.271, CI 95% 0.101– 0.723, P = 0.009) was associated with 30-day survival.
Conclusion: The expression of oxa-23 and the use of carbapenems were independent risk factors for carbapenem resistance. The use of cephalosporins combined with sulbactam was independently associated with 30-day survival. We recommend using cephalosporins combined with sulbactam in children infected with A. baumannii.
Keywords: β-lactamase geen, carbapenem resistance, Acinetobacter baumannii, prognosis, risk factors
Introduction
Acinetobacter baumannii is an opportunistic pathogen that is frequently found in debilitated inpatients and critically ill patients, especially in intensive care units.1,2 A. baumannii causes multi-system infections, particularly ventilator-associated pneumonia, bloodstream infections, wound infections, and is a significant cause of death.3,4 With increasing outbreaks and mortality, current therapeutic options are becoming useless because of drug resistance and the organism’s tenacious ability to survive. In 2017, the World Health Organization listed A. baumannii as a “priority pathogen” and called on clinicians to find ways to eradicate it.
According to statistics from the European Antibiotic Resistance Surveillance Network in 2018, about one-third of Acinetobacter species are resistant to carbapenem antibiotics. In the United States, the incidence of carbapenem-resistant A. baumannii (CRAB) increased from 20.6% in 2002 to 49.2% in 2008. In China, carbapenem resistance increased from 31% in 2005 to 66.7% in 2014.5,6 The combined incidence of CRAB infection in the intensive care unit (ICU) per 1000 patients was 41.7 cases (95% CI 21.7–78.7).7 In Asia, studies showed that the fatality rate of carbapenem-resistant A. baumannii infection was about 50%8.
There is currently no preferred treatment for CRAB which means multi-drug resistant, carbapenem nonsusceptibility is an independent risk factor for death from bacteremia in children.9 Longer in-hospital stays, antibiotic use, length of stay in the pediatric ICU, and surgery are risk factors for carbapenem-resistant A. baumannii infections.3,10 First-line treatments for CRAB in children include prolonged infusion of meropenem plus fluoroquinolone/aminoglycoside/colistin. Ampicillin-sulbactam/tigecycline are the second-line agents. The combination of first- and the second-line agents appeared to be a reasonable treatment11 Emergence of carbapenem nonsusceptibility made colistin and tigecycline the treatment of last resort. It is unknown if colistin is safe in children12.
Several mechanisms contribute to carbapenem resistance, including presence of carbapenemases and efflux pumps. The affinity with penicillin-binding protein is low. The expression of outer membrane channel proteins is downregulated or absent. Carbapenemases are among the most important and most frequently observed.13 There are several carbapenem enzyme classifications; the most common is A, B, C, and D. Class D β-lactamases are also called oxacillinases (OXAs) that hydrolyze oxacillin, the primary mechanism responsible for CRAB.14 Oxa-58, oxa-23, oxa-24, and oxa-51 like are the most prevalent subgroups worldwide.15,16 Oxa-23 was associated with mortality according to a recent report from Brazil.17
There is a lack of study of this problem in children, particularly those in the pediatric ICU (PICU). Nevertheless, knowledge of antibiotic resistance and the risk factors for CRAB infections can guide treatment strategies. Therefore, in the present study, we reported findings a group of 131 children in the PICU with hospital-acquired infections to determine which OXA genes participate in carbapenem-resistance and evaluate if gene expression is associated with mortality in the PICU.
Materials and Methods
Study Population and Eligibility Criteria
This retrospective study included 131 pediatric patients infected by A. baumannii in the PICU of Shengjing Hospital affiliated with China Medical University from January 2012 to December 2018. After the study was approved by the Research Ethics Committee (2019PS431K), we obtained consent from the hospital and the medical records of the patients considered for inclusion. There were a total of 266 patients and 541 strains from 2012 to 2018. To better explore the causes of carbapenem resistance and evaluate the outcomes of children as early as possible, considered the following inclusion criteria in Figure 1: 1) > 1 month and < 13 years old; 2) A. baumannii cultured from any sample, with clinical signs and symptoms consistent with hospital-acquired infection; and 3) retention of the first confirmed infection strain. Exclusion criteria are as follows in Figure 1; 1) absence of clinical data or loss of bacteria caused by improper preservation; 2) suspected infection strains: when culture at least two kinds of micros including A. baumannii, cannot clarify which one or both play a role; or colonies; and 3) community-acquired infection. We collected the basic clinical data of the patients with confirmed A. baumannii infections for the first time before drug sensitivity testing and Real-time reverse-transcription Polymerase Chain Reaction (Real-time PCR) testing of infected strains. These data included age, sex, underlying diseases, invasive procedures (mechanical ventilation, deep venous catheterization, thoracic puncture), changes in c-reactive protein levels, white blood cell counts within 24 hours after clinical diagnosis of infection and 48 hours before infection diagnosis (Δ), fever at culture time, and previous carbapenem treatment (meropenem/imipenem). The strain was stored at –80 °C, removed, resuscitated, and inoculated. The strains were transferred on blood plate culture medium using the plate marking method, and the strains were identified again using a VITEK-2 compact instrument.
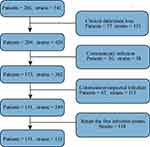 |
Figure 1 Exclusion criteria and inclusion criteria. |
The patients were divided into a carbapenem-resistant group and a carbapenem sensitive-group to determine the effect of oxa-23/24/51/58 gene expression on drug resistance as the primary outcome, and to explore the effect of gene expression on 30-day mortality as the secondary outcome.
Colonization was defined as positive culture without clinical signs of infection patients. Hospital-acquired infections were defined as infections that were not present at admission and occur within 48–72 hours after admission or up to 6 weeks after discharge, not during the incubation period.18 Infection was defined as cultured strains with relevant clinical symptoms and signs, and other infections excluded according to the definitions of infection in the intensive care unit.19 Sepsis and severe pneumonia were defined according to international definitions.20,21 Intra-abdominal infections: Patients typically present with rapid-onset abdominal pain and signs of local and systemic inflammation (pain, tenderness, fever, tachycardia, and/or tachypnea). Hypotension and hypoperfusion signs such as oliguria, acute alteration of mental status, and lactic acidosis are indicative of ongoing organ failure.22 Bacterial Meningitis: signs of meningeal irritability, fever, poor feeding, lethargy, irritability, seizure, with vomiting, photophobia, headache, and neck stiffness, positive CSF (Cerebrospinal fluid) Gram stain, CSF leukocyte count of at least 1000 cells per mm3, CSF protein level of at least 0.8 g per liter, peripheral blood leukocyte count of at least 10,000 cells per mm3,23,24 Urinary tract infection: clinical symptoms: fever, dysuria, urgency, and costovertebral angle tenderness and so on, a growth of more than 108 colony-forming units (CFU) per liter (105 per mL) of a unique bacterium is regarded most frequently as the cutoff between contamination and UTI.25
Antimicrobial Susceptibility Testing
We tested susceptibility of A. baumannii isolates according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints against amikacin/gentamicin, sulbactam and cefoperazone, cefepime/ceftazidime/ampicillin, ampicillin/sulbactam, piperacillin/tazobactam, compound sulfamethoxazole, and ciprofloxacin/levofloxacin/tetracycline/tigecycline (Supplementary Table 2). We performed the E-TEST method based on those defined by the Clinical and Laboratory Standards Institute.26–30 Carbapenem-non-susceptible A. baumannii was defined as isolates that exhibited in vitro resistance to imipenem or meropenem based on the E-TEST method (minimum inhibitory concentration [MIC] ≥ 8 µg/mL). A. baumannii was identified using the Vitek 2 System (France). The strains were Escherichia coli ATCC25922 and Pseudomonas aeruginosa ATCC27853.
Real-Time PCR Method
The Real-time reverse-transcription Polymerase Chain Reaction (Real-time PCR) method was as follows. We added appropriate amounts of sample to 1 mL TRIzol (9108, Takara Bio, Inc.), added 200 UL chloroform and mixed and incubated for 15 min. The mixture was centrifuged at 4 °C, 14,000 g for 15 min, after which we transferred the water phase to fresh test tubes, added 500 L isopropanol to precipitate, incubated on ice for 10 min, and centrifuged at 14,000 g for 15 min at 4 °C. The purity of the extracted RNA was tested (NanoPhotometer), and the samples with absorbance values between 1.8 and 2.0 were reverse transcribed. The unqualified samples underwent the procedure again. Extracted RNA was then reverse transcribed into cDNA using the PrimeScriptRT reagent kit with gDNA Eraser (RR047A, Takara Bio, Inc.). The reverse transcribed products were quantified using real-time PCR by 16s rRNA. Each targeted cDNA (2 µL) was amplified using the TB Green PCR Core kit (RR820A, Takara Bio, Inc.) via the ABI 7500 system, and the following primers: OXA-24 forward,5′-CCTTGCACATAACCGATTACCT-3′ and reverse,5′-CAGTCAACCAACCTACCTGTGG-3′; OXA-58 forward,5′-ATATCAAGAATTGGCACGTCGT-3′ and reverse,5′-TGTAATTGTCAAAGGCCCTTTC-3′; OXA-23 forward,5′-TCCCAGTCTATCAGGAACTTGC-3′ and reverse,5′-GGCGTAACCTTTAATGGTCCTA-3′; OXA-51 forward,5′-TCCAACAAGGCCAAACTCAAC-3′ and reverse,5′-CTTCTGTGGTGGTTGCCTTATG-3′; 16srRNA forward,5′-ATTAATGCAACTGCTCAACAAGC′ and reverse,5′-ATGTCTGCTAAGTGGGCAAGTTC-3′; The gene expression levels of the target gene and standard Baumannian ATCC19606 were compared. The reaction conditions were as follows: 95 °C pre-incubation 5 min, annealing 40 cycles: 95 °C for 3s, 60 °C for 20s. Then, 95 °C 15 s, 60 °C 15 s, 15 s, and 95 °C 15 s. The results were expressed as 2-delta CT value, and multiple changes of expression levels of these genes and the expression level of the housekeeping gene 16S were compared.
Statistical Analysis
The primary outcomes were risk factors of carbapenems resistance. The Secondary outcomes were risk factors related to 30-day mortality. The Chi-square test or Fisher exact test was used to analyze the categorical variables. The 2-tailed t-test and Mann–Whitney test were used to analyze the continuous variables. Logistic regression analysis was used to determine the risk factors for carbapenem resistance. Kaplan–Meier curves and univariate analysis were used to evaluate the proportional hazard hypothesis. Cox regression analysis was used in multivariate analysis, using SPSS 21. 0 statistical software for data processing. Differences with P < 0.05 were statistically significant.
Results
There were 131 patients (57 girls and 74 boys) aged from 1 month to 13 years. Of these 60 patients had underlying diseases: 68 patients with severe pneumonia, 17 with sepsis before infection, 12 with shock before infection, four with multiple organ dysfunction syndrome (MODS) before infection, 14 patients with sepsis after infection, 12 patients with shock after infection, and eight patients with MODS after infection. Twenty-seven patients died within 30 days after infection. Figure 2 shows there were 131 strains, 110 sputum samples, eight blood cultures, one urine culture, one cerebral fluid culture, two pleural fluid culture, three ascites cultures, and six bronchoalveolar lavage fluid samples. Of these 89 strains were carbapenem-resistant and 42 were carbapenem-sensitive.
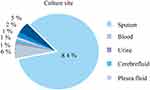 |
Figure 2 The different Specimen culture site including sputum, blood culture, urine culture, cerebra fluid, pleural fluid, ascites and bronchoalveolar lavage fluid from patients. |
Figure 3 shows that 67.94% of the isolates were resistant to carbapenems, followed by ceftazidime (64.12%). High susceptibility was detected for amikacin (83.21%) and tigecycline (82.44%), followed by cefoperazone sulbactam (69.47%). The sensitivity rate of gentamicin was 40.46% and that of levofloxacin was 39.69%.
Supplementary Table 1 shows Beta-lactamase gene expression. Among them, 14 strains of oxa-51 gene were not detected, 22 strains of oxa-23 gene were not detected, 25 strains of oxa-24 gene were not detected, and only oxa-58 gene was expressed 10 strains. In the CRAB group, 91% of the strains detected the OXA-23 gene, 93% of the strains detected OXA-51, and 81% of the strains detected OXA-24; in the non-CRAB, 67% of the strains detected OXA. Among 23 genes, OXA-51 was detected in 81% of the strains, and OXA-24 gene expression was detected in 83% of the strains.
Univariate analysis of the CRAB vs non-CRAB groups in Table 1 revealed the following: in-hospital days before infection (14 (18) vs 9 (13), P = 0.024), previous deep vein catheterization (40VS7 catheter, P = 0.001), previous urinary catheterization (56VS19 catheter, P = 0.042), fever (60vs21, P = 0.04), previous carbapenem (meropenem/imipenem) (30 vs 6, P = 0.017), oxa-24 (0.04 (0.33)vs 0.05(0.31), P =0.305), oxa-51 (0.9 (2.05)vs 0.10 (0.30), P < 0.000) and oxa-23 (0.29 (1.23)vs 0.00 (0.05), P < 0.000). The oxa-58-like gene was only detected in ten strains. Logistic regression analysis of factors associated with CRAB indicated that oxa-23 (HR = 0.005, CI 95% 0–0.153, P = 0.002) and previous carbapenem treatment (meropenem/imipenem) (HR = 0.031, CI 95% 0.1–0.959, P = 0.042) is shown in Table 2.
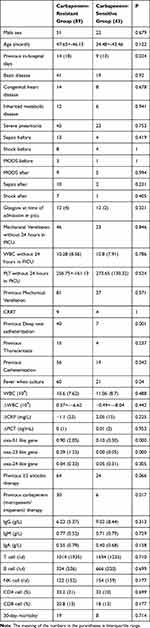 |
Table 1 Comparison Between Carbapenem-Resistant Group and Carbapenem-Sensitive Group |
 |
Table 2 Logistic Regression Analysis About Risk Factors Associated with Carbapenem Resistance |
Univariate analysis showed in Table 3 that there was no difference in age and sex expression between death group and survival group within 30 days. Differences between survivors and non-survivors were genetic metabolic diseases (12 vs 6, P = 0.041), sepsis after infection (4 vs 4, P = 0.02), shock after infection (6 vs 6, P = 0.015), MODS after infection (8 vs 6, P = 0.029), ΔCRP (mg/L) (3.34 (67) vs −1.04 (18), P = 0.012), albumin (g/L) (31.3 ± 5.04 vs 34.43 ± 4.46, P = 0.008), AST (μmol/L) (66.6 ± 68.3 vs 33.06 ± 22.42, P = 0.037), ALT (μmol/L) (129.91 ± 393.29 vs 44.88 ± 83.93, P = 0.017), and bilirubin (μmol/L) (8.43 ± 6.19 vs 6.64 ± 3.81 p = 0.004). However, sulbactam was used in combination with cephalosporins (6 vs 50, P = 0.023) was a protect factor for 30-day survivors. The expression of oxa-23, oxa-24, oxa-58, oxa-51 showed no significant difference between the those who survived and those who died: oxa-51 (0.47 (1.95) vs 0.11 (1.44), P = 0.65), oxa-24 (0.06 (0.73) vs 0.08 (2.41), oxa-23 (0.28 (2.4) vs 0.15 (0.22), P = 0.181).
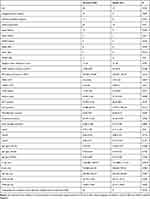 |
Table 3 Univariate Analysis Comparing Survivors and Non-Survivors at 30 Days from Infection Onset |
Cox regression analysis of factors associated with 30-day mortality showed that sulbactam in combination with cephalosporins (HR = 0.271, CI 95% 0.095–0.712, P = 0.009) was associated with 30-day survival in Table 4.
 |
Table 4 Cox Regression Analysis About Risk Factors Associated with 30-Day Mortality |
Discussion
To the best of our knowledge, this is the largest study about children of A. baumannii in northern China. We found that previous meropenem/imipenem therapy and oxa-23-like gene expression were independent risk factors for carbapenem resistance. Interestingly, carbapenem resistance was not associated with 30-day mortality. We explored the risk factors for 30-day mortality and found that ΔCRP, sepsis, albumin, transaminases, total bilirubin, and cephalosporins combined with sulbactam therapy were significant in univariate analysis. Only cephalosporins combined with sulbactam therapy were significant in Cox regression analysis.
Our carbapenem resistance rate was 67.94%. Amikacin and tigecycline had the highest sensitivity, followed by cefoperazone/sulbactam. Amikacin is an aminoglycoside that is ototoxic and nephrotoxic in children; tigecycline can increase mortality.31 Therefore, these treatments are not recommended. The low frequency of use may be the cause of sensitivity. A study showed that carbapenem-resistant A. baumannii infection was an independent risk factor for death from bacteremia in children.9 For children infected with A. baumannii, 7-day and 30-day mortality rates were 18.96% and 35.1%, respectively. Carbapenem resistance was an independent risk factor for 30-day mortality (18 vs 26, P = 0.034).9 Punpanich et al reported that the 30-day mortality in children with A. baumannii infection and bacteremia was 26.1%; carbapenem resistance was 4.76 (1.5 8–14.32), P = 0.005.32 We found that there was no significant difference in 30-day mortality between the carbapenem-resistant group and the carbapenem-sensitive group; the case-fatality rate was only 20.61% (carbapenem-resistant group vs carbapenem-sensitive group: 19 vs 8, P = 0.714), First, they studied bloodstream infections and no patients were in the pediatric intensive care unit. Second, we showed that cephalosporin/sulbactam for A. baumannii reduced the 30-day mortality rate. In our study, nearly half of the children with A. baumannii infection and were given appropriate treatment.
In summary, the differences in infection sites, departments, medication protection, and even regional differences may account for our low mortality rate and drug resistance with no difference in 30-day fatality rate between groups.
Sulbactam is a β-amidase inhibitor that has synergistic effects with other carbapenems. We found that cefoperazone/sulbactam was used most often (31/56). In a study of carbapenem-resistant A. baumannii infection-related hospital-acquired pneumonia, the 30-day survival rate among those treated with cefoperazone/sulbactam was 95.1%; for those treated without cefoperazone/sulbactam, the rate was 73.3%.33 Chi et al found that the use of cefoperazone/sulbactam had a protective effect on the 30-day mortality rate, and the mortality rate of children receiving cefoperazone/sulbactam treatment was lower than that of patients receiving tigecycline treatment (35.7% vs 51.9%, P = 0.001).34 The results of pharmacokinetic studies suggested that imipenem is more suitable than meropenem for severely ill patients.35,36 Cefoperazone/sulbactam combined with imipenem/cilastatin is recommended as routine treatment.
D-β-lactamase is closely related to carbapenem resistance and can hydrolyze carbapenem enzymes. The subgroups of oxa-23, oxa-24, oxa-51, and oxa-58 are prevalent worldwide, especially oxa-23.37 Liu et al detected oxa-23 expression in 28 hospitals in 18 provinces in China.38 A recent study suggested oxa-23 has a role in promoting sulbactam resistance.39 Many studies suggested that carbapenem resistance was related to oxa-23 expression, and the mortality rate of children with carbapenem resistance was high.9,40 An intensive care unit study in Brazil showed that oxa-23-producing A. baumannii strains belonged to the ST79 (CC79) clonal group, and patients infected or colonized with these isolates had high mortality rates (34.6%).17 14 strains in carbapenem-sensitive group absence oxa-23 expression. We found that the expression of oxa-23 was significantly different in the carbapenem-resistant group and the sensitive group (P = 0.002). Nevertheless, there was no significant difference in the 30-day mortality rate related to oxa-23 on further exploration. There are many factors that affect the mortality rate, and it is possible that the expression of a single gene plays a limited role.
Conclusions
In general, resistance to carbapenems is closely related to the expression of the oxa-23 gene. The expression of oxa-23 and the use of carbapenems are independent risk factors for carbapenem resistance. Cephalosporins combined with sulbactam are independently related to the 30-day mortality. A. baumannii were sensitive to tigecycline (82.44%) and amikacin (83.21%); however, they are not recommended for use in children because of ototoxicity and nephrotoxicity. We recommend using cephalosporins combined with sulbactam in children infected with A. baumannii. However, this study is a retrospective one, and it is impossible to distinguish which enzyme inhibitor is the best. There is a need to further explore treatment strategies for A. baumannii infections.
Abbreviations
MODS, multiple organ dysfunction syndrome; Real-time PCR, real-time reverse-transcription polymerase chain reaction; A. baumannii, Acinetobacter baumannii; ALT, alanine aminotransferase; AST, aspartate transaminase; CRP, C-reaction protein; PLT, platelet; WBC, white blood cell; Procalcitonin; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; T cell, T-lymphocyte; B cell, B-lymphocyte; NK cell, natural killer cell; CRRT, continuous renal replacement therapy; PICU, pediatric intensive care unit; CSF, cerebrospinal fluid; AMK, amikacin; GEN, gentamicin; SCF, sulbactam and cefoperazone; FEP, cefepime; CAZ, ceftazidime; AMP, ampicillin; SAM, ampicillin/sulbactam; TZP, piperacillin sodium/tazobactam sodium; SXT, compound sulfamethoxazole; CIP, ciprofloxacin; LVX, levofloxacin; TCY, tetracycline; TGC, tigecycline.
Ethical Consideration
The ethical approval was obtained from the Ethical Review Committee of Shengjing Hospital Affiliated to China Medical University. Since we reviewed the secondary data and obtained the clinical director’s informed consent, the patient’s informed consent was not required. Strict confidentiality during the data collection process, data processing and report writing process. The research was also conducted in accordance with the Declaration of Helsinki.
Acknowledgments
Thanks for the support of laboratory department.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding
National Natural Science Foundation of China (No. 81771621); Liaoning key R & D guidance Program (2019JH8/10300023); 345 talents Project (345 Talent Project).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Johnson J, Robinson G, Zhao L, Harris A, Stine O, Thom K. Comparison of molecular typing methods for the analyses of Acinetobacter baumannii from ICU patients. Diagn Microbiol Infect Dis. 2016;86(4):345–350. doi:10.1016/j.diagmicrobio.2016.08.024
2. Salehi B, Ghalavand Z, Mohammadzadeh M, Maleki D, Kodori M, Kadkhoda H. Clonal relatedness and resistance characteristics of OXA-24 and −58 producing carbapenem-resistant Acinetobacter baumannii isolates in Tehran, Iran. J Appl Microbiol. 2019;127(5):1421–1429. doi:10.1111/jam.14409
3. Baran G, Erbay A, Bodur H, et al. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis. 2008;12(1):16–21. doi:10.1016/j.ijid.2007.03.005
4. Dizbay M, Tunccan O, Sezer B, Hizel K. Nosocomial imipenem-resistant Acinetobacter baumannii infections: epidemiology and risk factors. Scand J Infect Dis. 2010;42(10):741–746. doi:10.3109/00365548.2010.489568
5. Hu F, Guo Y, Zhu D, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22:S9–14. doi:10.1016/j.cmi.2016.01.001
6. Mera R, Miller L, Amrine-Madsen H, Sahm D. Acinetobacter baumannii 2002–2008: increase of carbapenem-associated multiclass resistance in the United States. Microbial Drug Resistance (Larchmont, NY). 2010;16(3):209–215. doi:10.1089/mdr.2010.0052
7. Ayobami O, Willrich N, Harder T, Okeke I, Eckmanns T, Markwart R. Acinetobacter baumanniiThe incidence and prevalence of hospital-acquired (carbapenem-resistant) in Europe, Eastern Mediterranean and Africa: a systematic review and meta-analysis. Emerg Microbes Infect. 2019;8(1):1747–1759. doi:10.1080/22221751.2019.1698273
8. Kim Y, Kim S, Hong K, Kim Y, Park Y, Kang M. Risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia: impact of appropriate antimicrobial therapy. J Korean Med Sci. 2012;27(5):471–475. doi:10.3346/jkms.2012.27.5.471
9. Choe Y, Lee H, Choi E. Acinetobacter baumanniirisk factors for mortality in children with bacteremia in south korea: the role of carbapenem resistance. Microbial Drug Resistance (Larchmont, NY). 2019;25(8):1210–1218. doi:10.1089/mdr.2018.0465
10. Katragkou A, Kotsiou M, Antachopoulos C, et al. Acquisition of imipenem-resistant Acinetobacter baumannii in a pediatric intensive care unit: a case-control study. Intensive Care Med. 2006;32(9):1384–1391. doi:10.1007/s00134-006-0239-x
11. Hsu A, Tamma P. Treatment of multidrug-resistant Gram-negative infections in children. Clin Infect Dis. 2014;58(10):1439–1448. doi:10.1093/cid/ciu069
12. Abbas W, Acharya R, Pandit A, Gupta S, Rao R. The real-world experience with nivolumab in previously treated patients with advanced non-small cell lung cancer from a cancer center in India. South Asian J Cancer. 2020;9(1):50–52. doi:10.4103/sajc.sajc_111_19
13. Aarons L, Rowland M. Kinetics of drug displacement interactions. J Pharmacokinet Biopharm. 1981;9(2):181–190. doi:10.1007/BF01068081
14. Martinez T, Martinez I, Vazquez G, Aquino E, Robledo I. Genetic environment of the KPC gene in Acinetobacter baumannii ST2 clone from Puerto Rico and genomic insights into its drug resistance. J Med Microbiol. 2016;65(8):784–792. doi:10.1099/jmm.0.000289
15. Karampatakis T, Tsergouli K, Politi L, et al. Polyclonal predominance of concurrently producing OXA-23 and OXA-58 carbapenem-resistant Acinetobacter baumannii strains in a pediatric intensive care unit. Mol Biol Rep. 2019;46(3):3497–3500. doi:10.1007/s11033-019-04744-4
16. Froese M. [Significance of current life conflicts for psychological disorders]. Z Arztl Fortbild (Jena). 1985;79(4):167–170. Geraman
17. da Silva K, Maciel W, Croda J, et al. A high mortality rate associated with multidrug-resistant Acinetobacter baumannii ST79 and ST25 carrying OXA-23 in a Brazilian intensive care unit. PLoS One. 2018;13(12):e0209367. doi:10.1371/journal.pone.0209367
18. Horan T, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
19. Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33(7):1538–1548. doi:10.1097/01.CCM.0000168253.91200.83
20. Singer M, Deutschman C, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
21. Arora NK. Rational use of antibiotics for pneumonia. Indian Pediatr. 2010;47(1):11–18. doi:10.1007/s13312-010-0015-4
22. Sartelli M, Catena F, Abu-Zidan F, et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emergency Surgery. 2017;12:22.
23. Brouwer M, Tunkel A, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23(3):467–492.
24. Grief S, Loza J. Guidelines for the evaluation and treatment of pneumonia. Prim Care. 2018;45(3):485–503. doi:10.1016/j.pop.2018.04.001
25. Simões E, Silva A, Oliveira E, Mak R. Urinary tract infection in pediatrics: an overview. J Pediatr (Rio J). 2020;96:65–79. doi:10.1016/j.jped.2019.10.006
26. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing[S]. Twenty-fourth informational supplement; 2014. M100S, 24th Edition.
27. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing[S]. Twenty-fifth informational supplement; 2015. M100S, 25th Edition.
28. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing[S]. Twenty-sixth informational supplement; 2016. M100S, 26th Edition.
29. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing[S]. Twenty-seventh informational supplement; 2017. M100S, 27th Edition.
30. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing[S]. Twenty-eighth informational supplement; 2018. M100S, 28th Edition.
31. Prasad P, Sun J, Danner R, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54(12):1699–1709. doi:10.1093/cid/cis270
32. Punpanich W, Nithitamsakun N, Treeratweeraphong V, Suntarattiwong P. Risk factors for carbapenem non-susceptibility and mortality in Acinetobacter baumannii bacteremia in children. Int j Infect Dis. 2012;16(11):e811–815. doi:10.1016/j.ijid.2012.07.006
33. Xia J, Zhang D, Xu Y, Gong M, Zhou Y, Fang X. A retrospective analysis of carbapenem-resistant Acinetobacter baumannii-mediated nosocomial pneumonia and the in vitro therapeutic benefit of cefoperazone/sulbactam. Int J Infect Dis. 2014;23:90–93. doi:10.1016/j.ijid.2014.01.017
34. Niu T, Luo Q, Li Y, Zhou Y, Yu W, Xiao Y. Acinetobacter baumanniiComparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant. Antimicrob Resist Infect Control. 2019;8:52. doi:10.1186/s13756-019-0502-x
35. Kiratisin P, Apisarnthanarak A, Kaewdaeng S. Synergistic activities between carbapenems and other antimicrobial agents against Acinetobacter baumannii including multidrug-resistant and extensively drug-resistant isolates. Int J Antimicrob Agents. 2010;36(3):243–246. doi:10.1016/j.ijantimicag.2010.04.011
36. Novelli A, Adembri C, Livi P, Fallani S, Mazzei T, De Gaudio A. Pharmacokinetic evaluation of meropenem and imipenem in critically ill patients with sepsis. Clin Pharmacokinet. 2005;44(5):539–549. doi:10.2165/00003088-200544050-00007
37. Mugnier P, Poirel L, Naas T, Nordmann P. Worldwide dissemination of the blaOXA-23 carbapenemase gene of Acinetobacter baumannii. Emerg Infect Dis. 2010;16(1):35–40. doi:10.3201/eid1601.090852
38. Liu L, Ji S, Ruan Z, et al. Dissemination of blaOXA-23 in Acinetobacter spp. in China: main roles of conjugative plasmid pAZJ221 and transposon Tn2009. Antimicrob Agents Chemother. 2015;59(4):1998–2005. doi:10.1128/AAC.04574-14
39. Yang Y, Xu Q, Li T, et al. OXA-23 is a prevalent mechanism contributing to sulbactam resistance in diverse acinetobacter baumannii clinical strains. Antimicrob Agents Chemother. 2019;63(1). doi:10.1128/AAC.01676-18.
40. Schuertz K, Tuon F, Palmeiro J, et al. Bacteremia and meningitis caused by OXA-23-producing Acinetobacter baumannii - molecular characterization and susceptibility testing for alternative antibiotics. Brazilian J Microbiol. 2018;49:199–204. doi:10.1016/j.bjm.2018.04.002
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

