Back to Journals » Drug Design, Development and Therapy » Volume 12
Best tigecycline dosing for treatment of infections caused by multidrug-resistant pathogens in critically ill patients with different body weights
Authors Ibrahim MM, Abuelmatty AM, Mohamed GH, Nasr MA, Hussein AK, Ebaed MED, Sarhan HA
Received 29 July 2018
Accepted for publication 24 October 2018
Published 7 December 2018 Volume 2018:12 Pages 4171—4179
DOI https://doi.org/10.2147/DDDT.S181834
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Mohamed M Ibrahim,1 Abdulla M Abuelmatty,2 Gehan H Mohamed,3 Mohsen A Nasr,4 Amal K Hussein,5 Mohy El Deen Ebaed,6 Hatem A Sarhan5
1Department of Clinical Pharmacy, Faculty of Pharmacy, Minia University, Minia, Egypt; 2Department of Pharmacy, Jahra Hospital, Jahra, Kuwait; 3Department of Internal Medicine, Faculty of Medicine, Cairo University, Cairo, Egypt; 4Department of Internal Medicine, Faculty of Medicine, Ain Shams University, Cairo, Egypt; 5Department of Pharmaceutics, Faculty of Pharmacy, Minia University, Minia, Egypt; 6Department of Biochemistry, Egyptian Ministry of Interior, Cairo, Egypt
Background: The intensive care unit (ICU) is a center of multidrug-resistant (MDR) pathogens. This is due to overuse of antibiotics in the treatment of critically ill patients. Tigecycline is a broad-spectrum antibiotic that belongs to the glycylcycline group. Tigecycline has been indicated in treatment of complicated intra-abdominal infections (cIAIs) and complicated skin and soft-tissue infections (cSSTIs).
Objective: This study was done to discover the best dose regimen of tigecycline in treatment of cSSTIs and cIAIs, especially in patients who are critically ill and obese, for clinical outcomes and safety.
Setting: The study was conducted in an adult ICU that consists of 25 beds in a general hospital and was conducted within 2 years. A total of 954 patients were screened in this study.
Methods: This was a retrospective cohort study that compared the clinical outcomes of patients: mortality, ICU stay, and safety of using two different dose regimens of tigecycline between patients with different body weight who were treated for infections caused by MDR pathogens in the ICU. The study was conducted within 2 years. All results were collected from patients’ files and were analyzed with SPSS version 20.
Main outcome: The study was implemented to figure out the best dose regimen of tigecycline to achieve a reduction in mortality, ICU stay, treatment duration, and secondary septic-shock incidence with minimum side effects in treatment of cSSTIs and cIAIs in patients with different body weight.
Results: There was a significant improvement in mortality, ICU stay, recurrent infection by the same organism, duration of tigecycline treatment, number of patients who had first negative culture after starting treatment, secondary bacteremia, and secondary septic shock with patients who used high-dose regimens of tigecycline in different subgroups of body weight, with no significant difference in side effects.
Conclusion: The use of high-dose tigecycline resulted in a significant enhancement in all clinical outcomes, especially mortality and ICU stay when used in treatment of overweight and obese patients with cSSTIs and cIAIs.
Keywords: tigecycline, obese patients, intensive care unit, complicated intra-abdominal infections, complicated skin and soft-tissue infections
Introduction
Treatment of nosocomial infection and hospital-acquired bacterial infections is becoming the biggest challenge for health-care professionals, due to the continuous increase in prevalence of multidrug-resistant (MDR) bacteria like methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus spp., Acinetobacter baumannii, Klebsiella pneumoniae, carbapenemase-producing Enterobacteriaceae, and extended-spectrum β-lactamase-producing Enterobacteriaceae. MDR organisms increase morbidity, mortality, duration of hospitalization, and medical costs.1 Any delay in starting appropriate antimicrobial therapy is most likely to raise morbidity and mortality among infected patients, and inadequate therapy has been known to be accompanied by overblown mortality and increased length of hospitalization.2 A huge rate of resistance is found to well-known antimicrobial agents, such as β-lactams (penicillin, cephalosporins, and carbapenems), glycopeptides, aminoglycosides, and fluoroquinolones, which may reduce the effectiveness of such drugs,3 so we need to investigate the safety and efficacy of new antimicrobial molecules, such as tigecycline.
The first new molecule in the glycylcycline-antibiotic class is tigecycline, which has excellent activity on a wide range of bacteria: aerobic or anaerobic and also MDR.4,5 Tigecycline has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for the treatment of complicated intra-abdominal infections (cIAIs) and complicated skin and soft-tissue infections (cSSTIs).6,7 The FDA also recently approved it for the treatment of community-acquired pneumonia.8 We conducted a retrospective study to investigate the best dose regimen of tigecycline in treatment of patients infected by MDR pathogens, such as methicillin-resistant S. aureus, extended-spectrum β-lactamase, Acinetobacter, and others.
Methods
Study design and participants
This was a retrospective cohort study conducted in an adult intensive care unit (ICU) between 2013 and 2014. This ICU consists of 14 medical beds that receive patients from medical wards and 11 surgical beds that receive patients from surgical wards, and it is located in a tertiary hospital that has about 1,000 beds. A total of 1,430 patients were admitted within the period of the study: 696 patients in 2013 and 734 in 2014. Based on inclusion criteria, only 68 patients were enrolled. These patients were classified into four subgroups based on body weight by body-mass index (BMI; Figure 1): underweight, BMI <18.5 kg/m2; normal weight, BMI 18.5–24.9 kg/m2; overweight BMI 25–29.9 kg/m2; and obese, BMI 30–34.9 kg/m2.9 All results were collected retrospectively from patients’ medical files and analyzed statistically to figure out which dose was suitable for each subgroup of patients. In this study, we used the Kolmogorov–Smirnov test to value variable distributions. Data with abnormal distribution were assessed with the Mann–Whitney U test, data with normal distribution with Student’s t-test, and categorical variables with χ2 or Fisher’s exact test. There were very few missing data, and our method for handling these was pairwise deletion,10 because they were missing at random.11
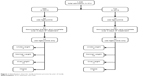  | Figure 1 Distribution chart for study-inclusion process by year of study. |
Antimicrobial agent
This study estimated the microbiological and clinical outcomes of different dosing regimens of one of the most powerful broad-spectrum antibiotics that we have in our hospital to treat MDR Gram-negative (except Pseudomonas) and Gram-positive pathogen. It is a tigecycline molecule that we use in the treatment of SSTIs, IAIs, and community-acquired pneumonia. We excluded patients who had any hepatic problems, because tigecycline is usually used with caution for these patients: loading dose 100 mg, then maintenance dose 25 mg twice daily. Tigecycline was given in 2013 as per its normal dose regimen of 100 mg loading dose, then 50 mg every 12 hours, which is recommended by the FDA,12 but the high-dose regimen – 100 mg every 12 hours – was given based on the recommendation of our antibiotic committee to overcome MDR Gram-negative bacteria.
Ethics approval
As this was a retrospective cohort study, formal consent from the patients was not required, because all patient data collected came from their medical files without any interference in treatment plans. The ethics committee of Minia University judged that this study did not require ethical approval or patient consent. Patient confidentiality has been maintained, and all patient data were anonymized.
Study-entry criteria
Inclusion criteria were: patients with microorganisms treated by tigecycline, with documented culture; those with pathogens that were susceptible to tigecycline in SSTIs, IAIs, and community-acquired infections; and those who had been on tigecycline treatment >3 days. Patients received tigecycline alone as single therapy, were admitted to the adult ICU, and were aged ≥13 years. Exclusion criteria were: patients receiving tigecycline combined with other antibiotics; those who had not completed ≥3 days on tigecycline; those with microorganisms not treated with tigecycline; those using tigecycline in non-FDA-indicated diseases, such as ventilator-associated pneumonia; and those with any hepatic problems at the start of the study.
Results
Finally, results were gathered from 33 patients who received the normal dose in 2013 and 35 who received the high dose in 2014, as shown in Figure 1. It was uncovered that the most common site of infection was SST in all subgroups, as shown in Tables 1 and 2. The most associated frequent comorbidity in all subgroups was cardiovascular disorders, diabetes disorders, renal impairment disorders, and hematological disorders with averages of 89%, 78%, 51%, and 26%, respectively. Average baseline sepsis-related organ-failure assessment (SOFA) and systemic inflammatory response syndrome (SIRS) scores were almost the same in all subgroups in the 2 years of the study: 9.5 and 3.4, respectively, as shown in Tables 3 and 4.
The most prevalent organism in all subgroups was Acinetobacter, with average 41% prevalence, as shown in Tables 5 and 6. The average age was 57.5 years, and almost half the patients were female (51%). Ethnicity was mainly Middle Eastern (>65%), as shown in Tables 7 and 8.
  | Table 5 Organism prevalence in underweight and normal-weight subgroups |
  | Table 6 Organism prevalence in overweight and obese subgroups |
  | Table 7 Demographic data for underweight and normal-weight subgroups |
  | Table 8 Demographic data for overweight and obese subgroups |
The most exciting clinical outcome was in the overweight subgroup, where mortality on the normal-dose regimen was 38%, but with high-dose regimen was 9% (P<0.01). Treatment duration with the normal dose was 17.34±2.98 days, but with the high dose was 6.19±1.05 days with P-value (0.01). ICU hospital stay with the normal dose was 29.65±4.76 days, but with the high dose was 12.02±2.11 days (P=0.01). Patients who had negative results from the next culture on the normal dose comprised 31%, but on the high dose 73% (P=0.02). Patients who had septic shock on the normal dose comprised 23%, but on the high dose this was 9% (P=0.03). Patients who had secondary bacterial infection with the same first organism on the normal dose comprised 8%, but 0 on the high dose (P<0.01), as shown in Table 10.
Results were similar in the obese subgroup, where mortality on the normal dose was 40%, while on the high dose it was 0 (P<0.01). Treatment duration with the normal dose was 17.98±3.75 days and with the high dose 6.01±0.55 days (P=0.02). ICU stay on the normal dose was 30.98±3.05 days and with the high dose 13.55±2.65 days (P=0.01). Patients with negative results from the first culture after treatment initiation comprised 40% in the regular-dose subgroups, while with the high dose this was 83% (P=0.02). Patients who had septic shock with the normal dose comprised 20%, but with the high dose this was 0 (P<0.01). Patients who had secondary bacterial infections with the same first organism with normal dose comprised 20%, but with the high dose this was 0 (P<0.01), as shown in Table 10. Also, with the underweight and normal-weight subgroups, improvement in clinical outcomes on the high-dose regimen was significant in mortality, ICU stay, percentage of patients cured by the first culture, secondary septic shock, and secondary bacterial infection, as shown in Table 9.
  | Table 9 Clinical outcomes for underweight and normal-weight subgroups |
  | Table 10 Clinical outcomes for overweight and obese subgroups |
All subgroups had almost the same associated side effects: nausea, vomiting, and diarrhea. Nearly all patients in the different subgroups had the same low incidence of skin, hepatic, renal, and hematological side effects. Not even one patient had another infection with another organism within the treatment period. Only one patient in normal-weight subgroup was stopped from continuing the treatment, because of vomiting with high dose regimen, as shown in Tables 11 and 12.
  | Table 11 Side effects associated with underweight and normal-weight subgroups |
  | Table 12 Side effects associated with overweight and obese subgroups |
Discussion
All results were collected during 2013–2014, after which comparison was done between the results collected to assess which dose regimen would be effective in different body-weight subgroups. After statistical analysis, it was found that there were significant differences among all subgroups in mortality, whereas patients who received high-dose tigecycline had a low mortality rate compared with patients who received normal doses. There were also significant differences among all subgroups in tigecycline duration of treatment and ICU stay, where the duration of tigecycline treatment and ICU stay was less in patients receiving high doses than those receiving normal doses.
Moreover, significant differences among almost all subgroups in number of patients who had direct negative first culture after starting therapy, secondary septic shock, and secondary bacterial infection with the same microorganism, while the number of patients who had negative results in the first culture after starting therapy was higher with the high-dose regimen, but fewer patients had secondary septic shock and secondary bacterial infection with the same organism with the high-dose regimen. All results were found to be in agreement with those of de Pascale et al at a teaching hospital in Rome, where he tried to conduct a retrospective study of prospectively collected data in the ICU and found that tigecycline was well tolerated at a higher-than-standard dose in a cohort of critically ill patients with severe infections.13
On the other hand, patients who were treated with the high-dose regimen had more nausea and vomiting, strongly noticed within underweight and normal-weight patients, but no significant differences were noticed with other side effects like hepatic and renal function, infection with another organism, or hematological/dermatological side effects. Only one patient was dropped out of the study in the normal-weight subgroup with the high-dose regimen because of side effects. This meant that the two dose regimens of tigecycline had the same safety profile. These results strongly agree with those observed by Ramirez et al, who found higher efficacy with tigecycline 100 mg twice daily relative to lower doses of tigecycline and imipenem–cilastatin in the treatment of hospital-acquired pneumonia. The safety profile of the higher doses of tigecycline was similar to the known safety profile of the approved dose of tigecycline.14
All subgroups had the same duration of ICU stay before getting an infection, as well as the same duration between receiving culture results and starting therapy. No subgroup showed a significant difference in baseline SOFA or SIRS scores, which showed that all patients in the four subgroups had the same illness status before starting tigecycline treatment. Also, age, ethnicity, sex, comorbidities, organism prevalence, and site of infection were almost the same in all groups and with insignificant differences, which showed that nearly all patients had the same status before starting tigecycline treatment. Based on these results, clinicians have to consider high-dose tigecycline in MDR pathogen-infection treatment, especially when patients have a high BMI.
Limitations
Firstly, this was a retrospective, single-center study with a medium number of patients. Secondly, few patients were treated with tigecycline alone as monotherapy. This has an impact on research conclusions. Furthermore, we were not able to perform tissue-level or pharmacokinetic testing due to financial issues.
Conclusion
Tigecycline use in this study improved clinical outcomes and mortality and reduced ICU stay significantly in obese patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10 Suppl 2):S165–S193. | ||
Hyle EP, Lipworth AD, Zaoutis TE, Nachamkin I, Bilker WB, Lautenbach E. Impact of inadequate initial antimicrobial therapy on mortality in infections due to extended-spectrum beta-lactamase-producing enterobacteriaceae: variability by site of infection. Arch Intern Med. 2005;165(12):1375–1380. | ||
Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. | ||
Bratu S, Tolaney P, Karumudi U, et al. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J Antimicrob Chemother. 2005;56(1):128–132. | ||
Hawkey P, Finch R. Tigecycline: in-vitro performance as a predictor of clinical efficacy. Clin Microbiol Infect. 2007;13(4):354–362. | ||
Babinchak T, Ellis-Grosse E, Dartois N, et al; Tigecycline 301 Study Group; Tigecycline 306 Study Group. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin Infect Dis. 2005;41(Suppl 5):S354–S367. | ||
Ellis-Grosse EJ, Babinchak T, Dartois N, et al; Tigecycline 300 cSSSI Study Group; Tigecycline 305 cSSSI Study Group. The efficacy and safety of tigecycline in the treatment of skin and skin-structure infections: results of 2 double-blind phase 3 comparison studies with vancomycin-aztreonam. Clin Infect Dis. 2005;41(Suppl 5):S341–S353. | ||
Tanaseanu C, Milutinovic S, Calistru PI, et al; 313 Study Group. Efficacy and safety of tigecycline versus levofloxacin for community-acquired pneumonia. BMC Pulm Med. 2009;9:44. | ||
Douketis JD, Paradis G, Keller H, Martineau C. Canadian guidelines for body weight classification in adults: application in clinical practice to screen for overweight and obesity and to assess disease risk. CMAJ. 2005;172(8):995–998. | ||
Kim JO, Curry J. The treatment of missing data in multivariate analysis. Sociol Methods Res. 1977;6(2):215–240. | ||
Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–592. | ||
TYGACIL® (tigecycline) [prescribing information]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2010. | ||
De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90. | ||
Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother. 2013;57(4):1756–1762. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.