Back to Journals » Journal of Inflammation Research » Volume 15
Bergaptol Alleviates LPS-Induced Neuroinflammation, Neurological Damage and Cognitive Impairment via Regulating the JAK2/STAT3/p65 Pathway
Authors Wu J , Zhang J, Xie Q, He X, Guo Z, Zheng B, Wang S, Yang Q, Du C
Received 30 July 2022
Accepted for publication 3 November 2022
Published 9 November 2022 Volume 2022:15 Pages 6199—6211
DOI https://doi.org/10.2147/JIR.S383853
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Jianbing Wu,1,* Jie Zhang,1,* Qiangli Xie,2 Xiaohuan He,3 Zhangchao Guo,1 Bo Zheng,4 Sisong Wang,5 Qiumei Yang,6 Chunfu Du1
1Department of Neurosurgery, Ya’an People’s Hospital, Ya’an, 625000, People’s Republic of China; 2Department of Cardiovascular Medicine, Chengdu Qingbaijiang District People’s Hospital, Chengdu, 610300, People’s Republic of China; 3Department of the Fifth Dispatched Outpatient, The General Hospital of Western Theater Command, Chengdu, 610083, People’s Republic of China; 4Department of Neurology, Ya’an People’s Hospital, Ya’an, 625000, People’s Republic of China; 5Department of Neurosurgery, the Chengdu 363 Affiliated Hospital of Southwest Medical University, Chengdu, 610041, People’s Republic of China; 6Department of Geriatrics, Luzhou People’s Hospital, Luzhou, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chunfu Du, Department of Neurosurgery, Ya’an People’s Hospital, 358 Chenghou Road, Ya’an, Sichuan, 625000, People’s Republic of China, Tel +86-835-2862065, Email [email protected]
Purpose: Neuroinflammation is considered a critical pathological process in various central nervous system (CNS) diseases and is closely related to neuronal death and dysfunction. Bergaptol is a natural 5-hydroxyfurocoumarin found in lemon, bergamot and other plants. Some studies have confirmed its anti-cancer, anti-inflammatory and anti-atherogenic functions, indicating that it may have significant medicinal value. In this study, we investigated the potential effect of Bergaptol in vitro and in vivo neuroinflammatory models.
Methods: Mice were injected with LPS (40 μg/kg) into the hippocampal CA1 region and then injected intraperitoneally with Bergaptol (10, 20 and 40 mg/kg) once a day for two weeks. In addition, to verify the effect of Bergaptol on BV2 cells, Bergaptol with different concentrations (5, 10 and 20 μg/mL) was firstly incubated for 1 hour, then LPS with a concentration of 1 μg/mL was added and incubated for 23 hours.
Results: Bergaptol treatment significantly improved the cognitive impairment induced by LPS. In addition, Bergaptol significantly inhibited the reduction of dendritic spines and the mRNA level of inflammatory factors (TNF-α, IL-6 and IL-1β) in hippocampal induced by LPS. In vitro, Bergaptol inhibited the production of TNF-α, IL-6 and IL-1β from LPS-treated BV-2 cells. In addition, Bergaptol treatment significantly reduced the phosphorylation levels of JAK2, STAT3 and p65 in LPS-stimulated BV-2 cells.
Conclusion: In conclusion, our results suggest that Bergaptol alleviates LPS-induced neuroinflammation, neurological damage and cognitive impairment by regulating the JAK2/STAT3/P65 pathway, suggesting that Bergaptol is a promising neuroprotective agent.
Keywords: Bergaptol, neuroinflammation, LPS, JAK2/STAT3/p65
Introduction
Neuroinflammation, as the immune response of the central nervous system (CNS), plays a vital role in protecting brain function in response to various adverse stimuli such as infection and trauma. However, prolonged excessive neuroinflammation may be destructive and is important pathogenesis of a variety of CNS diseases, such as cognitive impairment,1 Alzheimer’s disease (AD)2 and Multiple Sclerosis (MS).3 Microglia are the resident macrophages in CNS that function as crucial in neuroinflammation leading to abnormal nervous system function.4 In response to intense stimulation, microglia migrate rapidly and release inflammatory factors (TNF-α, IL-6 and IL-1β). Abnormally elevated pro-inflammatory mediators could cause neuronal death and synaptic dysfunction.5 Therefore, inhibition of abnormal neuroinflammation and reduction of inflammatory factors are promising methods for prevention and treatment of various CNS diseases.
Lipopolysaccharides (LPS) is a component of the outer wall of gram-negative bacteria, consisting of lipid and polysaccharide. A large number of studies have shown that LPS could stimulate microglia to produce inflammatory mediators and cause behavioral symptoms such as cognitive impairment and depression.6,7 Therefore, LPS administration has been widely recognized as a viable and stable model for studying neuroinflammation. In this manuscript, we simulated an animal model of neuroinflammation by injecting LPS (40 μg/kg) into the hippocampal CA1 region of mice.
Bergaptol, a natural furocoumarin, is especially abundant in Citrus products such as Lemon and Bergamot. Recent studies have found that Bergaptol has potential antitumor and anti-inflammatory effects.8,9 However, whether Bergaptol can inhibit neuroinflammation and play a neuroprotective role has not been reported. We used the intracranial injection of LPS and the LPS-stimulated BV-2 microglial cells to mimic the inflammatory response in vivo and in vitro to investigate the potential effect of Bergaptol. Bergaptol treatment significantly alleviated neurological damage and cognitive impairment induced by LPS. In addition, we determined that Bergaptol suppressed LPS-induced neuroinflammation via regulating the JAK2/STAT3/p65 pathway.
Materials and Methods
Chemicals and Reagents
Bergaptol (purity ≥ 98%) was purchased from Shanghai Ronghe Medical Technology Development Co. Ltd. (Shanghai, China), diluted with normal saline to 4mg/mL, filtered and stored at 4°. Lipopolysaccharide was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animal and Treatment
The experimental procedures of this project were approved by the Animal Experimentation Ethics Committee of Sichuan Kangcheng Biotechnology Co., LTD (No. IACUC-202011-r-001). All animal procedures were performed according to the Sichuan Provincial Administrative Measures for Laboratory Animals (GB/T35892-2018). The animals were raised in cages (indoor temperature 22 ± 2°C and 12/12 hours light-shedding cycle) with free access to water and food. C57BL/6 male mice (6 weeks) were randomly divided into five groups as follows: (1) Sham group, (2) LPS vehicle sham group, (3) LPS + Bergaptol (10 mg/kg) group, (4) LPS + Bergaptol (20 mg/kg) group, and (5) LPS + Bergaptol (40 mg/kg) group. Mice were anesthetized by 1% pentobarbital and fixed to a stereotaxic apparatus. The anterior fontanelle and posterior fontanelle were exposed by incision of the scalp about 2 cm along the sagittal suture of the mouse. Mice were injected with LPS (40 μg/kg) or equivalent volume of normal saline (0.9% NaCl) into the hippocampal CA1 region by microsyringe (coordinates: 0.8 mm posterior to the bregma, ±1.4 mm lateral to the sagittal line, and the depth of 4 mm) with a micro syringe. After surgery, the wound was sutured, and the animals were waked up under a roasting lamp. Bergaptol was dissolved in normal saline to 4mg/mL, and treated mice were intraperitoneally injected once a day for two weeks. Mice in sham and LPS groups were given the same amount of normal saline. The treatment schedule and dose of LPS and Bergaptol were selected based on the results of our preliminary study (data not shown). The experimental design and treatment schedule are shown below (Figure 1).
 |
Figure 1 The experimental design and treatment schedule. |
Cell Culture and Treatment
The BV-2 cell line was obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co. Ltd. (Shanghai, China), cultivated as described. Bv-2 cells were inoculated at a density of 5×103 cells/well on a 96-well plate and kept stable overnight in an incubator. Firstly, Bergaptol with different concentrations was used to incubate for 1 hour, and then LPS (1 μg/mL)2 was added for 23 hours.
Morris Water Maze (MWM) Test
The MWM test was performed to test learning and memory as described earlier.2 The detection system consists of a circular pool filled with water (150 cm in diameter and 40 cm in height) and an automatic image acquisition system. Divided the pool into four equal quadrants and placed a hidden platform (10 cm in diameter, 1 cm underwater) in the center of one of the quadrants. The mice were trained to find the hidden platform and successfully save themselves in the first part. Each mouse was lowered from a different quadrant into the pool for 60 seconds and then placed on the platform to rest for 15 seconds. The spatial probe test followed six consecutive days of training tests. The hidden platform in the swimming pool was removed, and the swimming track in 60 seconds was recorded. The mice were sacrificed 24 hours after the MWM test and collected brain tissue.
Cell Viability Assay
The potential cytotoxicity of Bergaptol in BV-2 cells was measured by Cell Counting Kit-8 (CCK8) (EpiZyme, China). Bv-2 cells were inoculated at a density of 5×103 cells/well on a 96-well plate and kept stable overnight in an incubator. After the cells were treated with different concentrations of Bergaptol (0–160 μg/mL), 10 ul CCK8 reagent was added to each well and incubated at 37°C for 2 hours. Measure each well’s optical density (OD) at 450 nm using a microplate meter.
Golgi-Cox Assay
Golgi staining was performed according to the instructions of the FD rapid Golgi staining kit (FD NeuroTechnologies, America). In short, the prepared brain tissue was immersed in equal volume mixed solutions A and B, placed at room temperature away from light for two weeks, and replaced with a new liquid every three days. The brain tissue was transferred to solution C and soaked at room temperature for 72 hours. The liquid was changed every 24 hours. Under the condition of −20°C, the brain tissue was cut into 100um thick slices with a frozen microtome. Then the prepared working solution was used to dye the sections. The soaking sequence of slices was as follows: 50% ethanol for 4 minutes, 75% ethanol for 4 minutes, 95% ethanol for 4 minutes, absolute ethanol for 20 minutes and xylene for 20 minutes. Finally, the slices were sealed with a resin sealing agent and observed with a microscope.
Haematoxylin-Eosin and Nissl Assay
Haematoxylin-eosin staining was performed according to the conventional protocol. Briefly, paraffin sections of brain tissue were first prepared and then stained with xylene dewaxing and hematoxylin solution. Nissl staining is a reliable histological examination and determines the extent of neuronal cell death. First routine dewaxing (15 minutes each for xylene I and xylene II, then gradient alcohol dehydration: 5 minutes each for 100%, 95%, 90%, 80%, 70%, 50%). Sections were rinsed three times with distilled water for 5 minutes each, then placed in a 60°C incubator and stained with 1% tolridineblue for 40 minutes. Finally, they were dehydrated in graded ethanol (70, 95 and 100%) and then transparent with xylene. The sections were all observed and photographed by compound microscopy, and the results were analyzed by Image software.
Immunofluorescence Assay
Mice were perfused with PBS and 4% paraformaldehyde to remove blood and fixation. The brains were then cut in the coronal plane into 100 um thick slices. Sections were permeabilized in 0.3% Triton X for 30 minutes and then washed three times with PBS for 5 minutes. Sections were closed with blocking solution at room temperature, washed three times with PBS for 5 minutes and incubated with primary antibodies (rabbit anti-Iba1: CST17198T, 1:500; rabbit anti-PSD95: CST3450T, 1:200; rabbit anti-synapsin-1: CST5297T, 1; 200) at 4°C overnight. Sections were rinsed with PBS and incubated with Alexa Fluor™ 647 donkey anti-rabbit IgG (ab150077, 1:500) secondary antibody overnight at 4°C. Finally, the slices were sealed with ProLong™ Diamond Antifade Mountant with DAPI (Solarbio), and the results were observed with a confocal microscope (Nikon).
Real-Time RT-PCR and Enzyme-Linked Immunosorbent Assay (ELISA)
Total RNA of the tissues was extracted according to the RNA extraction reagent instructions (Vazyme, R401-01). RT-PCR was performed with Archimed-X6 real-time PCR (Rocgene, China) equipment after preparing the reaction solution according to the SYBR Green PCR kit (Accurate Biology, AG11701).
Hippocampal samples were homogenized in a tenfold volume of RIPA lysis buffer. The supernatant was then obtained by centrifuging the homogenates at 4°C and 12,000 g for 15 min. Finally, the level of TNF-α (Bioswamp, MU30030), IL-6 (Bioswamp, MU30044) and IL-1β (Bioswamp, MU30369) in supernatant was measured by ELISA analysis.
Western Blotting
Proteins were obtained using RIPA lysis buffer with a 1 mM phosphatase and protease inhibitor mixture. The groups of proteins were separated onto polyvinylidene fluoride (PVDF) membranes by 10% SDS-PAGE gel electrophoresis. After blocking for 2 hours, incubate overnight with the following primary antibody (rabbit anti-phospho-JAK2 (ab32101, 1:500), rabbit anti-JAK2 (ab108596, 1:500), rabbit anti-phospho-STAT3 (ab267373, 1:1000), rabbit anti-STAT3 (ab68153, 1:1000), rabbit anti-phospho-NF-ΚB p65 (Cst3033T, 1:1000), mouse anti-NF-ΚB p65 (Cst6956T, 1:1000), rabbit anti-TNF-α (ab215188, 1:500), rabbit anti-IL-6 (ab233706, 1:500), rabbit anti-IL-1β (ab234437, 1:500), mouse anti-GAPDH (AG019, 1:1000)). Finally, the bands were incubated with the corresponding secondary antibodies (goat anti-mouse IgG, Beyotime, 1:2000; goat anti rabbit IgG, Beyotime, 1:2000) for 1 hour at room temperature and detected with ECL reagents.
Statistical Analysis
In this study, statistical analysis was performed using GraphPad Prism 8.0.1 statistics software. All data are shown as the mean ± SEM. Data from the behavioural tests were analyzed using two-way ANOVA. Other data were analyzed by one-way ANOVA. P < 0.05 was considered statistically significant.
Results
Effect of Bergaptol on LPS-Induced Cognitive Impairment in Mice
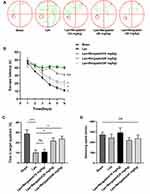
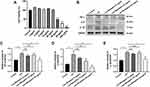
To verify whether Bergaptol treatment is beneficial for cognitive impairment induced by LPS in mice, we performed the Morris water maze test. Escape trajectories of LPS-treated mice were found to be more complex and the incubation period of they were significantly prolonged compared to the sham group (Figure 2A and B). This is a good indication that the injection of LPS into the CA1 region can successfully induce cognitive impairment in mice. In the spatial probe test, the time spent in the target quadrant was significantly reduced in the LPS group compared to the sham group, but the time spent in the target quadrant was significantly longer in mice after Bergaptol (20 and 40 mg/kg, not 10 mg/kg) treatment (Figure 2C). Analysis of the swimming speed of the mice revealed no statistically significant differences between the different groups (Figure 2D). These results suggest that Bergaptol treatment significantly improved the cognitive impairment induced by LPS in mice.
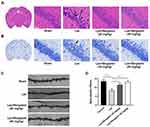
Effects of Bergaptol on the Hippocampal CA1 Neurons and Dendritic Spines
Behavioral experiments revealed that Bergaptol concentrations too low (10 mg/kg) might have no significant beneficial effect on LPS mice. In the following study, we examined the effects of Bergaptol (20 and 40 mg/kg) on LPS-induced neuronal damage. It was found by HE staining that Bergaptol treatment significantly reduced LPS-induced fixation and cleavage of neuronal nuclei in the CA1 region of the hippocampus (Figure 3A). Nissl staining revealed that Bergaptol treatment rescued LPS-induced the reduction of Nissl bodies in the CA1 region of the hippocampus (Figure 3B). In addition, mice CA1 region pyramidal neuron dendritic spines were analyzed by Golgi-Cox staining (Figure 3C). Statistical analysis showed that the density of dendritic spines of cone neurons in the CA1 region of mice in the LPS group was significantly lower than that in the sham group. Furthermore, compared with the LPS group, the dendritic spine density of mice in the Bergaptol-treated group was significantly increased, especially at higher concentrations of Bergaptol (40 mg/kg) (Figure 3D). These data suggested that Bergaptol has a mitigating effect on LPS-induced dendritic injury.
Effects of Bergaptol Treatment on Synaptic-Related Proteins
PSD-95 and synapsin-1 are the two main synaptic-related proteins. We analysed their changes in the hippocampus of mice. The results showed a tendency towards a decrease in the expression of PSD95 and synapsin-1 in the hippocampus of the LPS group compared with the sham group. However, Bergaptol treatment significantly improved the expression reduction of PSD-95 and synapsin-1 compared with the LPS group (Figure 4A and B). These data suggested that Bergaptol increased the expression of synaptic-related proteins in the hippocampus of LPS-treated mice.
Effect of Bergaptol on LPS-Induced Neuroinflammation in Mice
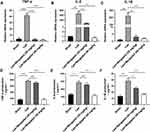
In this study, it was found that the mRNA levels of TNF-α, IL-6 and IL-1β were significantly increased in the hippocampus of LPS-treated mice compared to the sham group. However, Bergaptol (20 and 40 mg/kg) treatment significantly reduced the LPS-induced mRNA levels of TNF-α, IL-6 and IL-1β (Figure 5A–C). On the other hand, ELISA analysis revealed that TNF-α, IL-6, and IL-1β levels were significantly increased in the hippocampal tissue of LPS-treated mice. Similarly, Bergaptol treatment (20 and 40 mg/kg) significantly reduced the release of TNF-α, IL-6 and IL-1β in the hippocampus of LPS-treated mice (Figure 5D–F). These data suggest that Bergaptol inhibits LPS-induced neuroinflammation in mice.
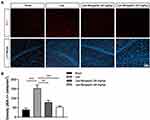
Effect of Bergaptol on Microglia in the Hippocampus of LPS-Treated Mice
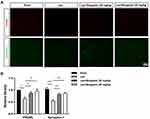
Microglia, resident macrophages of the central nervous system (CNS), play an important role in neuroinflammation and maintaining CNS homeostasis.10 Previous studies suggest that inhibition of excessive activation of stimulated microglia in the mouse brain could be an effective way to prevent and treat neurodegenerative diseases.11,12 In the present study, we investigated the effect of Bergaptol on microglia in the hippocampus of LPS-treated mice by immunofluorescence (Figure 6A). The density of Iba −1 positive microglia in the hippocampal region of LPS-treated mice was significantly increased compared to the sham group. Bergaptol treatment significantly reduced the density of Iba −1 positive microglia in the hippocampal region of lps-treated mice compared with LPS-treated group (Figure 6B).
Effect of Bergaptol on LPS-Induced Inflammatory Factor Production in BV-2 Cells
To explore the effect of Bergaptol on LPS-induced inflammation produced by BV-2 cells, we first determined the appropriate concentration of Bergaptol by CCK8. BV-2 cells were treated with different concentrations (0–160 μg/mL) of Bergaptol, and cellular activity was measured after 24 hours (Figure 7A). No significant difference in cell viability was observed for Bergaptol concentrations up to 20 μg/mL compared to the sham group, and subsequent experiments used Bergaptol concentrations of 5, 10 and 20 μg/mL, respectively. BV-2 cells were pretreated with Bergaptol (5, 10 and 20 μg/mL) for 1 hour, then then added LPS and continued to incubate for 23 hours. Compared with the sham group, the protein expression levels of TNF-α, IL-6 and IL-1β were significantly increased in LPS-treated BV-2 cells. However, pretreatment with Bergaptol (20 μg/mL) significantly reduced TNF-α, IL-6 and IL-1β levels in LPS-stimulated BV-2 cells compared with the LPS-treated group (Figure 7B–E).
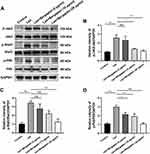
Effects of Bergaptol on the LPS-Induced Activation of JAK2/STAT3/p65 Pathway in BV-2 Cells
In neuroinflammation, the JAK2/STAT3/p65 pathway activation is one of the critical mechanisms.13 To investigate whether Bergaptol regulates LPS-induced inflammatory responses in BV2 cells by inhibiting excessive activation of the JAK2/STAT3/p65 pathway, we detected the relevant proteins by Western blotting (Figure 8A). The JAK2/STAT3/p65 pathway was significantly activated in LPS-stimulated BV-2 cells compared to the sham group. Bergaptol pretreatment significantly restored the LPS-induced elevated p-JAK2/JAK2, p-STAT3/STAT3 and p-p65/p65 ratios in BV-2 cells (Figure 8B–D).
Discussion
In recent years, the medicinal value of natural compounds has received increasing attention due to the scarcity of medicinal resources. Bergaptol, which is particularly abundant in citrus products, has been found to possess good antiatherogenic functions.8 In this study, we report for the first time that Bergaptol exerts neuroprotective effects by modulating the JAK2/STAT3/p65 pathway to inhibit LPS-induced neuroinflammation. This study suggests that Bergaptol may become a promising therapeutic drug for neuroinflammation-related diseases.
Neuroinflammation has long been considered the core of neurodegenerative diseases and is closely related to cognitive impairment caused by neuronal injury and dysfunction.14,15 In previous studies, systemic inflammatory models have been widely used to induce neuroinflammation. However, the contribution of systemic inflammation to neurodegenerative disease remains controversial. In addition, recent studies have found that the damage to hippocampal neurons caused by neuroinflammation is the key to the occurrence of cognitive impairment and other manifestations.16,17 In this study, we abandoned systemic inflammation to induce neuroinflammation and chose to inject LPS directly into the hippocampus to induce neuroinflammation. We found that the mice injected with LPS had apparent cognitive impairment and noticeable morphological changes in the hippocampus. It might be a new model for studying neuroinflammation, but its reliability may need to be confirmed by more research.
Microglia are the innate immune cells of the CNS and play a key role in mediating neuroinflammation. Microglia-mediated neuroinflammation is a highly complex pathophysiological process, and activated microglia differentially express a variety of protein molecules involved in inflammation. These protein molecules are not only directly related to the neuroinflammatory response but also closely related to other pathophysiological processes such as cell necrosis and neuronal functional status.18–20 It has been shown that under neuroinflammatory conditions, activated microglia release large amounts of inflammatory factors such as TNF-α, IL-6 and IL-1β, leading to neuronal death and synaptic dysfunction.21,22 On the other hand, microglia also play a key role in synaptic refinement, and abnormal microglia show dysfunctional synaptic pruning ability, which is an important cause of neural dysfunction.23 In our study, Bergaptol treatment significantly improved cognitive impairment in LPS-treated mice. Further analysis of changes in hippocampal neuron morphology in mice revealed that Bergaptol treatment significantly reduced the level of nucleus fixation in hippocampal neurons of LPS-treated mice and alleviated the reduction in hippocampal neuron spine density. On the other hand, Bergaptol treatment significantly alleviated the reduction of synaptic-related proteins (PSD95 and synapsin-1) in the hippocampus of LPS-treated mice. In conclusion, we evaluated the neuroprotective function of Bergaptol from the microscopic to the macroscopic level. Importantly, our immunofluorescence and ELISA results showed that Bergaptol treatment reduced the activation of microglia (Iba-1) and the release of inflammatory factors (TNF-α, IL-6 and IL-1β) in the hippocampus of LPS-treated mice. These results suggest that Bergaptol can inhibit neuroinflammation in mice’s hippocampus to protect hippocampal neurons and improve cognitive impairment in mice.
JAK2/STAT3 is a crucial regulator that induces the expression of various genes involved in the inflammatory response and is considered one of the essential inflammatory pathways.24,25 In addition, STAT3 can also crosstalk with other transcriptional factors such as NF-κB p65 and regulate various critical physiological processes, including the inflammatory response.26,27 Previous studies have shown that other LPS models can induce significant neuroinflammation through activation of the JAK2/STAT3/p65 pathway.28,29 In the present study, we found that Bergaptol pretreatment significantly restored LPS-induced elevation of p-JAK2/JAK2, p-STAT3/STAT3 and p-p65/p65 ratios in BV-2 cells. Therefore, we suggest that Bergaptol can control neuroinflammation by regulating the JAK2/STAT3/p65 pathway.
Conclusion
In conclusion, our in vivo and in vitro results demonstrated that Bergaptol alleviates LPS-induced neuroinflammation, neurological damage and cognitive impairment via regulating the JAK2/STAT3/p65 pathway. Bergaptol might be a promising candidate for the treatment of neuroinflammation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. d’Avila JC, Siqueira LD, Mazeraud A, et al. Age-related cognitive impairment is associated with long-term neuroinflammation and oxidative stress in a mouse model of episodic systemic inflammation. J Neuroinflammation. 2018;15(1):28. doi:10.1186/s12974-018-1059-y
2. Yang W, Liu Y, Xu QQ, Xian YF, Lin ZX. Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/Akt/GSK-3β pathway in experimental models of alzheimer’s disease. Oxid Med Cell Longev. 2020;2020:4754195. doi:10.1155/2020/4754195
3. Kempuraj D, Thangavel R, Natteru PA, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine. 2016;1(1):1003.
4. Gogoleva VS, Drutskaya MS, Atretkhany KS. The role of microglia in the homeostasis of the central nervous system and neuroinflammation. Mol Biol. 2019;53(5):790–798. doi:10.1134/S0026893319050054
5. Bairamian D, Sha S, Rolhion N, et al. Microbiota in neuroinflammation and synaptic dysfunction: a focus on Alzheimer’s disease. Mol Neurodegener. 2022;17(1):19. doi:10.1186/s13024-022-00522-2
6. Naeem K, Tariq Al Kury L, Nasar F, et al. Natural dietary supplement, carvacrol, alleviates LPS-induced oxidative stress, neurodegeneration, and depressive-like behaviors via the Nrf2/HO-1 pathway. J Inflamm Res. 2021;14:1313–1329. doi:10.2147/JIR.S294413
7. Ebeid MA, Habib MZ, Mohamed AM, et al. Cognitive effects of the GSK-3 inhibitor “lithium” in LPS/chronic mild stress rat model of depression: hippocampal and cortical neuroinflammation and tauopathy. Neurotoxicology. 2021;83:77–88. doi:10.1016/j.neuro.2020.12.016
8. Shen CY, Wang TX, Jiang JG, Huang CL, Zhu W. Bergaptol from blossoms of Citrus aurantium L. var. amara Engl inhibits LPS-induced inflammatory responses and ox-LDL-induced lipid deposition. Food Funct. 2020;11(6):4915–4926. doi:10.1039/C9FO00255C
9. Uto T, Tung NH, Taniyama R, Miyanowaki T, Morinaga O, Shoyama Y. Anti-inflammatory activity of constituents isolated from aerial part of angelica acutiloba kitagawa. Phytother Res. 2015;29(12):1956–1963. doi:10.1002/ptr.5490
10. Frost JL, Schafer DP. Microglia: architects of the developing nervous system. Trends Cell Biol. 2016;26(8):587–597. doi:10.1016/j.tcb.2016.02.006
11. Elbaz EM, Senousy MA, El-Tanbouly DM, Sayed RH. Neuroprotective effect of linagliptin against cuprizone-induced demyelination and behavioural dysfunction in mice: a pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicol Appl Pharmacol. 2018;352:153–161. doi:10.1016/j.taap.2018.05.035
12. Jo SH, Kim ME, Cho JH, et al. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways. Arch Pharm Res. 2019;42(8):695–703. doi:10.1007/s12272-019-01174-5
13. Hongyan L, Mengjiao Z, Chunyan W, Yaruo H. Rhynchophyllin attenuates neuroinflammation in Tourette syndrome rats via JAK2/STAT3 and NF-κB pathways. Environ Toxicol. 2019;34(10):1114–1120. doi:10.1002/tox.22813
14. Voet S, Srinivasan S, Lamkanfi M, van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med. 2019;11(6):e10248. doi:10.15252/emmm.201810248
15. Moyse E, Krantic S, Djellouli N, et al. Neuroinflammation: a possible link between chronic vascular disorders and neurodegenerative diseases. Front Aging Neurosci. 2022;14:827263. doi:10.3389/fnagi.2022.827263
16. Tyrtyshnaia A, Bondar A, Konovalova S, Sultanov R, Manzhulo I. N-docosahexanoylethanolamine reduces microglial activation and improves hippocampal plasticity in a murine model of neuroinflammation. Int J Mol Sci. 2020;21(24):9703. doi:10.3390/ijms21249703
17. Liu Q, Sun YM, Huang H, et al. Sirtuin 3 protects against anesthesia/surgery-induced cognitive decline in aged mice by suppressing hippocampal neuroinflammation. J Neuroinflammation. 2021;18(1):41.
18. Wang Y, Liu J, Li Y, Yang Y, Li K. Systematic characterization of heterogeneity caused by neuroinflammation in alzheimer’s disease based on integrative network analysis. Curr Alzheimer Res. 2021;18(13):1041–1056. doi:10.2174/1567205019666211216104449
19. de Wit NM, den Hoedt S, Martinez-Martinez P, Rozemuller AJ, Mulder MT, de Vries HE. Astrocytic ceramide as possible indicator of neuroinflammation. J Neuroinflammation. 2019;16(1):48. doi:10.1186/s12974-019-1436-1
20. Li W, Ali T, Zheng C, et al. Fluoxetine regulates eEF2 activity (phosphorylation) via HDAC1 inhibitory mechanism in an LPS-induced mouse model of depression. J Neuroinflammation. 2021;18(1):38. doi:10.1186/s12974-021-02091-5
21. Gonzalez-Reyes RE, Nava-Mesa MO, Vargas-Sanchez K, Ariza-Salamanca D, Mora-Munoz L. Involvement of astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front Mol Neurosci. 2017;10:427. doi:10.3389/fnmol.2017.00427
22. Han KM, Kang RJ, Jeon H, et al. Regorafenib regulates AD pathology, neuroinflammation, and dendritic spinogenesis in cells and a mouse model of AD. Cells. 2020;9(7):1655. doi:10.3390/cells9071655
23. Meng J, Han L, Zheng N, et al. Microglial Tmem59 deficiency impairs phagocytosis of synapse and leads to autism-like behaviors in mice. J Neurosci. 2022;42(25):4958–4979. doi:10.1523/JNEUROSCI.1644-21.2022
24. Zhou K, Chen J, Wu J, et al. Atractylenolide III ameliorates cerebral ischemic injury and neuroinflammation associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial fission in microglia. Phytomedicine. 2019;59:152922. doi:10.1016/j.phymed.2019.152922
25. Xiang S, Huang R, He Q, Xu L, Wang C, Wang Q. Arginine regulates inflammation response-induced by Fowl Adenovirus serotype 4 via JAK2/STAT3 pathway. BMC Vet Res. 2022;18(1):189. doi:10.1186/s12917-022-03282-9
26. Gao M, Liu L, Zhang X, Li Z, Zhang M. Interleukin-6 reverses Adriamycin resistance in nasal NK/T-cell lymphoma via downregulation of ABCC4 and inactivation of the JAK2/STAT3/NF-κB/P65 pathway. Environ Toxicol Pharmacol. 2021;85:103639. doi:10.1016/j.etap.2021.103639
27. Saravanan S, Islam VI, Babu NP, et al. Swertiamarin attenuates inflammation mediators via modulating NF-κB/I κB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur J Pharm Sci. 2014;56:70–86. doi:10.1016/j.ejps.2014.02.005
28. Millot P, San C, Bennana E, et al. STAT3 inhibition protects against neuroinflammation and BACE1 upregulation induced by systemic inflammation. Immunol Lett. 2020;228:129–134. doi:10.1016/j.imlet.2020.10.004
29. Zeng KW, Wang S, Dong X, Jiang Y, Tu PF. Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-κB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine. 2014;21(3):298–306. doi:10.1016/j.phymed.2013.08.016
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.