Back to Journals » Clinical Ophthalmology » Volume 8
Benefits of omega-3 fatty acid dietary supplementation on health-related quality of life in patients with meibomian gland dysfunction
Authors Oleñik A, Mahillo-Fernández I, Alejandre-Alba N, Fernández-Sanz G, Pérez M, Luxan S, Quintana S, de Carneros Llorente AM, García-Sandoval B, Jiménez-Alfaro I
Received 14 February 2014
Accepted for publication 20 March 2014
Published 30 April 2014 Volume 2014:8 Pages 831—836
DOI https://doi.org/10.2147/OPTH.S62470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Andrea Oleñik, Ignacio Mahillo-Fernández, Nicolás Alejandre-Alba, Guillermo Fernández-Sanz, María Alarcón Pérez, Sol Luxan, Silvia Quintana, Alfonso Martínez de Carneros Llorente, Blanca García-Sandoval, Ignacio Jiménez-Alfaro
Department of Ophthalmology, Fundación Jiménez Díaz, Madrid, Spain
Background: We assessed the impact of a dietary supplement based on the combination of omega-3 essential fatty acids and antioxidants on health-related quality of life in patients with meibomian gland dysfunction (MGD).
Methods: Patients of either sex (aged 18–85 years) diagnosed with MGD according to criteria identified at a 2011 International Workshop on Meibomian Gland Dysfunction participated in this randomized, double-masked, placebo-controlled study. Group A patients (controls) received an oral placebo supplement and group B patients received the oral study supplement (Brudysec® 1.5 g; Brudy Laboratories, Barcelona, Spain). At baseline and at 3-month follow-up, the patients completed the 36-Item Short Form Health Survey questionnaire using a Spanish validated version. The Physical (PCS) and Mental (MCS) Component Summary scores were the main outcome variables.
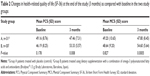
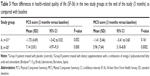
Results: A total of 61 patients completed the study (group A, n=31; group B, n=30). There were no significant differences in PCS and MCS scores at baseline between the two study groups, but after 3 months of treatment, significantly higher mean PCS and MSC scores were observed in patients treated with the active omega-3 dietary supplement as compared with controls (mean [standard deviation] PCS score 53.33±5.57 versus 47.46±7.31, P=0.008; mean MCS score 54.60±5.64 versus 47.80±8.45, P=0.0005). Moreover, mean differences between values at 3 months as compared with baseline were statistically significant for patients in group B (PCS score 7.14±5.81, 95% confidence interval 4.97–9.31, P=0.000; MCS score 5.96±7.64, 95% confidence interval 3.10–8.81, P=0.0002), whereas mean differences in patients assigned to group A were not statistically significant.
Conclusion: Dietary supplementation with a combination of omega-3 essential fatty acids and antioxidants had a significant beneficial effect on HRQoL (health-related quality of life) in patients with MGD.
Keywords: antioxidants, Brudysec®, nutraceuticals, polyunsaturated fatty acids
Introduction
Meibomian gland dysfunction (MGD) may well be the leading cause of dry eye disease throughout the world,1 and is the most common reason for ophthalmology consultation in daily practice. Increased meibum viscosity and hyperkeratinization of the ductal epithelium are the main mechanisms of obstructive MGD.2 Also, the tear film lipid layer is the major barrier to evaporation from the ocular surface. The tear film contains a complex mixture of proteins, enzymes, lipids, mucins, and salts that allow the tear film to perform its functions.3 A decrease in its thickness or functional integrity may cause evaporative dry eye. Although the etiology of MGD may differ from that of aqueous-deficient dry eye disease (due to insufficient lacrimal gland production), the two conditions share many clinical features, including symptoms of ocular surface irritation and visual acuity fluctuation, altered tear film stability, and potential compromise of the ocular surface.
Population-based and clinical cohort studies have shown that the prevalence of MGD varies widely from 3.5% to almost 70%,4,5 which may be explained by differences in the ethnicity of samples, methodological discrepancies regarding the clinical signs used to define MGD or its severity and assessment of clinical symptoms, and differences in age distribution across the studies. However, MGD is often underdiagnosed and overlooked. The International Workshop on Meibomian Gland Dysfunction in 2011 defined MGD as a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in glandular secretion. This may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.6
The goal of treatment for MGD is to improve the flow of meibomian gland secretions, thus leading to normal tear film stability.7 Overall, treatment varies widely, and includes warm compresses, lid massage, lid hygiene, artificial lubricants, eye (lipid) ointments, topical antibiotic and anti-inflammatory agents, and tetracycline derivatives, although in many cases treatment may be frustrating for both patients and ophthalmologists.8,9 Oral supplementation with omega-3 essential fatty acids has been proposed as a possible therapeutic option for patients with MGD. Given that meibum in MGD is often abnormal, it is possible that the lipid composition of meibum can be influenced by changes in dietary lipid intake. Clinical studies have demonstrated an association between the use of oral omega-3 supplements and improvement in symptoms of dry eye, tear stability, and tear secretion in different scenarios, including in patients with MGD.10–19
On the other hand, dry eye has been reported to negatively impact both general quality of life and vision-related daily life.20,21 In a previous randomized double-masked trial of 3 months’ duration, dietary supplementation based on a combination of omega-3 essential fatty acids and antioxidants (1.5 g/day) resulted in significant improvement in the mean Ocular Surface Disease Index, tear breakup time, Schirmer I test, lid margin inflammation, and meibomian gland expression (secretion) by means of a slit-lamp examination, as compared with baseline.19 In this study, the 36-Item Short Form Health Survey (SF-36)22 was used as a generic instrument for assessment of health-related quality of life (HRQoL).
The objective of this study was to assess the impact of oral supplementation with a combined formulation of omega-3 essential fatty acids and antioxidants on HRQoL in patients with MGD when compared with placebo. As far as we are aware, extension of the potential benefits of oral omega-3 nutritional supplements beyond ocular symptoms, especially in patients with MGD, has not been previously evaluated.
Materials and methods
Patients of either sex, aged 18–85 years and diagnosed with MGD according to the criteria identified at a 2011 International Workshop on Meibomian Gland Dysfunction23 were invited to participate in a randomized, double-masked study during a routine ophthalmologic appointment at the University Hospital Jiménez Díaz Foundation, in Madrid, Spain. Patients were recruited between March 2012 and December 2012 during ophthalmologic appointments at the study center. Patients with blepharitis but without an MGD diagnosis, and those with ocular disorders and using eye drops other than artificial tears, atopic or allergic disorders, systemic diseases (eg, Sjögren’s syndrome) and general treatments (eg, ocular hypertension), wearers of contact lenses, and pregnant women were excluded from the study. The study protocol was approved by the ethics committee of the hospital: Comité Ético de la Fundación Jiménez Díaz (CEIC-FJD) and the institutional review board of the University Hospital Jiménez Díaz Foundation, and written informed consent was obtained from all participants. The tenets of the Declaration of Helsinki for the protection of human subjects in medical research were strictly observed.
The primary objective of the study was to assess whether an oral nutraceutical formulation taken over a 3-month period could improve HRQoL as compared with placebo. Patients were visited at baseline (visit 0) and at the end of the study (3 months later). At the baseline visit, the patient’s eligibility was assessed, the informed consent form was signed, and the participant was randomly assigned to group A or group B. Group A patients (controls) received an oral placebo supplement, ie, 500 mg capsules containing sunflower oil with no other components or excipients apart from gelatin (bovine) and titanium oxide colorants, iron oxide, and hydroxides. Participants in group B received the oral study supplement (Brudysec® 1.5 g; Brudy Laboratories, Barcelona, Spain). The composition of the supplement formulation is shown in Table 1. Patients were instructed to take three capsules of the study medication (Brudysec 1.5 g or placebo) daily.
At baseline and at the 3-month follow-up visit, patients completed the SF-36 questionnaire using a Spanish validated version.24,25 The SF-36 is a multipurpose, short-form health survey with 36 questions. It yields an eight-scale profile of functional health, including physical functioning, role limitation because of physical disability, bodily pain, general health, vitality, social functioning, emotional limitation because of emotional disability, and mental health. These eight scales can be aggregated into two summary measures known as the Physical (PCS) and Mental (MCS) Component Summary scores. The scores range between 0 and 100, with higher scores representing better self-reported health. For the purpose of the study, the summary scores were used to minimize problems with multiple comparisons.
Safety was evaluated by ophthalmological examination and any adverse events that occurred during the study period. Patients could withdraw from the study of their own free will at any time, and could be withdrawn in the event of adverse events identified by ophthalmological criteria or if they developed concomitant illness potentially requiring specific nutritional treatment.
Statistical analysis
The data are expressed as the mean ± standard deviation, with differences in PCS and MCS scores between baseline and after 3 months expressed as the mean ± standard deviation and 95% confidence intervals (CIs). Data from patients in groups A and B were compared using the Student’s t-test for paired independent samples. Statistical significance was set at P<0.05. All statistical analyses were done using Statistical Package for the Social Sciences version 11.0 software (SPSS Inc., Chicago, IL, USA).
Results
Sixty-four patients met our inclusion criteria and were recruited into the study. Thirty-one patients were assigned to group A (controls) and 33 to group B. However, three patients from group B (9.1%) withdrew from the study, two because of fish-tasting regurgitation (n=2) and one for reasons not related to the study formulation, leaving 61 patients who completed the trial, comprising 31 (50.8%) in group A and 30 (49.2%) in group B. There were no significant differences between patients assigned to group A and those assigned to group B with regard to sex distribution (women comprising 71% versus 72.8%, respectively), mean age (54 years versus 58 years) or signs and symptoms of MGD, as Oleñik et al presented in a previous study.19 Both investigators and patients were blinded to treatment assignment throughout the study.
As shown in Table 2, there were no significant differences in PCS and MCS scores at baseline between the two study groups, but after 3 months of treatment, significantly higher mean PCS and MSC scores were observed in patients treated with the active omega-3 dietary supplement as compared with controls (mean PCS score 53.33±5.57 versus 47.46±7.31, P=0.008; mean MCS score 54.60±5.64 versus 47.80±8.45, P=0.0005). Moreover, compared with baseline, scores at 3 months were significantly improved for patients in group B (mean PCS score 7.14±5.81, 95% CI 4.97–9.31, P=0.000; mean MCS score 5.96±7.64, 95% CI 3.10–8.81, P=0.0002), but not for patients in group A (Table 3). No serious adverse events occurred during the study.
Discussion
This randomized, double-masked, 3-month clinical study demonstrates the positive impact of daily oral supplementation with a nutraceutical formulation (Brudysec 1.5 g) containing omega-3 fatty acids, vitamins, and essential trace elements on HRQoL in patients with MGD. Differences when compared with patients assigned to a placebo group were statistically significant for both the physical and mental components of the SF-36 weighed against baseline. In the present study, we used a generic HRQoL instrument instead of generic visual function or specific dry eye questionnaires.26 A number of studies have shown the detrimental effect of dry eye syndrome on vision-related quality of life.27–29 On the other hand, impairment of vision-related quality of life has shown a significant correlation with the severity of dry eye syndrome.30 Blepharitis and MGD also deserve attention because they present substantial quality of life issues for patients, eg, the need to apply warm compresses and cleanse the eye with diluted baby shampoo on a daily basis. However, HRQoL has not previously been examined in a homogeneous group of patients with MGD.
A number of mechanisms have been proposed via which supplementation with omega-3 essential fatty acids can alleviate MGD.12 It has been hypothesized that overwhelming the fatty acid metabolic pathway with omega-3 fatty acid molecules results in competitive inhibition of omega-6 fatty acid metabolism (omega-6 fatty acids produce molecules promoting inflammation), thus leading to decreased inflammation in the eyelid margin and in the meibomian gland region. Further, the present study was done in Spain, where the diet is based on sunflower oil and olive oil, so the intake of 1.5 g sunflower oil (placebo), which is high in omega-6 fatty acids, should be considered residual in inflammation study. Given that blepharitis, MGD, and dry eye are thought to be inflammatory diseases,23,31 a reduction in the systemic inflammatory state may alleviate blepharitis, MGD, and dry eye-associated discomfort. Another hypothesis concerns the composition of tear film. It has been suggested that unstable tear film results from abnormal meibomian gland secretions and can result in evaporative dry eye.
Supplementing the diet with high amounts of omega-3 fatty acids is likely to change the fatty acid composition and therefore the properties of meibomian gland secretions. This change may be beneficial in tear stabilization and may prevent inflammation from blocking the meibomian gland ducts and stagnation of meibum. Decreased inflammation of the lid margins and recovery of homeostasis of the ocular surface, along with significant improvement in HRQoL, was observed as a result of improved tear film stability in patients with MGD assigned to the omega-3 dietary supplement. Thus, omega-3 fatty acids inhibit proinflammatory factors. On the other hand, dietary antioxidants can decrease the oxidizing stress (potentially improving quality of life), but in this case the antioxidant does not decrease the MGD inflammation process. Therefore, 3 months of treatment with the supplement, prescribed as three capsules a day, was associated with statistically significant differences for all comparisons (PCS and MCS scores) between baseline and the end of treatment. Moreover, the mean differences in scores at 3 months versus baseline were significantly greater in the group assigned to active omega-3 supplementation as compared with controls. However, because omega-3 cannot be synthesized in the body, dietary supplementation with omega-3 should be encouraged in clinical practice to ameliorate symptoms of dry eye related to MGD19 and to improve HRQoL.
A limitation of this study was the lack of control over patients’ dietary intake. However, the randomized and double-masked design is a strength of the study, along with the fact that the data obtained are relevant to daily clinical practice.
Conclusion
This study was carried out in a homogeneous group of patients with MGD and shows that dietary supplementation with a combination of omega-3 essential fatty acids and antioxidants is an effective adjunctive treatment for improvement of quality of life when a generic HRQoL instrument (SF-36) is used. Further studies of longer duration and including larger patient populations are needed to assess whether the benefits of omega-3 supplementation on quality of life can be maintained in the long-term.
Acknowledgments
The authors thank Marta Pulido for editing the manuscript and providing editorial assistance. The food supplements used in this trial were provided by Brudy Laboratories, Barcelona, Spain.
Disclosure
The authors report no conflicts of interest in this work.
References
Nichols KK, Foulks GN, Bron AJ, et al. The International Workshop on Meibomian Gland Dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. | ||
Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–165. | ||
Green-Church KB, Butovich I, Willcox M, et al. The International Workshop on Meibomian Gland Dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52:1979–1993. | ||
Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of meibomian gland dysfunction. Optom Vis Sci. 1990;67:710–712. | ||
Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The International Workshop on Meibomian Gland Dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci. 2011;52:1994–2005. | ||
Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The International Workshop on Meibomian Gland Dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52:1930–1937. | ||
Geerling G, Tauber J, Baudouin C, et al. The International Workshop on Meibomian Gland Dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:2050–2064. | ||
Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–1803. | ||
Miljanovic´ B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–893. | ||
Kokke KH, Morris JA, Lawrenson JG. Oral omega-6 essential fatty acid treatment in contact lens associated dry eye. Cont Lens Anterior Eye. 2008;31:141–146. | ||
Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma-linolenic acid on meibomian gland dysfunction. Cornea. 2007;26:260–264. | ||
Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Trans Am Ophthalmol Soc. 2008;106:336–356. | ||
Kangari H, Eftekhari MH, Sardani S, et al. Short-term consumption of oral omega-3 and dry eye syndrome. Ophthalmology. 2013;120:2191–2196. | ||
Ong NH, Purcell TL, Roch-Levecq AC, et al. Epithelial healing and visual outcomes of patients using omega-3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea. 2013;32:761–765. | ||
Pinazo-Durán MD, Galbis-Estrada C, Pons-Vázquez S, Cantú-Dibildox J, Marco-Ramírez C, Benítez-del-Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin Interv Aging. 2013;8:139–148. | ||
Galbis-Estrada C, Pinazo-Durán MD, Cantú-Dibildox J, Marco-Ramírez C, Díaz-Llopis M, Benítez-del-Castillo J. Patients undergoing long-term treatment with antihypertensive eye drops responded positively with respect to their ocular surface disorder to oral supplementation with antioxidants and essential fatty acids. Clin Interv Aging. 2013;8:711–719. | ||
Ribelles-Villalba A, Galbis Estrada C, Pinazo Duran MD, Parras Cortes MA. Ojos eco: alternativas terapéuticas frente a los lubricantes oculares a propósito de una prueba piloto [Eco Eyes: therapeutic alternatives to the oculars lubricants: a pilot trial]. Medicina del Trabajo. 2013;22:120–129. Spanish. | ||
Oleñik A. Effectiveness and tolerability of dietary supplementation with a combination of omega-3 polyunsaturated fatty acids and antioxidants in the treatment of dry eye symptoms: results of a prospective study. Clin Ophthalmol. 2014;8:169–176. | ||
Oleñik A, Jiménez-Alfaro I, Alejandre-Alba N, Mahillo-Fernández I. A randomized, double-masked study to evaluate the effect of omega-3 fatty acids supplementation in meibomian gland dysfunction. Clin Interv Aging. 2013;8:1133–1138. | ||
Li M, Gong L, Chapin WJ, Zhu M. Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci. 2012;53:5722–5727. | ||
Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1:51–57. | ||
McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. | ||
Tomlinson A, Bron AJ, Korb DR, et al. The International Workshop on Meibomian Gland Dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52:2006–2049. | ||
Alonso J, Prieto L, Antó JM. La versión Española del SF-36 Health Survey (Cuestionario de Salud SF-36): un instrumento para la medida de los resulta dos clínicos [The Spanish version of the SF-36 Health Survey (SF-36 Health): An instrument for measuring clinical results]. Med Clin (Barc). 1995;104:771–776. Spanish. | ||
Vilagut G, Ferrer M, Rajmil L, et al. El cuestionario de salud SF-36 Español: una década de experiencia y nuevos desarrollos [The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments]. Gac Sanit. 2005;19:135–150. Spanish. | ||
Grubbs JR Jr, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33:215–218. | ||
Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157:799–806. | ||
Le Q, Zhou X, Ge L, Wu L, Hong J, Xu J. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol. 2012;12:22. | ||
García-Catalán MR, Jerez-Olivera E, Benítez-Del-Castillo-Sánchez JM. Ojo seco y calidad de vida [Dry eye and quality of life]. Arch Soc Esp Oftalmol. 2009;84:451–458. Spanish. | ||
Le Q, Ge L, Li M, et al. Comparison on the vision-related quality of life between outpatients and general population with dry eye syndrome. Acta Ophthalmol. 2014;92:e124–e132. | ||
[No authors listed]. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.