Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Benefits of different intensities of pulmonary rehabilitation for patients with moderate-to-severe COPD according to the GOLD stage: a prospective, multicenter, single-blinded, randomized, controlled trial
Authors He GX, Li N, Ren L, Shen HH , Liao N, Wen JJ, Xu YM, Wang J, Li QY
Received 7 May 2019
Accepted for publication 29 August 2019
Published 8 October 2019 Volume 2019:14 Pages 2291—2304
DOI https://doi.org/10.2147/COPD.S214836
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Guo Xia He,1,2,* Ning Li,1,3,* Lei Ren,2,4,* Hong Hua Shen,2 Ning Liao,5 Jian Jun Wen,6 Yi Min Xu,2 Jing Wang,4 Qing Yun Li1,3
1Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China; 2Department of Respiratory Rehabilitation, The Fourth Rehabilitation Hospital of Shanghai (Shanghai Jingan Geriatric Hospital), Shanghai 200042, People’s Republic of China; 3Institute of Respiratory Disease, Shanghai Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China; 4Department of Respiratory Medicine, The Second Affiliated Hospital of Suzhou University, Jiangsu 215004, People’s Republic of China; 5West-Nanjing Road Community Health-care Center of Shanghai, Shanghai 200040, People’s Republic of China; 6Caojiadu Community Health Service of Shanghai, Shanghai 200042, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Yun Li
Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, No. 197 Ruijin 2nd Road, Shanghai 200025, People’s Republic of China
Tel +86 1 350 173 8686
Fax +86 215 764 3271
Email [email protected]
Purpose: Pulmonary rehabilitation (PR) is essential to manage patients with COPD. The aim of this study was to investigate the appropriate intensity of PR exercise training for patients with moderate-to-severe COPD.
Patients and methods: A prospective multicenter randomized controlled trial was conducted from January 2014 to October 2018. The subjects were randomly assigned to three groups with different intensities of PR, according to their maximum oxygen uptake percentage determined by cardiopulmonary exercise testing. After 20 weeks of exercise training, the effects of low-, moderate-, and high-intensity exercise interventions on patients were compared to determine the most appropriate PR prescription.
Results: For patients with moderate COPD, all the measured parameters were significantly improved in the moderate- and high-intensity PR groups (P<0.01), while there was no significant difference in the frequency of acute exacerbations and the mMRC questionnaire after 20 weeks of PR exercise in the low-intensity PR group. For patients with severe COPD, all variables were also improved in the high-intensity PR group (P<0.05), while the mean differences of pre- and post-PR were lower than those in patients with moderate COPD. Moreover, the Hamilton Anxiety Scale and body mass index showed no significant difference in low-intensity PR group (P>0.05).
Conclusion: High-intensity PR exercise is helpful for patients with moderate to severe COPD. Moderate COPD patients need to receive intensive PR training; the improvement degrees from PR intervention were higher than those of the severe COPD patients. For patients with severe COPD, high-intensity PR exercise may be more beneficial if patients can tolerate it.
Keywords: chronic obstructive pulmonary disease, cardiopulmonary exercise testing, exercise therapy, pulmonary rehabilitation
Introduction
COPD is a common disease, seriously endangering the public health.1 Pulmonary rehabilitation (PR) has been proved to be beneficial for patients with COPD; however, its application is currently in development phase worldwide.2 In addition, PR is a comprehensive intervention, involving exercise training, counseling, instructional training, and behavioral change tailored to each patient.2,3 Improving the COPD patients’ quality of life is of great significance,4 and the purpose of PR is basically to reduce symptoms and improve exercise tolerance and functional capacity.4 However, the essential amount of PR is seriously underestimated by general practitioners and patients.5 Moore et al reported that 69,089 (64%) COPD patients in their cohort were eligible for PR, while only 6436 (9.3%) cases had been referred for rehabilitation.6 Another study demonstrated that if there is no targeted effort, the rate of early PR referrals for patients with COPD is extremely low.7
The 2019 GOLD report emphasized on the prominent role of PR in COPD management.8,9 The conclusions of the updated meta-analysis presented a strong argument that PR is beneficial for improving the subjective impact of symptoms and health status.10,11 However, lack of research data hampers progress on the specific frequency intensity of exercise, the degree of supervision, and the appropriate duration of PR exercise. Aerobic exercise is the core of lung rehabilitation, while a limited number of studies have recommended specific exercise intensities for indicating an optimal duration of PR to achieve the most promising results for patients with different severities of COPD.12,13 Although the new GOLD strategy provides the most appropriate exercise training program, it is based on the resources being available at each rehabilitation location and the patients’ specific capabilities. Therefore, the appropriate intensity of PR interventions to improve the patients’ quality of life with different severities of COPD remains elusive. To the best of our knowledge, there is no report concerning the comprehensive PR effect of patients with COPD based on the GOLD staging. It is noteworthy that most COPD patients with a high degree of severity (GOLD 4) require noninvasive positive pressure ventilation during daily activities, which might be extremely severe to tolerate high-intensity exercises. Therefore, we administered a 20-week PR exercise training for patients with moderate (GOLD 2)-to-severe (GOLD 3) COPD to compare exercise capacity (BODE) index, acute exacerbation (AE) of COPD (AECOPD), the psychological features of anxiety, and depression. We also discussed how to select an appropriate PR intervention in accordance with severity of COPD patients.
Materials and methods
Design
This is a prospective, multicenter, single-blinded, randomized controlled trial (Figure 1) conducted at five medical centers, including the Fourth Rehabilitation Hospital (Shanghai, China), Ruijin Hospital Affiliated to Shanghai Jiaotong University Medical College (Shanghai, China), the Second Affiliated Hospital of Suzhou University (Suzhou, China), West-Nanjing Road Community Health-care Center of Shanghai (China), and Caojiadu Community Health Service of Shanghai (China) from January 2014 to October 2018. Briefly, COPD patients who met the inclusion criteria were first contacted by visiting them in the inpatient room. Those who agreed to participate were provided with an explanation of rehabilitation program at the medical centers mentioned above and invited to sign written informed consent forms. All those patients were free to withdraw from the study at any time with no negative consequences. The performance testing was conducted in the same order at baseline (before randomization) and at 20 weeks.
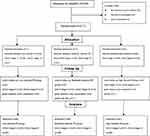 |
Figure 1 Flowchart of the randomized controlled trial. Abbreviations: PR, pulmonary rehabilitation; AECOPD, acute exacerbation of COPD. |
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of The Fourth Rehabilitation Hospital (Shanghai, China, Approval No. SP2015001), and written informed consent was obtained from each participant.
Inclusion and exclusion criteria
All subjects were included in the study based on the following criteria: 1) age ≥40 years; 2) clinical features of COPD and moderate-to-severe COPD, an FEV1/FVC ratio <0.70, and an FEV1 measurement between 30% and 80% with respect to the theoretical value based on postbronchodilator spirometry (GOLD 2 and GOLD 3); 3) no clinical features of asthma and/or evidence of bronchodilator responsiveness on spirometry; and 4) stable COPD with no recent history of exacerbation requiring hospitalization during the last 3 months. In order to minimize inaccuracy of diagnosis, COPD diagnosis was reviewed for each participant at baseline prior to enrolment and in accordance with GOLD strategies.14 Exclusion criteria were 1) coexisting active pulmonary tuberculosis, pulmonary fibrosis, pneumothorax, or lung cancer; 2) physically illness or mental incapacitation that prevented participation; 3) active microbial infections; and 4) lack of medical records; 5) clinical examinations consistent with coronary artery disease (eg, coronary angiography with stenting, coronary artery bypass grafting, myocardial infarction, admission to coronary care unit (CCU), etc.) or congestive heart failure; 6) neuromuscular, orthopedic and/or medical diseases precluding exercise testing; and 7) undergoing a cardiopulmonary rehabilitation program within the last year. The patients were followed-up for 24 months after active participation in a PR program.
Exacerbations of COPD
Exacerbations of COPD are defined as14 an acute worsening of respiratory symptoms, which result in additional therapy. Exacerbations of COPD play a substantial role in the rehabilitation of patients because they negatively impact health status, rates of hospitalization and readmission, and disease progression. Exacerbations of COPD are also complex events typically associated with increased airway inflammation, enhanced mucus production, and remarkable gas trapping. These changes may contribute to amplify dyspnea, which is a key symptom of an exacerbation. Other symptoms include increased sputum purulence and volume, together with increased cough and wheeze.
They are classified as follows:
- Mild (treatment with short-acting bronchodilators only, SABDs)
- Moderate (treatment with SABDs plus antibiotics and/or oral corticosteroids) or
- Severe (patients requiring hospitalization or visiting the emergency room). Severe exacerbations may be associated with acute respiratory failure as well.
The COPD patients received information required to highlight the importance of understanding of exacerbation symptoms and time of seeking professional health care for therapy. In addition, all patients were trained under the supervision of physiotherapists. Once dyspnea and sputum volume were intensified in a patient, a doctor attempted to report those symptoms.
Grouping
Initially, all subjects were assigned to GOLD 2 and GOLD 3 based on the criteria of GOLD.14 The participants were randomly assigned to the following groups: 1) low-intensity PR group, 2) moderate-intensity PR group, and 3) high-intensity PR group. Participants were allocated to each group on a random basis, which was defined by a computerized generator and was independent of the control of the principal investigator. The allocation sequence of the 89 participants of GOLD 2 and 128 participants of GOLD 3 was defined through a computer generator prior to the start of the study. After the generation of this sequence, 217 envelopes were created, numbered in the appropriate order, and contained the result of the allocation. The order of the envelopes’ number was defined based on the order of participants’ enrolment. The principal investigator was not aware of the information contained within the envelopes, thereby maintaining a minimization randomization process. To ensure the accuracy of the use of the envelopes, the documents inside the envelope were signed by the Data Safety Monitoring Board (DSMB) and were returned by the researchers after participants’ allocation.
Evaluation
Data related to the history and the current status of the disease, current medications, smoking history, and general physical examination were collected. At this stage, exclusion was still possible, in case of any inability or incapacity. Eventually, the patients underwent the following assessments.
Primary endpoint
Primary outcome of COPD patients was assessed at baseline and at 20th week using the BODE index.15,16 The BODE index, a multidimensional scale that predicts the risk of death, was accordingly calculated. It consisted of the following variables: body mass index (BMI) (B), obstruction (O, ie, FEV1), dyspnea (D, measured using the mMRC questionnaire), and exercise (E; meters walked in 6-min walk test, 6MWT). The participants’ BMI was calculated as body weight (kg)/height (m2). We also used the mMRC questionnaire to measure the intensity of dyspnea during daily activities. The 6MWT is widely used for testing functional capacity and physical exercise tolerance based on the distance walked in 6 mins. None of the participants used oxygen during the test. At the beginning and end of the test, blood pressure, heart rate, respiratory rate, Borg Dyspnea Scale, fatigue in the legs, and oxygen saturation were measured. The 6MWT was undertaken in accordance with the American Thoracic Society (ATS)/European Respiratory Society (ERS) guidelines. The variables were assigned to points as follows: BMI (>21 kg/m2=0 point, ≤21 kg/m2=1 point), FEV1% predicted (≥65%=0 point, 50–64%=1 point, 36–49%=2 points, and ≤35%=3 points), mMRC questionnaire (0–1=0 point, 2=1 point, 3=2 points, and 4=3 points), and 6MWD (≥350 m=0 point, 250–349 m=1 point, 150–249 m=2 points, and <149 m=3 points), and the points were summed to determine score of the BODE index. The scores ranged between 0 and 10 points, and higher scores represented a greater risk of death.
Secondary endpoint
Frequent exacerbations were defined as moderate or severe exacerbations or one or more hospitalization due to exacerbation of COPD in the previous 12 months. The COPD patients received information required to highlight COPD exacerbation symptoms and were interviewed every 3 months as well. An investigator recorded the patients’ vital status and the frequency of AECOPD with or without hospitalization. The Hamilton Rating Scale for Depression (HRSD) was used to assess the patients’ depression,17 and the Hamilton Anxiety Scale (HAMA) was employed to identify the patients’ anxiety.18
Other variables collected at baseline
Pack-years of smoking were calculated as follows: number of cigarette/cigarettes packs smoked/day × number of years smoked (where a bidi pack was calculated as the number of cigarette/cigarettes/20).19
Sample size
Using values of the BODE index in COPD patients with similar severity as reference,20 we estimated that 168 patients (28 patients in each group) were recruited to detect a statistically significant difference of 1 point between groups, with assumption of a common SD of 2 points in the index, two-sided significance level (α) of 5%, statistical power (1-β) of 80%, and a common dropout rate of 10%. It has been shown that the difference in 1 point in the BODE index had clinical relevance and was also associated with an increased risk of mortality of 33%.
Measurements
Pulmonary function tests
Spirometry, plethysmography, and diffusion test were carried out (Master Screen, Care Fusion Germany 234 GmbH, Höchberg, Germany) by trained personnel in a quiet room. Each test was performed three times, and the best result for each test was recorded and used to obtain the FVC, FEV1, and the FEV1/FVC ratio. Spirometry was repeated 20 mins after inhaling 200 µg of salbutamol to obtain the PB FVC, FEV1, and FEV1/FVC ratios. All patients with an FEV1/FVC ratio <70% and fixed airway obstruction on spirometry (improved PB FEV1<200 mL or improved FEV1/FVC <12%) were included in the present study, while those patients with a history of wheezing, chest tightness, and allergies affecting the eyes, nose, or skin; those suffering from osteoarthritis; and those with oxygen saturation <90% were excluded from the study as well.
Cardiopulmonary exercise testing
In the present study, the intensity of PR was determined by cardiopulmonary exercise testing as follows: ≥70% of maximal oxygen uptake, which was defined as high-intensity PR exercise, 50~70% was defined as moderate-intensity PR exercise, and <50% was defined as low-intensity PR exercise. The cutoff values were based on the prognostic importance of maximal oxygen uptake in COPD patients as reported in a previous study.20
PR intervention
The intervention provided a 20-week supervised inpatient PR program, included 10 education sessions delivered by a multidisciplinary team, comprising medical care, respiratory therapy, education, nutritional and psychological counseling, and chest physiotherapy. The patients underwent conventional exercise training 5 days per week for 40 mins, with 10 mins of warm-up before training, as well as 10 mins of relaxation exercises after training. The exercise component of warm-up incorporated an individualized exercise program consisting of various types of interval endurance training, such as walking and functional strength exercises. Moreover, relaxation exercises included stretching and walking. The exercise program consisted of 20 mins of stationary cycling using an upper limb and lower limb coordination exercise machine (Jiangsu Tianrui Medical Equipment Co., Ltd., Nanjing, China), starting at 50% of the maximal load achieved during an exercise test. The load was progressively increased by 10 W, if the patient’s heart rate and oxygen saturation were stable and the exercise was well tolerated. The sessions were ended with the help of relaxation techniques. Electrocardiogram (ECG) signals and blood-oxygen saturation level were monitored during the exercise session and within 1 hr after the exercise. To ensure patient’s safety, if the blood-oxygen saturation was <85%, blood pressure was >200/100 mmHg (1 mmHg=0.133 kPa), or the heart rate reached 85% of the maximum value during the cardiopulmonary exercise testing, the exercise was stopped. Furthermore, once a patient had severe shortness of breath and could not tolerate exercise subjectively, the physiotherapist attempted to report the case. Patients were given a 5-min rest before continuing their training as well. The duration of exercise training was defined as the sum of the time to reach the target intensity. Patients, who were repeatly unable to tolerate rehabilitation training for more than three times, were removed from this study.
In this study, 3 patients in the low-intensity PR group, 4 patients in the moderate-intensity PR group, and 5 patients in the high-intensity PR group were lost the follow-up because of poor cooperation.
Adverse events (AEs)
Definition
AEs occurred after patients received rehabilitation training, while they were not necessarily causally related to rehabilitation intervention. Thus, an AE can be an undesirable and unexpected physical sign (eg, including laboratory abnormalities, etc.), a symptom, or a time-related illness during training, regardless of whether there is a causal relationship with training.
Serious AE (SAE) refers to any event during rehabilitation training, that requires hospitalization, prolonged hospitalization, disability, impact on workability, or endangers life or death.
Determination of the severity of AEs
- Mild: A patient can endure it, without influencing continuation of treatment, with no need for special treatment, and no impact on COPD patient’s clinical conditions;
- Moderate: A patient is unbearable and needs to stop taking the medicine or do special treatment, containing a direct impact on COPD patient’s clinical conditions;
- Severe: An event endangering patient’s life, disability, or death; thus, the patient must stop that event immediately or do an emergency treatment.
AEs’ handlers
Any AEs, eg, patients’ subjective discomfort in training and abnormal laboratory testing, should be effectively treated seriously and carefully analyzed; besides, corresponding measures should be taken immediately, and patients should be examined on time according to their clinical conditions.
AEs included shortness of breath, dyspnea, arrhythmias, increased heart rate that reached 85% of the maximum value during the cardiopulmonary exercise test, fingertip blood-oxygen saturation of less than 85%, and blood pressure <200/100 mmHg (1 mmHg=0.133 kPa), which were closely followed to observe the outcomes of events or suspension training. Generally, if mild acute exacerbations occur during rehabilitation, the patient needs to suspend scheduled intensity training for 4 weeks, and to be treated with short-acting, drug dosing adjustment, and receive symptomatic treatment as indicated by the protocol.
When severe AEs are basically judged, corresponding treatment or rescue measures shall be immediately taken according to the clinical manifestations and clinical treatment standards. If acute exacerbations (moderate or severe) occur during rehabilitation, a patient has to stop scheduled intensity training. During resting at bed, a patient performs some active exercises being consistent with his/her conditions, in which the patient lost the follow-up (Figure 1).
All AEs should be tracked until they are properly resolved or stable.
In this study, one patient of GOLD 3, who allocated to moderate-intensity PR group, with multiple comorbidities died due to AEs. Besides, one patient of GOLD 3, who allocated to high-intensity PR group, was withdrawn from the study because of AEs.
Adherence
Adherence was defined in accordance with the prescribed regimen of PR classes. To monitor adherence in each group, we quantified the activity on a self-reported card registered by the patients, and attendance in the scheduled visits was every 3 months with physician over the 1-year follow-up. Moreover, an investigator recorded the patients’ vital status and the frequency of AECOPD with or without hospital admission.
Statistical analysis
Data were analyzed by using SPSS 22.0 software (IBM, Armonk, NY, USA). Prior to statistical analysis, the Kolmogorov–Smirnov test and the Shapiro–Wilk test were carried out to assess the normality of continuous data. Descriptive statistics (mean ± SD) were used to determine the participants’ characteristics. Normally distributed baseline demographic variables were compared by one-way ANOVA. Non-normally distributed variables were compared by using the Kruskal–Wallis test, with an alpha level of significance of 0.05. In addition, Δ expressed the mean difference between before and after PR treatment in the same group of patients. The outcomes of each variable were measured. If the one-way ANOVA showed a significant interaction for each variable, Bonferroni’s post hoc test was performed to identify the specific mean differences. An unconditional logistic regression analysis was applied to assess the influence of PR. For all analyses, P<0.05 (2-tailed) was considered statistically significant.
Results
Participants’ characteristics
Of the 316 patients with moderate-to-severe COPD who were referred to respiratory services for PR, a total of 217 patients were eventually randomized into three groups, 14 of whom lost follow-up (rate of losing the follow-up =6.45%). Among those patients who lost the follow-up, one in moderate-intensity PR group died due to acute exacerbations, one in high-intensity PR group died due to acute exacerbations, and 12 were due to poor cooperation. The distribution of participants is shown in Figure 1. Baseline characteristics are documented in Table 1. The subjects’ mean age was 65.3±6.2 years. No significant difference was found in FEV1, BODE index, and other baseline characteristics obtained at admission in the three groups.
 |
Table 1 Baseline characteristics of the participants |
Additionally, there were no significant differences in comorbidities between patients with moderate and severe COPD (coronary heart disease: 34% vs 33%, diabetes mellitus: 25% vs 32%, chronic renal failure: 2% vs 5%, noncirrhotic liver disease: 2% vs 4%, respectively).
Table 2 summarizes the changes in BMI, lung function, and other parameters after three interventions. The 6MWD, mMRC, BODE index, and HRSD were improved, while the frequency of AECOPD was significantly decreased among all the groups after 20-week of PR exercise training. However, FEV1, BMI, and HAMA were notably increased only in moderate- and high-intensity PR groups (P<0.05). The comparison analysis between groups showed that the 6MWD, BODE index, and HRSD were improved in parallel with the increase of PR exercise intensity. Compared with low- and moderate-intensity PR groups, the FEV1 was remarkably higher and frequency of AECOPD was markedly decreased after 20 weeks of high-intensity PR exercise training. Compared with the mild PR group, the mMRC of the high-intensity PR group was significantly higher, and the HAMA of the moderate-intensity PR group and high-intensity PR group was notably improved as well.
 |
Table 2 Comparison of parameters before and after different intensities of PR among all the participants |
PR intervention for GOLD 2 COPD patients
The changes between pre- and post-PR intervention of three different intensive exercise training programs for moderate COPD patients (GOLD 2) are summarized in Table 3. All variables improved in the high-intensity PR group between preintervention and postintervention. Significant differences were also found in all parameters except for FEV1 and HAMA in the moderate-intensity PR group. Besides, 6MWD and frequency of AECOPD were improved in low-intensity PR group after 20 weeks of PR intervention.
 |
Table 3 Comparison of parameters before and after different intensities of PR in the COPD patients with GOLD 2 |
The comparison analysis between groups showed that significant differences were found in the improved degrees of 6MWD and BODE index among the three groups (Δ6MWD: 54.5 vs 25.8 vs 10.3, ΔBODE index: 1.6 vs 0.8 vs 0.0, respectively; Table 3). Additionally, FEV1, HRSD, HAMA, and frequency of AECOPD were significantly improved in high-intensity PR group compared with moderate-intensity PR group and low-intensity PR group (ΔFEV1: 3.2 vs 0.3 vs 0.8, ΔHRSD: 3.3 vs 1.5 vs 0.4, ΔHAMA: 2.3 vs 0.0 vs 0.2, frequency of AECOPD 1.2 vs 0.7 vs 0.6, respectively). Compared with the low-intensity PR group, the mMRC of the high-intensity PR group and the moderate-intensity PR group (ΔmMRC: 1.4 vs 0.9 vs 0.0) was remarkably improved. Compared with the low-intensity PR group (ΔBMI: 1.2 to 0.4), the BMI of the high-intensity PR group was notably increased.
PR intervention for GOLD 3 COPD patients
Table 4 summarizes the changes between preintervention to postintervention for patients of GOLD 3 within the three groups. All variables were significantly improved in the high-intensity PR group between preintervention and postintervention. Significant differences were also noted in all parameters in the low-intensity PR group except for BMI and HAMA. In addition, only BMI was not markedly increased in the moderate-intensity PR group.
 |
Table 4 Comparison of parameters before and after different intensities of PR in COPD patients with GOLD 3 |
The comparison analysis between the groups revealed that FEV1 and 6MWD were considerably improved in the high-intensity PR group compared with the moderate-intensity PR group and low-intensity PR group (ΔFEV1: 2.0 vs 0.6 vs 0.7, Δ6MWD: 32.4 vs 23.0 vs 14.5, respectively). Significantly higher improvement of frequency of AECOPD was only found in the high-intensity PR group compared with the low-intensity PR group (frequency of AECOPD 1.4 vs 0.5). Compared with the low-intensity PR group, HAMA was markedly decreased in the high-intensity PR group and moderate-intensity PR group (ΔHAMA 1.8 vs 2.4 vs 0.2). Significant differences were also found in the improved degree of HRSD among the three groups (ΔHRSD 4.3 vs 3.0 vs 2.2).
The effects of PR intervention
This study was conducted to assess the effects of PR. An unconditional logistic regression analysis was applied, in which the variables were selected by backward procedure. The results are presented in Table 5. It was revealed that age, intensity of PR, GOLD 2/GOLD 3, HRSD, and frequency of AECOPD were statistically significant (P<0.05), and ORs were 2.338, 10.339, 3.146, 1.114, and 2.701, respectively. Additionally, their corresponding 95% CIs) were (1.052,5.200), (3.215,33.244), (2.173,4.555), (1.030,1.205), and (1.503,4.854), respectively.
 |
Table 5 Univariate analysis for predicting the effects of pulmonary rehabilitation on COPD patients (BODE≤–1) |
Discussion
To the best of our knowledge, this is the first study comparing the results of different intensive PR regimens in patients with moderate-to-severe COPD. The findings of the present study are summarized as follows: 1) it is recommended that patients with moderate COPD undergo moderate- and high-intensity PR exercises, especially high-intensity PR exercise, which may have higher physiological advantages; 2) for patients with severe COPD, high-intensity PR exercise may be more beneficial, although moderate- and low-intensity PR training programs can sufficiently decrease the frequency of AECOPD and could be helpful to significantly improve the symptoms of dyspnea and quality of life; 3) the improved degree of PR intervention for moderate COPD patients was higher than that of severe COPD patients.
The evaluation of COPD has shifted from spirometry to a focus on patients’ overall health.21 The BODE index and the frequency of AECOPD were major determinants of quality of life in COPD patients.22 A previous study showed that the total BODE index converted by recentering is more promising in the prediction of mortality risk and quality of life than lung function, as well as being simple and feasible.23 AECOPD also plays a substantial role in this disease because acute exacerbations can rapidly reduce patients’ quality of life, aggravate their symptoms, accelerate the decline in lung function, and increase the social and economic burden of the family. Matsui et al24 reported that early PR was associated with reduced 90-day readmission and shortened the length of stay in hospital (LOS) in patients with AECOPD. Our results showed that in all public health training groups, the BMI and frequency of AECOPD were improved, while the high-intensity PR group had a significantly higher level of benefit. Those results are in line with previously reported findings (eg, Moore et al6), demonstrating that PR can reduce hospital admissions for AECOPD. A number of researches25–27 also showed a significant relationship between GOLD stages and the BODE index, and the capacity of exercise in patients was associated with the severity of COPD. It was confirmed that geriatric COPD rehabilitation in the setting of a nursing home may reduce hospital admissions in frail COPD patients and increase exercise tolerance as well.
Furthermore, COPD has significant extrapulmonary effects; the most important effect of these systemic manifestations is dysfunction of skeletal muscle, especially in the lower limb muscles involved in walking, leading to a progressive decline in daily activities. Studies28,29 revealed that in a remarkable proportion of COPD patients, fatigue in skeletal muscle, rather than breathing difficulty, limits the patient’s exercise capacity. Symptoms of fatigue and dyspnea may hinder patients to perform physical activities, set a vicious cycle of worsening muscle endurance, as well as further erosion of exercise tolerance. Moreover, PR is one of the most effective non-pharmacological management programs for patients with COPD. The majority of current PR guidelines recommend that higher intensity is a key component of any exercise program. However, in PR, the role of exercise intensity in improving athletic performance and extending athletic training outcomes remains elusive. The FEV1 was significantly improved in this study, because the patients were not adequately stable at baseline, or PR improved respiratory muscle strength, which led to improvement of lung function.
Morris et al20 found that there is insufficient evidence to suggest that high-intensity exercise training program provides additional benefits compared with the low-intensity exercise training, although they only compared high-intensity exercise training program with low-intensity one. Therefore, further efforts should be made to tailor specific therapeutic approaches to individuals’ needs.30 Our results demonstrated that severe-intensity PR exercise training results in a greater improvement in exercise capacity for GOLD 2 and GOLD 3 patients. Physical activity may increase the confidence and willingness of COPD patients to further participate in intense physical activities, and may serve as an intermediate target to increase uptake of PR.31,32 One possible explanation for this effect is that high-intensity training extends the patient’s movement time.33 If the patients of GOLD 3 can tolerate hypopnea during PR, they may require high-intensity exercise and provide more physiological results. The better the lung function and the higher the exercise intensity, the more beneficial the exercise will be for the patient.
Anxiety and depression are major complications of COPD as well.34 In addition, PR may help reduce anxiety and depression. Our results revealed that depression and anxiety were significantly improved in the PR-based groups, especially in the high-intensity PR group, which is consistent with previous studies.35–37
Several limitations should be taken in interpreting our results into account. First, this is a small sample size study; therefore, further well-designed, multicenter, prospective interventional clinical trials with PR treatment need to be conducted in the future. Second, the participants of the present study only confined to GOLD 2 and GOLD 3 patients, because the patients of GOLD 1 had no obvious symptoms, which resulted in poor compliance, while different rehabilitation strategies being consistent with their conditions for patients with GOLD 4 are highly required.
In summary, high-intensity PR exercise should be adopted for patients with moderate-to-severe COPD. Moderate COPD (GOLD 2) patients need to further receive intensive PR training; the improved degrees of PR intervention for the GOLD 2 patients were further considerable compared with those of GOLD 3 patients. For severe COPD (GOLD 3) patients, high-intensity PR exercise might be more beneficial if patients can tolerate it.
Acknowledgment
This study was financially supported by the Shanghai Health and Family Planning Commission (Grant No. 201540060), the Key Construction Projects of Shanghai Health and Family Planning on Weak Discipline (Grant No. 2015ZB0401), Health and Family Planning Commission of Jing’an District, Shanghai (Grant No. 2018MS21).
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
2. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST
3. Spruit MA. Pulmonary rehabilitation. Eur Respir Rev. 2014;23(131):55–63. doi:10.1183/09059180.00008013
4. Halding AG, Grov EK. Self-rated health aspects among persons living with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1163–1172. doi:10.2147/COPD.S129325
5. Galera O, Grimal G, Bajon D, Darolles Y. Barriers to referral to pulmonary rehabilitation in COPD patients from the perspective of general practitioners. Rev Pneumol Clin. 2017;73(3):115–119. doi:10.1016/j.pneumo.2017.03.002
6. Moore E, Newson R, Joshi M, et al. Effects of pulmonary rehabilitation on exacerbation number and severity in people with COPD: an historical cohort study using electronic health records. Chest. 2017;152(6):1188–1202. doi:10.1016/j.chest.2017.05.006
7. Morso L, Jensen MS, von Plessen C, Qvist P. Rehabilitation of discharged patients with chronic obstructive pulmonary disease-are new strategies needed? Health Serv Res Manag Epidemiol. 2017;4:2333392816687704.
8. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.5-FINAL-04Nov2018_WMS.pdf.
9. Saunders T, Campbell N, Jason T, et al. Objectively measured steps/day in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Phys Act Health. 2016;13(11):1275–1283. doi:10.1123/jpah.2016-0087
10. Rugbjerg M, Iepsen UW, Jorgensen KJ, Lange P. Effectiveness of pulmonary rehabilitation in COPD with mild symptoms: a systematic review with meta-analyses. Int J Chron Obstruct Pulmon Dis. 2015;10:791–801. doi:10.2147/COPD.S78607
11. Corhay JL, Dang DN, Van Cauwenberge H, Louis R. Pulmonary rehabilitation and COPD: providing patients a good environment for optimizing therapy. Int J Chron Obstruct Pulmon Dis. 2014;9:27–39. doi:10.2147/COPD.S52012
12. Torres-Sanchez I, Valenza MC, Cebria IIMDA, Lopez-Lopez L, Moreno-Ramirez MP, Ortiz-Rubio A. Effects of different physical therapy programs on perceived health status in acute exacerbation of chronic obstructive pulmonary disease patients: a randomized clinical trial. Disabil Rehabil. 2018;40(17):2025–2031. doi:10.1080/09638288.2017.1323236
13. Sandoz JS, Roberts MM, Cho JG, Wheatley JR. Magnitude of exercise capacity and quality of life improvement following repeat pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1085–1091. doi:10.2147/COPD.S131778
14. Disease. GIfCOL. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global initiative for chronic obstructive lung diseases (GOLD). 2014. Available from: http://www.goldcopd.org.
15. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi:10.1056/NEJMoa021322
16. Cote CG, Celli BR. Pulmonary rehabilitation and the BODE index in COPD. Eur Respir J. 2005;26(4):630–636. doi:10.1183/09031936.05.00045505
17. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi:10.1136/jnnp.23.1.56
18. Predescu V, Ciurezu T, Romila A, et al. The “double-blind” procedure in study of the anxiolytic effects of the preparation Wy 3498 (Oxazepam). Evaluation of anxiety states with the Hamilton scale (H.S.). Neurol Psihiatr Neurochir. 1969;14(2):153–165.
19. Chhabra SK, Rajpal S, Gupta R. Patterns of smoking in Delhi and comparison of chronic respiratory morbidity among beedi and cigarette smokers. Indian J Chest Dis Allied Sci. 2001;43(1):19–26.
20. Morris NR, Walsh J, Adams L, Alision J. Exercise training in COPD: what is it about intensity? Respirology. 2016;21(7):1185–1192. doi:10.1111/resp.12864
21. Horita N, Koblizek V, Plutinsky M, Novotna B, Hejduk K, Kaneko T. Chronic obstructive pulmonary disease prognostic score: a new index. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(2):211–218. doi:10.5507/bp.2016.030
22. Corlateanu A, Botnaru V, Covantev S, Dumitru S, Siafakas N. Predicting health-related quality of life in patients with chronic obstructive pulmonary disease: the impact of age. Respiration. 2016;92(4):229–234. doi:10.1159/000448625
23. Neo HY, Xu HY, Wu HY, Hum A. Prediction of poor short-term prognosis and unmet needs in advanced chronic obstructive pulmonary disease: use of the two-minute walking distance extracted from a six-minute walk test. J Palliat Med. 2017;20(8):821–828. doi:10.1089/jpm.2016.0449
24. Matsui H, Jo T, Fushimi K, Yasunaga H. Outcomes after early and delayed rehabilitation for exacerbation of chronic obstructive pulmonary disease: a nationwide retrospective cohort study in Japan. Respir Res. 2017;18(1):68. doi:10.1186/s12931-017-0552-7
25. Khan NA, Daga MK, Ahmad I, et al. Evaluation of BODE index and its relationship with systemic inflammation mediated by proinflammatory biomarkers in patients with COPD. J Inflamm Res. 2016;9:187–198. doi:10.2147/JIR.S108783
26. Ramon MA, Esquinas C, Barrecheguren M, et al. Self-reported daily walking time in COPD: relationship with relevant clinical and functional characteristics. Int J Chron Obstruct Pulmon Dis. 2017;12:1173–1181. doi:10.2147/COPD.S128234
27. Blindenbach S, Vrancken J, van der Zeijden H, et al. Effects of Geriatric COPD rehabilitation on hospital admissions and exercise tolerance: a retrospective observational study. Tijdschr Gerontol Geriatr. 2017;48(3):112–120. doi:10.1007/s12439-017-0214-8
28. Layec G, Hart CR, Trinity JD, et al. Oxygen delivery and the restoration of the muscle energetic balance following exercise: implications for delayed muscle recovery in patients with COPD. Am J Physiol Endocrinol Metab. 2017;313(1):E94–E104. doi:10.1152/ajpendo.00075.2017
29. Cannon DT, Coelho AC, Cao R, et al. Skeletal muscle power and fatigue at the tolerable limit of ramp-incremental exercise in COPD. J Appl Physiol (1985). 2016;121(6):1365–1373. doi:10.1152/japplphysiol.00660.2016
30. Bisca GW, Camillo CA, Cavalheri V, Pitta F, Osadnik CR. Peripheral muscle training in patients with chronic obstructive pulmonary disease: novel approaches and recent advances. Expert Rev Respir Med. 2017;11(5):413–423. doi:10.1080/17476348.2017.1317598
31. Cheng SWM, Alison J, Dennis S, et al. A behaviour change intervention to reduce sedentary time in people with chronic obstructive pulmonary disease: protocol for a randomised controlled trial. J Physiother. 2017;63(3):182. doi:10.1016/j.jphys.2017.04.001
32. Mesquita R, Meijer K, Pitta F, et al. Changes in physical activity and sedentary behaviour following pulmonary rehabilitation in patients with COPD. Respir Med. 2017;126:122–129. doi:10.1016/j.rmed.2017.03.029
33. Miki K, Maekura R, Kitada S, et al. Pulmonary rehabilitation for COPD improves exercise time rather than exercise tolerance: effects and mechanisms. Int J Chron Obstruct Pulmon Dis. 2017;12:1061–1070. doi:10.2147/COPD.S131061
34. Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–349. doi:10.1183/09059180.00007813
35. Coventry PA, Bower P, Keyworth C, et al. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta-analysis. PLoS One. 2013;8(4):e60532. doi:10.1371/journal.pone.0060532
36. Bolton CE, Bevan-Smith EF, Blakey JD. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax. 2013;68(Suppl 2):ii1–ii30. doi:10.1136/thoraxjnl-2013-203808
37. Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491–496. doi:10.15537/smj.2017.5.17965
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
