Back to Journals » Clinical Interventions in Aging » Volume 15
Benefits of Cochlear Implantation in Middle-Aged and Older Adults
Authors Völter C , Götze L , Haubitz I, Dazert S, Thomas JP
Received 25 March 2020
Accepted for publication 8 July 2020
Published 7 September 2020 Volume 2020:15 Pages 1555—1568
DOI https://doi.org/10.2147/CIA.S255363
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Christiane Völter, Lisa Götze, Imme Haubitz, Stefan Dazert, Jan Peter Thomas
Department of Otorhinolaryngology, Head and Neck Surgery, Katholisches Klinikum, Ruhr-University of Bochum, Bochum, Germany
Correspondence: Christiane Völter Tel +49 2345098390
Email [email protected]
Introduction: Nowadays cochlear implantation (CI) is the treatment of choice in adults in case conventional hearing devices fail. Besides speech perception, an improvement in quality of life and in cognitive performance has been reported. Thereby, the study focused on the impact of age.
Participants and Methods: Thirty middle-aged (MA) between 50 and 64 years and 41 older subjects (OA) aged 65 and older with bilateral severe hearing loss performed a comprehensive computer-based neurocognitive test battery (ALAcog) pre- and 12 months post-implantation. Besides, monosyllabic speech perception in quiet (Freiburg monosyllabic speech test), health-related quality of life (HR-QoL, Nijmegen Cochlear Implant Questionnaire) and depressive symptoms (GDS-15) have been assessed.
Results: Both age groups significantly improved in all three categories after 12 months. No differences were evaluated between MA and OA regarding speech perception and HR-QoL pre- and post-operatively. In contrast, cognitive performance differed between the age groups: pre-operatively OA performed worse in most neurocognitive subdomains like working memory (p=0.04), inhibition (p=0.004), processing speed (p=0.003) and mental flexibility (p=0.01), post-operatively MA outperformed OA only in inhibition (p=0.01). Age only slightly influenced cognitive performance in MA, whereas in OA age per se tremendously impacted on working memory (p=0.04), inhibition (p=0.02), memory (p=0.04) and mental flexibility (p=0.01). Educational level also affected processing speed, mental flexibility (p=0.01) and working memory (p=0.01). This was more pronounced in OA. In both age groups, hearing status had a strong effect on attentional tasks (p=0.01). In MA, depressive symptoms were more influential on cognitive functioning and on HR-QoL than in OA. Improvement in quality of life (p=0.0002) and working memory (p=0.001) was greater for those with a higher pre-operative depression score.
Conclusion: Speech perception and HR-QoL improved in hearing impaired, independently of age. Pre-operative differences in cognitive performance between OA and MA clearly attenuated 12 months after CI. Impact of comorbidities differed between age groups.
Keywords: cochlear implantation, age-related hearing loss, benefit, outcome, cognitive domains, quality of life, depression
Introduction
The growing number of the elderly population will be one of the major challenges in the future. Health-related disorders will become an increasing problem for the individual and a large socio-economic burden for the general population. Multiple chronic diseases including deficits in sensory abilities show a highly rising number. According to the World Health Organization (WHO) already now more than 450 million people worldwide suffer from disabling hearing loss and more than 50% of octogenarians are hereby affected.1
Recently consequences of age-related hearing loss have gained further interest.2–5 Due to changes in communication patterns, quality of life is strongly reduced in hearing-impaired subjects.2,6 According to the National council on the Aging Report comprising 4394 participants only 39% of hearing-impaired elderly have an excellent health compared to 68% in the general population.7
The reduced quality of life due to hearing loss has been largely described.2,8,9 In the Blue Mountain Hearing Study conducted by Chia who enrolled 2431 participants the physical and mental domain of the self-reported quality of life assessed by the Short Form-36 Questionnaire was associated with the severity of the hearing impairment and the use of hearing aids positively influenced outcome.10
Hearing-impaired subjects have a higher risk to suffer from social isolation or to fall into depression.11,12 This applies especially to women: female sexagenarian with a hearing loss of at least 25 dB have a 3.5-fold increased risk to suffer from isolation in comparison to age-matched normal hearing controls.5 This is in line with large epidemiologic longitudinal studies by Li et al analyzing data of more than 18,000 adults in the US and by Hsu et al on more than 20,000 subjects in Taiwan11,13 and with a recently published review and meta-analysis including a total of 147,148 participants from 35 studies. Hereby, hearing loss was associated with a significantly greater odds ratio for depression in older adults (OR = 1.479).14 Interestingly, mainly minor depressive symptoms and not severe depression seem to be highly associated with hearing impairment in the long-term follow-up as reported by Cosh in a study on 8340 adults.3 This might be even more pronounced in adults aged <65 years.15,16 Besides the negative impact of age on sensory abilities, aging is associated also with an enhanced cognitive decline starting already from the second decade of life.17 Further, executive functions decrease from 4% in the fourth decade to 11% for adults in their seventh decade as shown by Singh-Manoux et al in 4675 elderly.18 Retirement negatively impacts on cognitive functioning (such as memory) as described by Bonsang 2012 in a large data set of 82,462 elderly aged 51–75 years.19 Whereas fluid intelligence such as inhibition, interference control and working memory is highly vulnerable to age, crystalline intelligence persists mostly stable or even increases in ongoing life.20,21
The multisensory impact on cognition has been extensively studied by Humes in 2013 in 98 hearing-impaired persons with a mean age of 69.2 and a control group of normal hearing participants aged 22.7 years. Younger subjects outperformed the elderly in working memory and verbal processing speed. In contrast, elderly achieved better results in tests with a high context portion.6 In addition, sensory-cognitive associations were stronger if more than one sensory domain was affected.22
Thereby hearing and cognition cannot be considered as variables separately affected by age, but they are closely related and influenced by each other. Cognitive functioning is required to ensure speech perception especially in adverse listening conditions on one side.6,23,24 On the other hand, poorer hearing is associated with a worse cognitive performance, and hearing is proposed as one modifiable risk factor, which might diminish the risk of dementia if treated in the mid-life.25,26
However, psychological disorders as depression also have an effect on cognition and might be important mediators in the relationship between hearing loss and cognitive decline in the elderly.27,28 This has already been described by Huber in a study on 30 normal hearing (NH) and 30 hearing-impaired (HI) subjects between 60 and 80 years.29 HI subjects significantly differed in their performance in the clock drawing test, the word list learning (immediate and delayed) as well as in the Stroop and the TMT B tasks. In terms of anxiety assessed by the HADS (Hospital Anxiety and Depression Scale) no difference was obtained between the NH and HI subjects, but HI presented significantly higher levels of depressive symptoms. Furthermore, TMT B performance was mediated by the severity of depressive problems, whereas none of the other cognitive subtests did.
Nowadays, cochlear implantation has become the treatment of choice in severe to profound hearing-impaired subjects, which cannot be sufficiently treated by conventional hearing devices.30 Multiple studies have shown that also the older population has a substantial audiological benefit hereby.31–33 However, cochlear implantation is still less frequently performed in the aged hearing-impaired population than it should be done due to the audiological necessity even in industrialized nations as stated by Turunen-Taheri who studied the treatment of 1076 older subjects with severe to profound hearing loss at a mean age of 70.6.34
In general, hearing benefit regarding speech perception in quiet is quite similar between different age groups even if some studies point out that older adults need more time to adapt to the new auditory signal or do not reach the same speech perception in noise.33 Age differences in demanding listening situations might be due to the longer duration of hearing loss or to the influence of cognitive abilities, especially inhibitory control and attention. Besides pure audiological benefits also significant improvements in post-operative quality of life have been described. Patients aged 70 years and older even outperform younger adults aged between 19 and 67 in health-related questionnaires such as the Nijmegen Cochlear Implant Questionnaire (NCIQ).31
Recently there is an increasing interest in neurocognitive changes in older subjects after hearing rehabilitation.35–39 Mosnier was the first who investigated cognitive functions in older hearing-impaired subjects between 65 and 85 years before and after cochlear implantation and reported on benefits mainly regarding recall and attention.40 An improvement in attention has also been found by Sarant in 59 CI patients aged 72 that underwent testing with the Cogstate battery 18 months after cochlear implantation.38 Besides, working memory improves post-implantation as shown in a recent study by our group in 20 elderly 12 months after implantation .36 Similar findings have also been described by Jayakody in 16 CI recipients with a mean age of 61.7 that underwent testing with the Cambridge Neuropsychological Test Automated Battery (CANTAB).35
However, the impact of age has not been extensively evaluated in these studies although hearing, age and cognition are closely related to each other. Furthermore, the auditory-presented material used in some cognitive assessments might have partially influenced previous results in severely hearing-impaired subjects.41,42 Therefore, the presented study aimed to analyze the impact of age on the benefits of CI 12 months after implantation in a large sample size regarding speech perception and quality of life as well as cognitive performance using a comprehensive non-auditory test battery.
Participants and Methods
Participants
Age groups were defined in middle- (MA) and older-aged (OA) adults according to the definition of the WHO. Middle-aged was classified as people aged between 50 and 64 years, and old age starts at 65 years (WHO 2002) when individuals usually retire. Thirty MA and 41 OA with a bilateral severe to profound postlingual hearing loss were included (Table 1). The two groups did not differ with regard to gender (p=0.78), education (p=0.78) nor with regard to the duration of severe hearing impairment (p=0.74) or the amount of depressive symptoms (p=0.43). Inclusion criteria were defined as (1) age of 50 or older, (2) native or excellent German language speaker, (3) no uncorrected vision loss, (4) absence of a global cognitive impairment according to the Multiple Word Sentence Test (MWT-B),43 (5) no history of severe depression, (6) absence of central nervous system disease or treatment with anticholinergic medication, (7) postlingual bilateral hearing loss of 61dB or worse on average in the frequencies 0.5, 1, 2 and 4kHz on the better hearing ear.
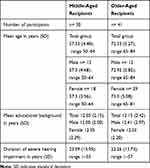 |
Table 1 Demographic Data |
All participants were recruited at the ENT-department at the Ruhr-University Bochum, Germany.
Audiometric Assessment
Pre-operatively, pure tone audiometry was measured separately for each ear at the frequencies between 0.25 and 8 kHz (DIN EN ISO 8253). The 4-pure tone average (4-PTA) was calculated separately for each ear using the mean of the frequencies 0.5, 1, 2 and 4 kHz.
The German Freiburg monosyllabic speech test in quiet was performed in all subjects pre- and 12 months post-operatively in free field at 65 dB sound pressure level (SPL) by an experienced audiologist in a soundproof booth. Additionally, in 37 patients, sentence recognition in noise was assessed 12 months postoperatively by the Oldenburg sentence test.44 For speech perception in noise, the signal-to-noise ratio (SNR) for 50% correct word recognition was assessed. An adaptive procedure was used with background noise fixed at 65 dB SPL and both speech and noise were presented from the front. A lower score indicated a better sentence comprehension in noise.
Health-Related Quality of Life
The Nijmegen Cochlear Implant Questionnaire (NCIQ) was used pre- and post-operatively to assess health-related quality of life (HR-QoL).45 Patients evaluated quality of life in 60 statements covering three domains which were further split into six subdomains: 1) physical domain: (a) basic sound perception and (b) advanced sound perception, (c) speech production; 2) psychological domain: (a) self-esteem; 3) social domain: (a) activity limitations, (b) social interactions. Answers range from 0 (not at all) to 100 (very good). A higher score reflected better health-related quality of life.
Neurocognitive Assessment
A visually based neurocognitive assessment tool (ALAcog), which is based on standardized paper and pencil versions adapted for hearing-impaired and already used in former studies, has been applied pre- and post-operatively.46,47 The test battery is composed of 11 subtests, covering various cognitive subdomains as previously described in detail:36,47
-M3 (according to the D2 test of attention by Brickenkamp 1962)48 to assess attention.
-Recall and Delayed Recall (according to the verbal learning and memory test by Helmstaedter 2001)49 for short and delayed memory.
-0- and 2-back (based on Kirchner 1958)50 to assess working memory.
-OSPAN (based on the Operation Span task by Conway 2005)51 is a dual task, which assesses working memory as well.
-Flanker (according to Eriksen and Eriksen 1974, Wild-Wall 2008)52,53 measures the ability to inhibit compatible (cFlanker) or incompatible (iFlanker) distractors.
-TMT A and B (based on the Trail Making Test by Reitan 1958):54 TMT A assesses processing speed, TMT B mental flexibility.
-Verbal fluency (according to the Chicago Word Fluency Test by Thurstone 1948)55 assesses verbal functioning and executive control.
For each test raw data and a total score, the inverse efficiency (IE) was calculated based on the time needed and the number of correct answers given.56 A better cognitive performance is indicated by a lower IE score. The bias of practice effects was minimized by the application of two different versions.
Psychosocial Comorbidities
Severe depression has been ruled out in all patients by precise questioning. Besides in 53 out of 71 subjects with a mean age of 65.87 (SD 9.34), the GDS-15-Assessment (Geriatric Depression Scale) was applied pre-operatively. This test is based on 15 dichotic questions on the attitude and mood of the subjects. A score of 0–5 indicates the absence of depressive symptoms, 6–10 indicates mild or moderate depressive symptoms and a score >10 might be a hint for profound depressive symptoms.57
Educational background has been assessed by the number of years participants attended school or did further studies.
Aural Rehabilitation
All participants took part in a standardized regular aural rehabilitation program once a week during the first weeks post-implantation (basic rehabilitation) with an experienced speech therapist in a face-to-face session in accordance with the guidelines of the German Society for Otorhinolaryngology, Head and Neck Surgery (Guidelines for cochlea implant supply, AWMF). After this, aural training was followed up to 2 years at a lower frequency.
Statistical Analysis
Data analysis was performed in Medas (C. Grund, Margetshoechheim, Germany). All results were presented using mean and standard deviation (SD). As dataset was partially not normally distributed, Wilcoxon– and Mann–WhitneyU-test were applied to compare cognitive and auditory abilities as well as quality of life pre- and post-operatively. TMT was calculated with linear models and rule of proportion in case participants were unable to finish the task within 90 seconds.
The effect size (EF) has been calculated adapted to Cohen’s d (up to 0.1 refers to a weak, 0.3 to a moderate and 0.5 to a strong effect). To address, whether speech perception at 65dB, age, gender or education were most predictive for cognitive performance 12 months post-implantation, multiple regression analysis was performed. Standardized Beta-weight (β) was used to compare the predictors.
Correlation analyses using Kendall’s tau (τ) were performed between different variables such as speech perception scores, quality of life measures, depressive symptoms, cognitive function and duration of deafness. The level of significance was set to p<0.05.
The study was in line with the requirements of the ethic institution of the Ruhr University Bochum (No 16–5727-BR) and the requirement of the declaration of Helsinki. All participants signed their informed consent.
Results
Hearing Status
Preimplantation both age groups did not significantly differ with regard to 4-PTA of the worse or the better hearing ear (p=0.07, p=0.9). Moreover, speech understanding before implantation was comparable between the groups without any significant difference (p=0.68). Twelve months after implantation patients of both age groups showed a significant improvement of monosyllabic speech perception (MA p=0.003 and OA p=0.005) with similar results regarding the speech perception scores in MA and OA recipients (p=0.45) (Table 2). Also, 12 months after implantation speech perception in noise was similar (p=0.55) in MA and OA.
 |
Table 2 Results of Hearing Assessment |
Correlation Analysis
No correlation was evaluated between speech perception in quiet and total quality of life. The only significant association found was with regard to the post-operative subscore self-esteem, but only in the elderly. Subjects who improved more in speech perception at 65 dB showed a significant better self-esteem (τ=0.25; p=0.03). However, speech perception in quiet as well as improvement in speech perception in quiet after 12 months was predicted by pre-operative inhibition of compatible stimuli (τ=−0.22, p=0.049; τ=−0.24; p=0.04, respectively).
Health-Related Quality of Life
Age had a partial influence on pre-operative quality of life. Older subjects scored better in basic sound perception (p=0.047). Twelve months post-implantation both groups revealed a significant improvement of the HR-QoL (p=0.0000) with similar total scores of the NCIQ between both age groups pre- and post-implantation (pre-implantation MA 46.02 vs OA 50.55; p=0.5 and post-implantation MA 65.33 vs OA 66.42; p=0.69) (Table 3).
 |
Table 3 Health-Related Quality of Life Assessed by the Nijmegen Cochlear Implant Questionnaire (n=71) |
Correlation Analysis
Twelve months post-operatively, no significant correlation could be detected between speech perception in quiet and total NCIQ-Score (p=0.19), but with regard to the improvement of quality of life in MA and OA (MA τ=−0.28; p=0.048 and OA τ=0.23, p=0.04). However, post-operative speech perception in noise correlated directly to the subdomains of advanced sound perception (τ=−0.42; p=0.04) and of self-esteem (τ=−0.31; p=0.02) in MA, but not in OA subjects.
Duration of deafness correlated only pre-operatively with NCIQ score in some subdomains such as in self-esteem (τ=0.30, p=0.03) in MA as well as in the physical domain (τ=0.23, p=0.04) and advanced sound perception in OA (τ=0.22, p=0.04). Post-operatively, there was no association between duration of hearing loss and quality of life (τ=−0.002, p=0.98). However, older subjects showed a larger improvement in post-operative advanced sound perception in case of a shorter duration of hearing loss (τ=−0.22, p=0.04).
Neurocognitive Performance
In total, a significant improvement could be observed in cognitive functioning post-operatively in the whole study group with the largest improvement in patients with poor baseline cognitive performance.
Pre-operatively OA subjects performed worse than MA in 4 out of 11 neurocognitive subtests. This was highly significant with regard to the TMT (TMT A p=0.003, EF=0.61 and TMT B p= 0.01, EF=0.56 and the inhibition (iFlanker p=0.004, EF=0.39). Furthermore, significant age-dependent differences could be detected in working memory as assessed by the OSPAN (p=0.04, EF=0.53). The effect size was between 0.39 and 0.61. Post-operatively, MA outperformed OA still in the ability to suppress inhibitory signals (iFlanker p=0.01), with a small effect size of 0.23 (Table 4). MA and OA improved similar in most subtests studied. Only in 0-back (p=0.04) as well as in TMT A (p=0.02) improvement was more pronounced in OA than in MA.
 |
Table 4 Pre- and Post-operative Neurocognitive Performance According to Age (n=71) |
In-Depth Analysis
Pre-operatively a slower reaction time and less correct items were found for OA than for MA subjects in the M3 task, but without any significance (reaction time MA 840.64 ms vs OA 945.65 ms; p=0.11 and correct letters MA 79.9 vs OA 73.6; p=0.16). Post-implantation the two groups significantly differed: OA completed only 85.78 trials, whereas MA were able to increase the performance to 95.77 trials (p=0.02). In addition, the number of correct labels was higher for MA than for OA (p=0.046) as well as the reaction time was shorter for the younger than for the older group (MA 759.41 ms vs OA 852.46 ms; p=0.03).
With regard to Recall and Delayed Recall, pre- and post-operative results were independent of the age groups. Patients of both age groups scored less in Delayed Recall than in Recall tasks by more than 1.5 words on average. Both groups equally improved pre- to post-implantation (Recall pre: p=0.91 vs post: p=0.12 and Delayed Recall pre: p=0.37 vs post: p=0.08).
The same was true for the 0- and the 2-back task; 0-back performance was comparable between OA and MA pre- and post-implantation (pre: MA 342.30 vs OA 324.53; p=0.79 and post: MA 314.16 vs OA 337.07; p=0.12).
Working memory assessed by the OSPAN significantly differed between OA and MA (p=0.04, EF=0.53) pre-operatively. This was due to the smaller number of correctly solved maths’ calculations in the OA (MA 38.96 vs OA 37.68; p=0.04). Post-operatively, OA required longer to solve the equations (MA 2734.27 ms vs OA 3244.22 ms; p=0.04), but they calculated as well as the MA (correct equations: p=0.93).
A highly significant difference could be detected between the two age groups in the pre-operative TMT (TMT A, p=0.003; TMT B, p=0.015) but no longer after implantation (TMT A, p=0.16; TMT B, p=0.1). Pre-operatively, the last label which has been clicked for TMT A was number 23 by MA and 20 by OA (p=0.02). Twelve months after CI MA achieved an equal result whereas OA improved by three additional letters (p=0.5). The pre-operative performance of TMT B significantly differed (MA 20 vs OA 16), after implantation the numbers were similar between the two age groups (p=0.15).
The ability to detect incompatible stimuli (Flanker task) was highly influenced by age pre- as well as post-operatively. OA missed a higher number of incompatible arrows pre- and post-implantation (pre: MA 1.33 vs OA 3.53; p=0.04 and post: MA 1.56 vs OA 1.52; p=0.04). OA responded slower to the target than MA although not significantly (pre: MA 410.34 vs OA 448.92; p=0.11 and post: MA 408.38 vs OA 442.93; p=0.01). In contrast, cFlanker responses were similar for both groups (pre: MA 363.93 vs OA 460.53; p=0.08 and post-implantation MA 363.60 vs OA 399.10; p=0.07). The number of missed compatible Flankers equally decreased pre- to post-implantation for both groups (pre: MA 0.80 vs OA 1.53; p=0.33 and post: MA 0.33 vs OA 0.95; p=0.21). Reaction time remained stable pre- to post-implantation in both age groups (pre: MA 342.16 versus OA 344.71; p=0.15 and post: MA 350.61 vs OA 369.65; p=0.11).
Verbal fluency did not show any difference related to age-groups (pre: p=0.75 and post-implantation p=0.89). The number of animals for MA pre-implantation was 6.5 and 6.68 for OA. Post-implantation MA named 7.03 and OA 7.86 correct animals.
Correlation Analysis
Pre-operative inhibitory performance assessed by incompatible Flanker was predictive of post-operative speech perception in quiet in older subjects, but not in the younger group. (OA: τ=−0.22, p=0.049, MA: τ=−0.1, p=0.48). In addition, post-operative speech perception in quiet was related to post-operative performance in attentional tasks in OA (τ=−0.26, p=0.02 vs MA τ=−0.19, p=0.16), whereas improvement of speech perception significantly correlated to an improvement of M3 in both age groups (MA: τ=−0.27, p=0.048 and OA: τ=−0.23, p=0.03).
Covariants on Neurocognitive Performance
Multiple regression analysis regarding post-operative cognitive performance was calculated separately for MA and OA subjects regarding age, gender, speech perception in quiet and education:
In MA age significantly predicted cognitive performance in 2 of 11 subtests: TMT A (β=0.45, p=0.01) measuring processing speed and M3 evaluating attention (β=0.32, p=0.049). Besides speech perception in quiet had the strongest influence on attentional performance assessed by the M3 (β=−0.47, p=0.01). Longer education times correlated with the results in the Recall (β=−0.39, p=0.03) and OSPAN tasks (β=−0.43, p=0.02), and female gender correlated with better results in verbal fluency (β=−0.4, p=0.049).
In OA age influenced 5 out of 11 subtests: 2-back (β=0.31, p=0.04), Recall (β=−0.3, p=0.04), TMT A (ββ=0.35, p=0.02), TMT B (β=0.39, p=0.01) and cFlanker (β=0.37, p=0.02). Monosyllabic speech perception also had an impact on attentional tasks (M3 β=−0.4, p=0.01) and on inhibition (iFlanker β=−0.32, p=0.04). Education was predictive for the Recall task (β=−0.3, p=0.02), the OSPAN (β=−0.4, p=0.01), the TMT A (β=−0.38, p=0.01) and the TMT B (β=−0.4, p=0.01).
Comorbidities
Subjects with severe depression were excluded from the study. However, a deeper analysis of 53 subjects revealed that 52.8% suffered from slight or moderate depressive symptoms pre-operatively (mean= 7.32, SD 1.4). Total preoperative GDS-15 score did not differ according to age (MA 6.25 (SD 2.29) and OA 5.73 (SD 1.36); p=0.43) or gender (men 5.77 (SD 2.1), women 6.05 (SD 1.71); p=0.66). Pre- and post-operative speech perception as well as improvement of speech perception showed no association to GDS-15 score in both age groups. Duration of hearing loss significantly related to mental health (τ=−0.24; p=0.01) in younger, but not in older subjects (τ=−0.10; p=0.43).
Depression score had an impact on cognitive performance (1) and quality of life (2). This was more pronounced for younger than for older subjects (Table 5):
 |
Table 5 Impact of Pre-operative Depressive Symptoms on Pre- and Post-operative Cognitive Performance, Quality of Life and Speech Perception According to Age |
(1) Pre-operative depressive score correlated with preoperative cognitive performance only in subjects aged 50–64 years. This was true with regard to attention (p=0.03), working memory (2-back: p=0.001 and OSPAN: p=0.01), inhibition (cFlanker: p=0.02 and iFlanker: p=0.03) and TMT (TMT A: p=0.01 and TMT B: p=0.03).
Post-operative cognitive outcome could be predicted by pre-operative mood mostly in the younger group. Patients reporting on a higher depressive score before implantation also obtained worse cognitive results 12 months post-implantation in incompatible Flanker (p=0.02), OSPAN (p=0.03) in MA and in TMT A for both age groups (MA p=0.03 and OA p=0.02).
Besides, subjects aged <65 showed a significantly greater improvement in M3 (p=0.03), 0-back (p=0.01) and 2-back (p=0.001) in case of higher depressive scores. This could not be observed in subjects of ≥65 .
(2) Pre-operative mental health correlated to the preoperative quality of life in both groups, but more pronounced in MA. Post-operative depression score was predictive for health-related quality of life in OA for physical sound perception (τ=−0.26, p=0.047). Besides, improvement in quality of life relied on psychosocial comorbidity only in the younger subjects (p=0.04).
Discussion
The target of the study was twofold (1) to evaluate the impact of age on cognitive and mental benefits of cochlear implantation in adult cochlear implant recipients and (2) to analyze the age-dependent interaction between speech comprehension, cognition and quality of life.
According to previous investigations also in our study adults of any age achieve a significant improvement in monosyllabic speech perception after cochlear implantation with no differences between the two age groups of middle-aged and older-aged adults.33,58–60
Besides restoration of speech perception, cochlear implantation has also a tremendous impact on the quality of life. In this study quality of life was equal between the age groups at any time despite in the subtest basic sound perception before cochlear implantation where elderly reported on better results. No correlation could be found between monosyllabic speech perception in quiet and quality of life. However, in older subjects with better self-esteem speech understanding in quiet was better. Similar results have been found by Moberly in 25 long-time CI users aged 50–83. Social interaction was correlated to an audio-visual task and to the recognition of complex sentences, but not to sentences in noise or to single words in quiet.61
However, as already stated by others improvement of quality of life after hearing rehabilitation is difficult to assess.62,63 Commonly used questionnaires that measure general quality of life such as the WHOQOL-BREF are time consuming and may not be suitable, because they do not encounter the impairment caused by hearing impairment64 and health-related questionnaires as the Nijmegen Cochlear Implant Questionnaire that is widely used in hearing-impaired subjects do not sufficiently include daily activities.31,45,62,63,65 Therefore, both subjective self-assessments show only limited correlations to objective measurements.66
This has also been found in a meta-analysis including 14 publications and covering 679 patients. Despite large post-operative improvements in quality of life only a low correlation could be observed between hearing in quiet or in noise and the Nijmegen Cochlear Implant Questionnaire, the Hearing Handicap Inventory or the Abbreviated Profile of Hearing Aid Benefit.63 Polku who investigated the effect of self-perceived hearing loss on quality of life measured by the WHOQOL-BREF observed that audiometrically assessed speech perception did not correlate to any QoL subdomain, whereas self-perceived impairment correlated to the total score and all subdomains (physical, psychological, environmental and social).67 Sorrentino analyzed 69 CI users divided into 2 groups according to age (one older group of 25 individuals and a younger one of 19 individuals) as well as 25 normal hearing controls ≥65 with a cognitive screening test (Mini-Mental Status Examination) and the Glasgow Benefit Inventory commonly used to evaluate post-operative outcome in Otorhinolaryngology. Total QoL and the subdomain of physical health was related to speech perception, but only in the elderly and not in the younger aged group.68
Concerning cognition, our findings demonstrate that neurocognitive functions significantly improve 12 months after cochlear implantation in middle-aged as well as in older adults with severe to profound hearing loss. But whereas prior to surgery OA performed worse in working memory, inhibition, processing speed and mental flexibility, only inhibition significantly differed between the two age groups post-operatively. This is in line with data published by Salthouse showing that the ability to inhibit stimuli decreases with age.69
Furthermore, Moberly found in a study on 30 hearing-impaired subjects with a mean age of 68.8 that the response time for inhibition assessed with a Stroop task was predictive for speech comprehension in noise.70 This could also be observed in our study. The post-operative monosyllabic speech perception 12 months after implantation could be predicted pre-operatively by the inhibitory Flanker task in the older, but not in the middle-aged group.
Including age, hearing, gender and education as possible confounders, it was shown that hearing is a predictor for attention in both age groups and for OA with regard to inhibition. Cognitively challenging tasks such as the OSPAN, but also the Recall and the TMT are predominantly determined by education. Looking at the two age groups, age had a high impact on post-operative neurocognitive performance mainly on the 2-back, the compatible Flanker and on the Recall in older subjects in contrast to subjects <65 years of age.
Besides, a relationship has been found between health quality of life and some cognitive subtests: in MA advanced sound perception correlated to the 2-back task and improvement in working memory to a post-operative improvement of social interactions in the total group (p=0.04). Duration of hearing loss was associated with the total NCIQ score. Subjects with a shorter history of hearing impairment suffered more. Moberly described a negative correlation between patient's age and the subdomain advanced sound perception and between a combination of patient´s age at the time of implantation, duration of CI use, duration of hearing loss and the total well-being score.61
Due to the close connectivity between the auditory cortex and the limbic system, reduced peripheral input does not only lead to deactivation in central auditory structures but also to increased activation of cognitive control networks and to dysregulation of the limbic system on the neural level. On the behavioral level, social isolation may lead to depression as well as to cognitive decline in hearing impaired and mental disorders may be a mediator between hearing loss and cognitive decline.29,71
CI candidates exhibit a wide range of psychological disorders as shown in a study by Brüggemann, who described affective or somatoform disorders in 81% of adult CI candidates in contrast to 32% in the general population.72 In a review including 66 studies, Besser and colleagues also reported that hearing-impaired subjects are prone to suffer from multiple psychological disorders such as anxiety, an increased suicidal risk and social isolation.73 Along with that Brewster observed in 3075 elderly aged 70–79 years that the risk to suffer from severe depression assessed by the CES-D scale (Centre for Epidemiologic Study Depression) increased 1.85-fold in hearing-impaired compared to normal hearing counterparts.74 The use of hearing devices had no influence on mood. However, hearing evaluation was based on patient’s report and not on detailed audiometric evaluation, and duration and frequency of hearing aid use were not encountered.74
Dawes studied the association of cognitive performance and hearing status in hearing aid users as well as the relationship to social isolation and/or depression by structural equation modelling on a subsample of the UK Biobank data set (n=164,770) of UK adults aged 40–69.75 Although a positive relationship has been detected between hearing aid use and cognition as well general health, there was no hint that this effect was mediated by social and psychological factors. Authors claim that hearing restoration might increase self-efficacy which is associated with better performance on challenging tasks. However, as subjects with higher cognitive scores might have searched more often for hearing devices a bias cannot be ruled out and longitudinal studies are missing.
In our study depression score was assessed only pre-operatively. Thereby the influence of hearing rehabilitation via cochlear implantation on depression could not be answered by this investigation. However, subjects who suffered from their hearing loss for a longer period of time showed less signs of depression in the younger aged group. Although severe depression has been excluded in our study population half of the subjects suffered from mild or moderate depressive symptoms. This is in line with a recent study by Tretbar in which only 22 out of 52 hearing impaired were without any psychiatric disorder, whereas 30 had been currently or previously treated, in 19 subjects due to symptoms of depression.76
Knopke also studied psychosocial factors that might have an impact on quality of life such as the level of anxiety, stress and depression in 62 cochlear implant recipients aged 70–80 and in 24 CI users over 80. Whereas in the younger group NCIQ was mainly predicted by depressive symptoms, anxiety was a positive predictor only for the octogenarians.77 Age-related differences have also been described in a study of 50,398 Norwegians aged 20–101. Younger adults 20–44 and middle-aged adults of 45–64 perceived a higher psychosocial burden in depression, self-esteem and anxiety in case of hearing loss. In middle-aged the level of depressive symptoms increased by 0.1 SD for every 10dB hearing loss in contrast to elderly who showed only a slight association (0.01 SD increase for 10dB hearing loss).15
In our study, total depression score did not differ between the two age groups, but the impact of depression on cognitive performance and on well-being. In general, quality of life was poorer for patients with a higher level of depression. But whereas MA were significantly more affected by depression and had a higher improvement in quality of life if depression was higher, this association could not be observed in the elderly in the same way. It might be speculated that middle-aged hearing impaired who experience severe limitations in their daily activities at home or at work are more vulnerable to depressive symptoms than subjects aged ≥65 who are no longer part of the workforce and who might have accepted hearing loss as a part of normal aging .71 However, social support can moderate the relationship between hearing loss and depression in later life.78 Additionally, the presence of depressive symptoms did not affect cognitive performance in the older group but in subjects <65 years of age.
A potential bias of the study might be due to the study design which evaluated the outcome mainly on questionnaires and behavioral investigation rather than on objective measures. At the moment normative scores or cut-off data for appropriate test-instruments for hearing impaired are still missing.79,80 Another limitation is that mental health assessed by the GDS-15 has only been done in a small sample size of 53 subjects and only prior to implantation. Other comorbidities such as anxiety were not studied.
To sum up cochlear implantation shows benefits in speech understanding and quality of life regardless of age. Neurocognitive functions which differ pre-operatively according to age also improve post-operatively. Subjects with worse pre-operative performance in cognitive and mental health show the greatest improvement. Quality of life is predicted by an interplay of various patients’ characteristics such as age, but also by duration of hearing loss and cognitive as well as psychosocial variables.
Up to now, there is still limited knowledge about the benefit of hearing rehabilitation in severe cognitive or depressive impaired older subjects.75,81,82 Further studies are required to figure out whether cochlear implantation might even improve quality of life in these patients as well as to identify the degree of impairment, which should not be exceeded to still achieve a benefit by cochlear implantation. To assess post-operative outcome, comorbidities should be taken into consideration as the impact of covariables strongly differs according to age.
Acknowledgments
We are very thankful to Prof. Dr. Michael Falkenstein (Institute for Work, Learning and Aging, Bochum) and Ludger Blanke for providing the ALAcog assessment battery and helpful technical support. Furthermore, to Janine Müther and Robert Käppeler who helped with data collection and to all the patients, who participated in the study. We further appreciate the support by the DFG Open Access Publication Funds of the Ruhr-University Bochum.
Author Contributions
CV, JPT, SD made substantial contributions to conception and design, LG, CV, IH to the acquisition of data, analysis and interpretation of data, CV, LG and JPT took part in drafting the article, IH and SD in revising it critically for important intellectual content; all authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
The Department of Otorhinolaryngology, Head and Neck Surgery at the Katholisches Klinikum in Bochum, Ruhr-University of Bochum, has received unrelated third-party funds from MED-EL. Christiane Völter, Jan Peter Thomas, Stefan Dazert have received travel expense support from MED-EL. The authors declare that the research was conducted in the absence of any commercial or financial relationships and report no other conflicts of interest in this work.
References
1. Arvin B, Prepageran N, Raman R. “High frequency presbycusis”-is there an earlier onset? Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 3):480–484. doi:10.1007/s12070-011-0356-x
2. Ciorba A, Bianchini C, Pelucchi S, Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin Interv Aging. 2012;7:159–163. doi:10.2147/CIA.S26059
3. Cosh S, Carriere I, Daien V, et al. The relationship between hearing loss in older adults and depression over 12 years: findings from the Three‐City prospective cohort study. Int J Geriatr Psychiatry. 2018;33(12):1654–1661. doi:10.1002/gps.4968
4. Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med. 2012;172(4):369–371. doi:10.1001/archinternmed.2011.728
5. Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngology. 2014;150(3):378–384. doi:10.1177/0194599813518021
6. Humes LE, Kidd GR, Lentz JJ. Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Front Syst Neurosci. 2013;7:55. doi:10.3389/fnsys.2013.00055
7. The National Council on the Aging. The Consequences of Untreated Hearing Loss in Older Persons. Washington, DC: the National Council on the Aging 1999. Available from: http://www.hearingoffice.com/download/UntreatedHearingLossReport.pdf. 24, 2020.
8. Davis A, McMahon CM, Pichora-Fuller KM, et al. Aging and hearing health: the life-course approach. Gerontologist. 2016;56(Suppl_2):S256–S267. doi:10.1093/geront/gnw033
9. Eisele M, Kaduszkiewicz H, König -H-H, et al. Determinants of health-related quality of life in older primary care patients: results of the longitudinal observational AgeCoDe Study. Br J Gen Pract. 2015;65(640):e716–e723. doi:10.3399/bjgp15X687337
10. Chia E-M, Wang JJ, Rochtchina E, Cumming RR, Newall P, Mitchell P. Hearing impairment and health-related quality of life: the Blue Mountains Hearing Study. Ear Hear. 2007;28(2):187–195. doi:10.1097/AUD.0b013e31803126b6
11. Hsu W-T, Hsu -C-C, Wen M-H, et al. Increased risk of depression in patients with acquired sensory hearing loss: a 12-year follow-up study. Medicine. 2016;95(44):e5312. doi:10.1097/MD.0000000000005312
12. Pronk M, Deeg DJH, Smits C, et al. Hearing loss in older persons: does the rate of decline affect psychosocial health? J Aging Health. 2014;26(5):703–723. doi:10.1177/0898264314529329
13. Li C-M, Zhang X, Hoffman HJ, Cotch MF, Themann CL, Wilson MR. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol Head Neck Surg. 2014;140(4):293–302. doi:10.1001/jamaoto.2014.42
14. Lawrence BJ, Jayakody DMP, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing loss and depression in older adults: a systematic review and meta-analysis. Gerontologist. 2020;60(3):e137–e154. doi:10.1093/geront/gnz009
15. Tambs K. Moderate effects of hearing loss on mental health and subjective well-being: results from the Nord-Trøndelag Hearing Loss Study. Psychosom Med. 2004;66(5):776–782. doi:10.1097/01.psy.0000133328.03596.fb
16. Keidser G, Seeto M. The Influence of Social Interaction and Physical Health on the Association Between Hearing and Depression With Age and Gender. Trends Hearing. 2017;21:1–15. doi:10.1177/2331216517706395
17. Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30(4):507–514. doi:10.1016/j.neurobiolaging.2008.09.023
18. Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344(jan04 4):d7622. doi:10.1136/bmj.d7622
19. Bonsang E, Adam S, Perelman S. Does retirement affect cognitive functioning? J Health Econ. 2012;31(3):490–501. doi:10.1016/j.jhealeco.2012.03.005
20. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cognitive Psychol. 2000;41(1):49–100. doi:10.1006/cogp.1999.0734
21. Lehto JE, Juujärvi P, Kooistra L, Pulkkinen L. Dimensions of executive functioning: evidence from children. Br J Dev Psychol. 2003;21(1):59–80. doi:10.1348/026151003321164627
22. Humes LE, Young LA. Sensory–Cognitive Interactions in Older Adults. Ear Hearing. 2016;37:52–61. doi:10.1097/AUD.0000000000000303
23. Pichora-Fuller KM, Kramer SE, Eckert MA, Edwards B. Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL). Ear Hearing. 2016;37(Suppl 1):5S–27S. doi:10.1097/AUD.0000000000000312
24. Rönnberg J, Rudner M, Foo C, Lunner T. Cognition counts: a working memory system for ease of language understanding (ELU). Int J Audiol. 2008;47(suppl. 2):S99–S105. doi:10.1080/14992020802301167
25. Dawes P, Emsley R, Cruickshanks KJ, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PLoS One. 2015;10(3):e0119616. doi:10.1371/journal.pone.0119616
26. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. doi:10.1016/S0140-6736(17)31363-6
27. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bulletin. 2013;139(1):81–132. doi:10.1037/a0028727
28. Vives M, López-Navarro E, García-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. 2015;43(5):187–193.
29. Huber M, Roesch S, Pletzer B, Lukaschyk J, Lesinski-Schiedat A, Illg A. Cognition in older adults with severe to profound sensorineural hearing loss compared to peers with normal hearing for age. Int J Audiol. 2020;59(4):254–262. doi:10.1080/14992027.2019.1687947
30. Lenarz M, Sönmez H, Joseph G, Büchner A, Lenarz T. Cochlear implant performance in geriatric patients. Laryngoscope. 2012;122(6):1361–1365. doi:10.1002/lary.23232
31. Olze H, Gräbel S, Förster U, et al. Elderly patients benefit from cochlear implantation regarding auditory rehabilitation, quality of life, tinnitus, and stress. Laryngoscope. 2012;122(1):196–203. doi:10.1002/lary.22356
32. Lally JW, Adams JK, Wilkerson BJ. The use of cochlear implantation in the elderly. Curr Opinion Otolaryngol Head Neck Surg. 2019;27(5):387–391. doi:10.1097/MOO.0000000000000569
33. Forli F, Lazzerini F, Fortunato S, Bruschini L, Berrettini S. Cochlear implant in the elderly: results in terms of speech perception and quality of life. Audiol Neurootol. 2019;24(1):1–7. doi:10.1159/000499176
34. Turunen-Taheri SK, Edén M, Hellström S, Carlsson P-I. Rehabilitation of adult patients with severe-to-profound hearing impairment–why not cochlear implants? Acta Oto-Laryngologica. 2019;139(7):604–611. doi:10.1080/00016489.2019.1607976
35. Jayakody DMP, Friedland PL, Nel E, Martins RN, Atlas MD, Sohrabi HR. Impact of Cochlear implantation on cognitive functions of older adults: pilot test results. Otol Neurotol. 2017;38(8):e289–e295. doi:10.1097/MAO.0000000000001502
36. Völter C, Götze L, Dazert S, Falkenstein M, Thomas JP. Can cochlear implantation improve neurocognition in the aging population? Clin Interv Aging. 2018;13:701–712. doi:10.2147/CIA.S160517
37. Claes AJ, van de Heyning P, Gilles A, et al. impaired cognitive functioning in cochlear implant recipients over the age of 55 years: a cross-sectional study using the Repeatable Battery for the Assessment of Neuropsychological Status for Hearing-Impaired Individuals (RBANS-H). Front Neurosci. 2018;12:580. doi:10.3389/fnins.2018.00580
38. Sarant J, Harris D, Busby P, et al. The Effect of cochlear implants on cognitive function in older adults: initial baseline and 18-month follow up results for a prospective international longitudinal study. Front Neurosci. 2019;13:789. doi:10.3389/fnins.2019.00789
39. Cosetti MK, Pinkston JB, Flores JM, et al. Neurocognitive testing and cochlear implantation: insights into performance in older adults. Clin Interv Aging. 2016;11:603–613. doi:10.2147/CIA.S100255
40. Mosnier I, Bebear J-P, Marx M, et al. Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolaryngol Head Neck Surg. 2015;141(5):442–450. doi:10.1001/jamaoto.2015.129
41. Jorgensen LE, Palmer CV, Pratt S, Erickson KI, Moncrieff D. The effect of decreased audibility on MMSE performance: a measure commonly used for diagnosing dementia. J Am Acad Audiol. 2016;27(4):311–323. doi:10.3766/jaaa.15006
42. Dupuis K, Marchuk V, Pichora-Fuller MK. Noise affects performance on the Montreal Cognitive Assessment. Can J Aging. 2016;35(3):298–307. doi:10.1017/S0714980816000313
43. Lehrl S. MWT-B—Mehrfach-Wortschatz-Intelligenztest. Göttingen, Germany: Hogrefe; 2005.
44. Kuehnel V, Kollmeier B, Wagener K. Entwicklung und Evaluation eines Satztests für die deutsche Sprache I: design des Oldenburger Satztests. DevEvaluation German Sent Test I 1999;38:4–15.
45. Hinderink JB, Krabbe PF, van den Broek P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: the Nijmegen cochlear implant questionnaire. Otolaryngology. 2000;123(6):756–765. doi:10.1067/mhn.2000.108203
46. Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta psychologica. 1999;101(2):267–291. doi:10.1016/S0001-6918(99)00008-6
47. Völter C, Götze L, Falkenstein M, Dazert S, Thomas JP. Application of a computer-based neurocognitive assessment battery in the elderly with and without hearing loss. Clin Interv Aging. 2017;12:1681–1690. doi:10.2147/CIA.S142541
48. Brickenkamp R. Test d2: Aufmerksamkeits-Belastungs-Test. Göttingen: Hogrefe Verl. für Psychologie; 1962.
49. Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest: VLMT; Manual. Göttingen: Beltz-Test; 2001.
50. Kirchner WK. Age differences in short-term retention of rapidly changing information. J Exp Psychol. 1958;55(4):352. doi:10.1037/h0043688
51. Conway MA. Memory and the self. J Memo Lang. 2005;53(4):594–628. doi:10.1016/j.jml.2005.08.005
52. Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Attention Perception Psychophys. 1974;16(1):143–149. doi:10.3758/BF03203267
53. Wild K, Howieson D, Webbe F, Seelye A, Kaye J. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement. 2008;4(6):428–437. doi:10.1016/j.jalz.2008.07.003
54. Reitan RM. Validity of the Trail Making Test as an Indicator of Organic Brain Damage. Percept Mot Skills. 1958;8(3):271–276. doi:10.2466/pms.1958.8.3.271
55. Thurstone LL. Primary mental abilities. Science. 1948;108(2813):585.
56. Wild-Wall N, Falkenstein M, Hohnsbein J. Flanker interference in young and older participants as reflected in event-related potentials. Brain Res. 2008;1211:72–84. doi:10.1016/j.brainres.2008.03.025
57. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17(1–2):37–49. doi:10.1016/0022-3956(82)90033-4
58. Lenarz T, Muller L, Czerniejewska-Wolska H, et al. Patient-Related Benefits for Adults with Cochlear Implantation: A Multicultural Longitudinal Observational Study. Audiol Neurootol. 2017;22(2):61–73. doi:10.1159/000477533
59. Jolink C, Helleman HW, van Spronsen E, Ebbens FA, Ravesloot MJL, Dreschler WA. The long-term results of speech perception in elderly cochlear implant users. Cochlear Implants Int. 2016;17(3):146–150. doi:10.1080/14670100.2016.1162383
60. Dillon MT, Buss E, Adunka MC, et al. Long-term speech perception in elderly cochlear implant users. JAMA Otolaryngol Head Neck Surg. 2013;139(3):279–283. doi:10.1001/jamaoto.2013.1814
61. Moberly AC, Harris MS, Boyce L, et al. Relating quality of life to outcomes and predictors in adult cochlear implant users: are we measuring the right things? Laryngoscope. 2017;128(4):959–966. doi:10.1002/lary.26791
62. Ambert-Dahan E, Laouénan C, Lebredonchel M, et al. Evaluation of the impact of hearing loss in adults: validation of a quality of life questionnaire. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1):25–31. doi:10.1016/j.anorl.2017.09.003
63. McRackan TR, Bauschard M, Hatch JL, et al. Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. Laryngoscope. 2017;128(4):982–990. doi:10.1002/lary.26738
64. Nordvik Ø, Laugen Heggdal PO, Brännström J, Vassbotn F, Aarstad AK, Aarstad HJ. Generic quality of life in persons with hearing loss: a systematic literature review. BMC Ear Nose Throat Disord. 2018;18(1):1–13. doi:10.1186/s12901-018-0051-6
65. Nagle S, Schmidt L. Computer acceptance of older adults. Work. 2012;41(Suppl 1):3541–3548. doi:10.3233/WOR-2012-0633-3541
66. Vasil KJ, Lewis J, Tamati T, Ray C, Moberly AC. How does quality of life relate to auditory abilities? A subitem analysis of the Nijmegen Cochlear Implant Questionnaire. J Am Acad Audiol. 2020;31(4):292–301. doi:10.3766/jaaa.19047
67. Polku H, Mikkola TM, Rantakokko M, et al. Hearing and quality of life among community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2018;73(3):543–552. doi:10.1093/geronb/gbw045
68. Sorrentino T, Donati G, Nassif N, Pasini S, Redaelli de Zinis LO. Cognitive function and quality of life in older adult patients with cochlear implants. Int J Audiol. 2020;59(4):316–322. doi:10.1080/14992027.2019.1696993
69. Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Society. 2010;16(5):754–760. doi:10.1017/S1355617710000706
70. Moberly AC, Houston DM, Castellanos I. Non-auditory neurocognitive skills contribute to speech recognition in adults with cochlear implants. Laryngoscope Invest Otolaryngol. 2016;1(6):154–162. doi:10.1002/lio2.38
71. Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. 2018;175(3):215–224. doi:10.1176/appi.ajp.2017.17040423
72. Brüggemann P, Szczepek AJ, Klee K, Gräbel S, Mazurek B, Olze H. In patients undergoing cochlear implantation, psychological burden affects tinnitus and the overall outcome of auditory rehabilitation. Front Hum Neurosci. 2017;11:226. doi:10.3389/fnhum.2017.00226
73. Besser J, Stropahl M, Urry E, Launer S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hear Res. 2018;369:3–14. doi:10.1016/j.heares.2018.06.008
74. Brewster KK, Ciarleglio A, Brown PJ, et al. Age-related hearing loss and its association with depression in later life. Am J Geriatr Psychiatry. 2018;26(7):788–796. doi:10.1016/j.jagp.2018.04.003
75. Dawes P, Pye A, Reeves D, et al. Protocol for the development of versions of the Montreal Cognitive Assessment (MoCA) for people with hearing or vision impairment. BMJ Open. 2019;9(3):e026246. doi:10.1136/bmjopen-2018-026246
76. Tretbar K, Basilowski M, Wiedmann K, et al. Lebensqualität und Depression bei Hörminderung: eine deutsche Bedarfsanalyse. HNO. 2019;67(1):36–44. doi:10.1007/s00106-018-0576-4
77. Knopke S, Häussler S, Gräbel S, et al. Age-dependent psychological factors influencing the outcome of cochlear implantation in elderly patients. Otol Neurotol. 2019;40(4):e441–e453. doi:10.1097/MAO.0000000000002179
78. West JS. Hearing impairment, social support, and depressive symptoms among U.S. adults: a test of the stress process paradigm. Soc Sci Med. 2017;192:94–101. doi:10.1016/j.socscimed.2017.09.031
79. Völter, C., Götze, L., Bruene-Cohrs, U., Dazert, S., & Thomas, J. P. (2020). Hören und Kognition: neurokognitive Testbatterien in der HNO-Heilkunde. HNO. 68(3):155–163.
80. Raymond, M., Barrett, D., Lee, D. J., Peterson, S., Raol, N., & Vivas, E. X. (2020). Cognitive Screening of Adults With Postlingual Hearing Loss: A Systematic Review. Otolaryngology–Head and Neck Surgery. doi:10.1177/0194599820933255
81. Regan J, Frison E, Collin F, et al. Individualised sensory intervention to improve quality of life in people with dementia and their companions (SENSE-Cog trial): study protocol for a randomised controlled trial. Trials. 2019;20(1):80. doi:10.1186/s13063-018-2973-0
82. Nguyen M-F, Bonnefoy M, Adrait A, et al. Efficacy of hearing aids on the cognitive status of patients with Alzheimer’s disease and hearing loss: a multicenter controlled randomized trial. J Alzheimers Dis. 2017;58(1):123–137. doi:10.3233/JAD-160793
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
