Back to Journals » Cancer Management and Research » Volume 12
Benefits and Limitations of a Multidisciplinary Approach in Cancer Patient Management
Authors Berardi R , Morgese F, Rinaldi S, Torniai M, Mentrasti G, Scortichini L, Giampieri R
Received 29 February 2020
Accepted for publication 26 May 2020
Published 30 September 2020 Volume 2020:12 Pages 9363—9374
DOI https://doi.org/10.2147/CMAR.S220976
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Rossana Berardi, Francesca Morgese, Silvia Rinaldi, Mariangela Torniai, Giulia Mentrasti, Laura Scortichini, Riccardo Giampieri
Clinica Oncologica, Università Politecnica delle Marche, Azienda Ospedaliera Universitaria Ospedali Riuniti di Ancona, Ancona, Italy
Correspondence: Rossana Berardi
Clinica Oncologica, Università Politecnica Delle Marche, AOU Ospedali Riuniti Di Ancona via Conca 71, Ancona 60126, Italy
Tel +39 071 596-5715
Fax +39 071 5965053
Email [email protected]
Abstract: Over the years, a growing body of literature has confirmed as beneficial the implementation of a multidisciplinary approach in the so-often-intricate scenario of cancer patients’ management. Together with the consolidation of tumor-board experience in clinical practice, certain aspects have emerged as controversial and a source of current debate. In this systematic literature review, we focused our attention on the impact of multidisciplinary tumor boards, assessing benefits and limitations as a result of the dissemination of such approaches. On the bright side, adherence to clinical guidelines, treatment outcomes, and overall improvement in decision-making processes have been recognized as advantages. On the other side, our analysis highlights a few limitations that should be taken into account to optimize cancer patients’ management. Of note, some issues, such as costs, legal responsibility, geographic barriers, and treatment delays, have yet to be resolved. In order partly to address this matter, software platforms and novel methods of computational analysis may provide the needed support. Therefore, the aim of our analysis was to describe the multidisciplinary approach in cancer care in terms of adherence to clinical guidelines, treatment outcomes, and overall improvement in decision-making processes through a systematic review of the literature.
Keywords: multidisciplinary, tumor board, cancer patients, benefits, limitations
Introduction
Management of cancer patients is becoming a worldwide challenge, due to rapidly changing evidence, new drugs approval, and scientific guideline updates. The introduction of the multidisciplinary approach has helped clinicians meet the growing needs of cancer patients. This can be achieved through multidisciplinary clinics, as breast units, or multidisciplinary tumor boards (MTBs), also known as multidisciplinary meetings. Breast units are working entities organized to ensure patients’ clinical examination, diagnostic procedures, including imaging and biopsies, and therapeutic planning, all in one visit. All these procedures are achieved through the combined efforts of different figures, such as clinical oncologists, radiologists, and surgeons, dealing with breast cancer.1,2
In the National Cancer Institute’s dictionary, a tumor board (or review) is defined as:
A treatment planning approach in which a number of doctors who are experts in different specialties (disciplines) review and discuss the medical condition and treatment options of a patient.3
In a cancer setting, this means that multidisciplinary teams discuss the management of cancer patients on a regular basis to provide them the best care, according to their experience and the latest guidelines. This latter approach is common in the US and accepted also in other countries.
Multidisciplinarity began >50 years ago, as reported in several reviews.4 For example, Milligan et al illustrated different cases of patients, not only cancer patients, discussed in laryngology multidisciplinary settings in the 1920s and reported patient anamnesis, clinical history, all specialists opinions given during discussion, and then a conclusive report, specifying how the patient was treated and his/her condition a few months later.5 O’Brien described his experience during his time at Baylor Hospital from the late 1960s to the early 1970s. Once weekly, medical oncologists, together with radiation oncologists and surgeons, discussed all different types of cancer cases.6 Then, in the 1990s, the multidisciplinary approach took hold in Europe’s clinical practice, as has happened in UK and in Germany. Other countries managed to introduce the multidisciplinary approach later (such as in Belgium, where it became mandatory from 2000).7-–9
Typically, multidisciplinary approaches are thought to be meetings where different specialists converge physically together to discuss several clinical cases. Actually, nowadays virtual meetings are also frequently used, allowing distant physicians to confer with each other and decide the right diagnostic and therapeutic path.10 Sometimes, resident hospital staff do not have access to have all required data to make the right decision for each patient, and thus mini–tumor boards are born with the intent to allow only a few specialists to take part in the discussion.1,11,12
A new variety of MTB is the so-called molecular tumor board. Due to the impact of molecular biology as a tool to support different therapeutic decisions, there was the need to add to the “standard” MTB a series of specialists focused on molecular biology, such as pathologists, oncologists, hematologists, basic scientists, and genetic counselors. In particular, due to the opportunity of using genetic cancer-cell profiling to predict drug sensitivity and resistance, molecular tumor boards provide clinicians with the right decision for each patient, due to their taking into account clinical factors and targetable genetic alterations and their relative weight in influencing patient outcomes.1
In an MTB, the “core team” is usually composed of oncologists, surgeons of different subspecialties, pathologists, radiotherapists, and other specialists, according to the type of cancer (eg, head and neck, breast, gastrointestinal, genitourinary). They are open to other members, too (“non–core team”), such as palliative-care physicians, medical students, psychologists, physicians in training or nursing-staff specialists, research nurses, and coordinators. Some countries consider the role of nurse staff to be crucial in influencing treatment decisions and have decided to include nurses in the core team rather than the non–core team1,8,13 (Figure 1).
Few studies have addressed the issue of patient participation in MTBs. Choy et al conducted a very interesting pilot study to assess the usefulness of involvement of breast cancer patients in multidisciplinary meetings, participating in their own treatment planning: 22 of 30 selected patients agreed to take part, seven refused, and another agreed, but was not present at the time of the meeting. The authors reported that patient involvement did not increase their anxiety and was helpful in improving their understanding of treatment choices. Even health-care professionals were satisfied with this involvement, although some admitted that patient participation in MTBs compelled them to be more alert and adjust their language so as to allow understanding of the dialogues by patients.14 In another paper, Butow et al found that physicians had some reservations about patients participating in MTBs, because they had to adjust their language for all participants, constraining discussion and delaying meetings.15 Finally, patient involvement may contribute to the diagnostic process and therapeutic choice, particularly when treatment decisions have a deep impact on their quality of life. In early prostate cancer, for example, patients are able to express their preference among treatments of similar value.8
Primary-care physicians are not considered an integral part of MTB. However, they can have a meaningful role in early identification of cancer, introducing patients to the team, and follow-up after hospital discharge. In addition, they are primarily involved in the management of a series of unrelated comorbidities and symptoms (such as pain) when the patient is at home, and their involvement can help in prompting identification of treatment-related side effects when the patient is discharged from hospital.16,17
Despite technology being able to provide valuable help in physicians’ interactions, it promotes a different way of communication that might influence MTB effectiveness. Mascia et al focused on this peculiar issue, comparing face-to-face vs electronic-based communication among members of an Italian MTB taking place at the Fondazione Policlinico Universitario Agostino Gemelli IRCCS, an Italian research hospital, treating hepatocellular carcinoma patients since 2007. The authors demonstrated that physicians still prefer face-to face communication to exchange work-related information, particularly if they belong to the same clinical unit and the same hospital building, highlighting that physical proximity helps in better knowledge exchange. Among new communication tools, MTB specialists seem to prefer WhatsApp messages, particularly members of the same clinical unit, probably given the informal relationship between workers and members with different expertise. As stated by Johnston et al, WhatsApp acts as a tool capable of relating junior and senior colleagues.18 Based on performance, Mascia et al underlined that members using face-to face communication showed better capability to coordinate and manage the implementation of discussed cases more promptly. Although easy to use, these tools might hamper the quality of MTB discussion.19
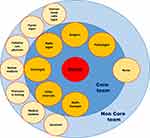 |
Figure 1 Multidisciplinary tumor board. |
Methods
We conducted a systematic literature search for available evidence on the benefits and limitations of a multidisciplinary approach in cancer patients. The aim of the present evaluation of the current evidence was to describe the multidisciplinary approach in terms of adherence to clinical guidelines, treatment outcomes, and overall improvement in the decision-making process. Selection was undertaken by searching PubMed for clinical practice guidelines, original articles, manuscript reviews and prospective and retrospective studies in English published from 1987 to November 2019. The search term used was “multidisciplinary tumor board”. This systematic review adheres to PRISMA guidelines.20 After analysis, we identified 194 potentially relevant articles: 126 were excluded due to not being in English, impertinence, duplicatation, unavailablility of the full article, and being case reports, studies on pediatric cancer patients, or surveys. Figure 2.
 |
Figure 2 PRISMA. |
Benefits
Over the years, a solid body of literature has advocated the implementation of a multidisciplinary approach for adherence to clinical guidelines, outcome improvement, and cancer patient management.21 As such, the present evaluation on MTB advantages was performed focusing on these major aspects.
Adherence to Clinical Guidelines
Clinical guidelines help to define the best therapeutic strategy for each cancer patient, based on high-quality evidence. Adherence to guidelines is associated with an improvement in cancer patient outcome, preventing over- and undertreatment and reducing mortality.22 In the last few years, diagnostic and therapeutic options have increased significantly for cancer patients. Therefore, the creation of MTBs has become necessary for interdisciplinary cooperation and better optimization and integration of all therapeutic resources. In fact, several studies have shown that MTBs implement multimodal treatment, ensuring greater adherence to guidelines and as a result an improvement in patient outcomes.23
MTBs offer benefits to patients, physicians, the community, and hospitals. In particular, they guarantee:
- uniformity of standards of care for cancer patients
- open communication lines to exchange information among physicians who can benefit from both the best scientific evidence and guidelines and the experience of others, improving the decision-making process thanks to case review, radiology- and pathology-report revision, and discussion of treatment options21
- a mechanism for review of the quality of professional care24
The aim of MTBs is improving patient management and outcomes. Most national and international guidelines recommend multidisciplinary management of cancer patients through the creation of MTBs. Adherence to MTB therapeutic indications to the best national and international guidelines is one of the most important parameters for assessing the quality of an MTB. Higher adherence to current guidelines has been observed for both staging and treatment.25
Several studies have analyzed the degree of adherence of MTB therapeutic decisions to guidelines, showing greater agreement compared to therapeutic decisions of individual clinicians. A retrospective study analyzed 3,815 cancer patient cases treated at the Centre for Integrated Oncology at the University Hospital Bonn. Therapeutic recommendations were formulated by three tumor boards, according to types of tumor and best guidelines. The study evaluated the degree of therapeutic recommendation implementation after MTB evaluation: 80% of all recommendations were implemented, with 8.3% of indications showing deviance, due to patient wishes (36.5%), patient death (26%), and physicians’ decisions, based to patient age, comorbidities, or adverse effects of the treatment (24.1%).26
A recent retrospective study on patients with head and neck cancers treated in a single urban academic medical center analyzed the level of concordance between a multidisciplinary team’s therapeutic indications and National Comprehensive Cancer Network (NCCN)-guideline recommendations. Adherence to NCCN guidelines was observed in about 98% of patients assessed in the MTB, while only 80% evaluated by a single specialist received a therapeutic indication in accordance with NCCN guidelines. Deviations from guidelines were mainly observed for a selected few patients, where MTB indication was based on patient age and comorbidities.27 Brauer et al conducted a prospective study aiming to evaluate the role of multidisciplinary teams in the management of patients with pancreatic or gastrointestinal cancer. They found an adherence rate to NCCN guidelines of 100%, while previous series had reported adherence by single physicians of 80%. However, clinician adherence to the treatment plan recommended by the MTB was not complete, due to the need for further diagnostic investigations or medical conditions.28
The therapeutic diagnostic algorithm of colorectal cancer patients is well specified in the guidelines, which suggest a multidisciplinary approach within an MTB to improve patient outcomes.29 A retrospective study analyzed the adherence of MTB decisions to NCCN guidelines on colorectal cancer, showing agreement of 97%. However, compliance of doctors with MTB recommendations was lower (87%), due to patient preference and doctor discretion.10 The management of patients with rare tumors is complex, and guidelines recommend management in expert centres within an MTB. A recent study evaluated the degree of concordance between MTB decisions on indication for postoperative radiotherapy andEuropean Society of Medical Oncology (ESMO) guidelines. MTB indications agreed with ESMO–Réseau Tumeurs Thymiques (RYTHMIC) guidelines in 92% of cases. However, only 85% of patients received postoperative radiotherapy, due to excessive delays after surgery for clinical conditions.30
In order to assess adherence to MTB therapeutic indications and guideline recommendations, an Indian group conducted a study evaluating the level of agreement between IBM’s Watson for Oncology (WFO) and MTB recommendations from the Manipal Comprehensive Cancer Center in Bangalore, India. WFO is an artificial intelligence (AI) system helping physicians in cancer-treatment decisions. WFO indications are processed from a body of knowledge comprising medical journals and textbooks, guidelines, and data on 550 breast cancer cases, including cancer characteristics and stage, patient characteristics and comorbidities, and laboratory exams. Treatment-recommendation concordance was demonstrated in 93% of breast cancer cases. Subgroup analysis showed greater agreement in patients with stage II and III, but low concordance for patients aged >75 years. Nonconcordance was observed especially in WFO indications of aggressive treatment approaches in frail patients. MTBs also considered demographic characteristics, comorbidities, patient preferences and level of social support in treatment choices. These aspects are not usually considered in guidelines, as there is a general lack of studies focused on these matters.31 Furthermore, the introduction of MTBs has been demonstrated to improveability to reach a decision, quality of information presentation, and quality of teamwork.32 In conclusion, MTBs ensure a high degree of concordance of therapeutic decisions with guidelines. However, the advantage of MTBs is to ensure individualized therapy, especially for the most complicated cases, taking into account patients’ clinical decisions and conditions. MTBs allow for the discussion, spread, application, and implementation of the best guidelines.1
In order to improve the decision-making power of MTBs, several instruments have been investigated. Shah et al investigated the quality of MTBs through an observational tool — Colorectal Multidisciplinary Team Metric for Observation of Decision-Making —evaluating quality and time used for presentation of patient history, radiological and pathological information, and contribution to decision-making of each team member. The authors identified areas for improving MTB procedures and optimizing the decision-making process.33 Another study used the MTB Metric for Observation of Decision-Making’ tool to evaluate the decision-making process of a MTB. This tool considered the quality of information presented at the MTB, team-member contributions, and number of case reviews. Analysis showed that psychosocial elements, comorbidities, and cancer nurses’ contributions should be used in decision-making processes and case reviews.34
Outcomes
Multidisciplinary teams increasingly provide treatment of cancer, but the effects of this approach on survival are unclear. Survival benefit from MTB meetings has been observed in a series of highly heterogeneous studies, usually with small numbers of patients included. There is a suggestion that multidisciplinary- and expert-care availability, particularly in cancer types where multimodal treatment is required, is crucial to optimize treatment choices and improve patient outcomes.
Serper et al performed a retrospective cohort study of all patients diagnosed with hepatocellular carcinoma (HCC) treated by 128 Veterans Affairs medical centers, demonstrating that MTB involvement was correlated with overall survival (HR 0.83, 95% CI 0.77–0.90).35 Agarwal et al reported a retrospective analysis comparing survival outcomes of 306 HCC patients managed in MTBs with survival outcomes for 349 patients who did not reach MTB discussion from 2002 to 2011. These patients were treated in a single tertiary-care center in Chicago. The two groups were essentially homogeneous, except that patients in the MTB group had less advanced HCC than those in the non-MTB cohort. The rate of treatment was higher among MTB patients (75%, OR 2.80, 95% CI 1.71–4.59) vs the others (61%; P<0.0001).The MTB seemed to be an independent predictor factor of better survival on multivariate analysis after stratification of tumor stage at onset. The MTB promoted a multimodal approach for HCC patients, allowing enhanced communication among the expert team and patient follow-up. This approach further reduced the potential of examination duplication and delayed or contradictory treatments.36
Liu et al conducted a retrospective analysis of 224 head–neck squamous-cell carcinoma patients treated at Temple University, Philadelphia, Pennsylvania between October 2006 and May 2015, comparing patients who were treated before introduction of an MTB in the hospital vs those who were discussed in MTBs. Median follow-up was 2.8 years, and a majority of patients were in the advanced stage (68%). Five-year overall survival and disease-specific survival were significantly better in the post-MTB cohort vs pre-MTB cohort (40% vs 61% and 52% vs 75%, respectively; P=0.008 and P=0.003).37 Blay et al examined the outcome of 9,646 sarcoma patients treated by a network of 26 reference sarcoma centers with specialized MTBs between 2010 and 2014. This research was funded by the French National Cancer Institute. Most cases presented to MTBs had a higher likelihood of having metastatic involvement at onset and more frequent unfavorable prognostic factors (ie, largerprimary tumors, greater depth, higher grading, and more retroperitoneal locations; all P<0.001). Presentation to MTBs before treatment was correlated with significantly lower 2-year local relapse-free survival (65.4% vs 76.9%, P<0.001) and 2-year relapse-free survival (46.6% vs 51.7%, P<0.001).38
Kesson et al included 13,722 breast cancer patients in a retrospective, comparative, nonrandomized interventional cohort study conducted at an NHS hospital in Scotland. Diagnosis of invasive breast cancer had been done between 1990 and 2000. After the introduction of multidisciplinary care, breast cancer mortality was 18% lower than neighboring areas performing traditional care (HR 0.82, 95% CI 0.74–0.91, P=0.004).39 Instead, Brauer et al analyzed the impact of MTBs on the outcomes of 470 prospectively collected cases of pancreatic and upper gastrointestinal diseases (presented during a 12-month period). Mean overall survival was not significantly different between cases with a change in plan as a result of MTBs vs no modifications in treatment choice (12.1±5.6 months vs 9.0±5.4 months, P=0.154).28 This concept was further confirmed by a wide-ranging literature review by Croke et al.21
Improvements in Clinical Decision-Making and Patient Management
Several studies have confirmed that MTB discussion results in a change in diagnostic or treatment plan in a considerable proportion of cases. Focusing on breast cancer, Newman et al retrospectively described a change in predefined surgical plans after MTB revision of pathological slides by dedicated breast pathologists in 13 patients (9%) pertaining to their center from an outside institution. Additionally, reexamination of previously acquired breast imaging led to surgery in 11% of cases where surgery was not considered a first option before MTB presentation. On the contrary, independently of pathological and radiological reevaluation, the MTB discussion suggested different surgical approaches in a remarkable portion of patients (32%) (eg, sentinel lymph–node biopsy vs axillary lymph–node dissection, mastectomy vs conservative surgery).40 Along these lines, a survey conducted by the Memorial Sloan Kettering Cancer Center showed that sharing individual surgical inclinations in a cross-sectoral setting might reduce unnecessary invasive procedures, such as the adoption of axillary lymph–node dissection in early breast cancer cases.11
With regard to other malignancies, Lee et al observed that modifications in formerly indicated diagnostic workup and treatment strategies at data evaluation occurred in almost half the gynecological tumors discussed within their MTB meeting. Interestingly, the authors found the percentage of recommended changes to be higher than previous findings from a head–neck tumor prospective study.41 In this respect, Wheless et al described a variation of approximately 27% in therapy, diagnosis, or diagnostic procedures. More importantly, a major proportion of patients (65%) experienced a multimodal intensification of their treatment strategy following MTB presentation.42 Similarly, both a cohort chart review and a prospective observational study respectively revealed change in management in 36% and 25% of gastrointestinal and pancreatic tumors after MTB discussion.28,43
According to a study on colorectal cancer patients with stage IV disease, recommendations for preoperative chemotherapy have increased significantly in cases of oligometastatic disease (limited to one site) due to input from MTB discussion. Lowes et al observed that after MTB confrontation, physicians were considerably more prone to refer elderly patients (>70 years) for treatment.44 A retrospective study by Pawlik et al investigated the role of a multidisciplinary approach in respect to pancreatic cancer. The study showed that some cases of declared unresectable disease profited from MTB-enriched surgical experience. Notably, radiological reevaluation caused an upstaging to metastatic disease in almost 70% of cases, requiring an adjustment of the patient’s plan of care.45 In a single-center experience reported by Jury et al, the value of implementing an MTB approach was marked by an increasing number of patients for whom multimodality therapy was indicated after access to their clinic.23 As stated by Ioannidis et al, gathering health-care professionals from different branches has been very beneficial in rectal cancers. Multimodal treatment constitutes the standard of care for these patients, and is partially accountable for outcome improvements achieved in this setting.29
Within this MTB framework, the opportunity to gain new and wide-ranging information is another central aspect to take into consideration. On this point, Deressa et al observed that patients discussed at MTB were characterized by exhaustive staging, resulting in more accurate treatment plans. In contrast, those who had undergone surgery prior to MTB discussion had inadequate and poor staging information.46 The quality of shared information (case history, radiological information) has been related to high-standard decision-making in terms of recommendations given,47 and team members are expected to cooperate as constant supervisors for the level of patient care provided by the group.24 Additionally, when formal consolidated guidelines are lacking, a multisectoral approach might guide health-care professionals in the decision-making process, as advocated by Wotman et al in papillomavirus-positive oropharyngeal squamous-cell carcinoma with incomplete postchemoradiation node response.48
A multimodal and interdiscipline-centered approach might similarly compensate, as underlined by Herlemann et al, the absence of consolidated recommendations on timing and best-treatment sequence in metastatic hormone-sensitive prostate cancer.49 To corroborate this, Fazio et al suggested that an integrated multispecialty strategy might be helpful also to optimize the management of lung neuroendocrine tumors, since the wider armamentarium available for this subgroup compared to the poorly differentiated counterpart.50 Furthermore, some evidence supports the implementation of MTB in surveillance. On behalf of the American College of Chest Physicians, Rubins et al highlighted the importance of multidisciplinary management for early detection of treatment complications, recurrences, or metachronous tumors in follow-up lung cancer patients after curative treatment.51 Taken altogether, the work of Gambazzi et al agrees on multidisciplinary radiological surveillance in posttreatment non-small-cell lung cancer.52 Finally, there have also been reports of increased clinical trial screening and patient recruitment in clinical settings where patient recommendations are discussed by an MTB, as opposed to trial accrual counting exclusively on a dedicated research team.45,53 As to standardizing multidisciplinary management of cancer patients in Europe, implementation of existing recommendations has been done through the creation of consensus documents based on the Delphi method.54––56
Focus on Rare Tumors
Rare cancers often require multimodal therapy. A few rare cancer types (Merkel-cell carcinoma, sarcoma, and HCC) require multidisciplinary management to offer the best treatment choice. On top of being discussed in MTBs, these cases should be referred to high-volume centres to tailor the best treatment strategy for each patient.57––61
Limitations
A multidisciplinary approach certainly provides benefits in cancer patient management, mostly resulting from the sharing of decision-making processes in diagnostic and therapeutic settings. However, various aspects of the multidisciplinary approach might affect the applicability of MTBs in clinical practice, especially in suboptimal settings. These potential limitations still represent the subject of notable controversies and current debates.
In this systematic review, ten of the papers selected identified serious limitations regarding the multidisciplinary approach in cancer patients.24,28,30,32,44,60,62–65 Among these, the role of MTBs in patient outcomes might deserve special attention, and remains a matter of debate. In this regard, Brauer et al reviewed 470 cases of patients with benign and malignant pancreatic and gastrointestinal diseases that had led to MTB discussion and been recorded in a prospectively collected database. Despite strong adherence to NCCN guidelines, multidisciplinary discussion produced a change in patient management in a minority (about a quarter) of cases. Nevertheless, survival time was no different between these cases and patients without any variation in plan, suggesting that MTB discussion might not have a significant impact on outcome.28 In the same paper, the authors also focused on institutional resource utilization for MTBs, estimating total time expenditure of 16.5 hours and a cost of US$2,035 weekly.28 On the basis of these not-negligible expenses, MTBs should be available only in those settings where justified by a high number of cases that require critical decisions, and regular assessment of their effectiveness should be performed.
Another potential limitation of the multidisciplinary approach concerns the quality of the information presented to MTBs that might play a crucial role in team decision-making. Through a cross-sectional, observational study conducted at University Cancer Center Hamburg, a German hospital hosting 16 MTBs, Hahlweg et al evaluated the quality of single-case information using a scoring system with six main variables. Despite high variability among the 16 examined MTBs, data concerning comorbidities and psychosocial context were almost always missing or superficially presented, affecting teams’ final decisions and recommendations.62 Furthermore, low-quality information presented might render the MTB unable to make a decision, especially when there is a lack of fundamental reporting (ie, imaging performed at external centers), as deduced from an analysis of 68 consecutive cases presented at the Lung Oncology MTB of Peter MacCallum Cancer Centre (Melbourne, Australia) between March and May 2011. In three of 68 patients, inadequacy of administrative support in quickly finding missing information significantly reduced the effectiveness of a multidisciplinary approach.63 Lamb et al achieved similar results in their prospective longitudinal study evaluating the quality of decision-making processes in 1,421 urological cancer patients presented to MTBs of Whipps Cross University Hospital (London, UK) over 2 years (from 2009 to 2011). Despite significant growth in teamwork quality and effectiveness due to improvement interventions, lacking anamnestic, radiological, or pathological information still represented obstacles to reaching clinical decisions.32 On the other hand, an excessive amount of not strictly clinical information might lead to team members expressing contrasting opinions and the MTB producing more than one recommendation.63
Another potential limitation of the multidisciplinary approach is related to legal issues. MTBs represent an instrument of peer review for cancer patients. Due to the confidential nature of the relationship between patient and their physician, it might be not so simple to maintain the same confidentiality within an MTB. Already in 1987, Gross et al analyzed the prickly question of legal issues related to tumor boards, focusing on team members’ responsibilities in confidentiality and anonymity of every patient presented to an MTB.24
Furthermore, geographical barriers might represent concrete impediments to achieving an effective multidisciplinary approach in oncology settings. Regarding extra-European regions, MTBs still do not represent a common reality in Africa or the Middle East. A consensus of 22 urologists and oncologists from these areas firstly met in Quatar (February 2012) and then in Dubai (March 2013) to discuss local management of renal cell–carcinoma patients, frequently in the absence of an MTB. Zekri et al wrote a report on the consensus of opinion reached, identifying the main barriers to the multidisciplinary approach and interdisciplinary referral as financial issues, patients’ social conditions, and deficiency of surgeons.64
Geographical origin and socioeconomic conditions might limit accessibility to national networks and MTBs, even in European countries, with significant urban–rural inequalities, especially in the field of rare cancers. As reported in a recent paper by Lowes et al, MTBs are not yet widespread, despite national guidelines recommending a multidisciplinary approach in the majority of neoplasms, representing a real cornerstone in modern oncology.44 Fayet et al evaluated efforts of French sarcoma networks in reducing geographical disparities that still affect cancer patients.65 Despite centralization representing an essential requirement in rare cancer management, with a significant correlation with prognosis, Sandrucci et al focused on its disadvantages for patients, as the obligation to move to referral centers caused notable discomfort.60
Finally, a multidisciplinary approach might be associated with treatment delays due to MTB-meeting schedules and frequently longer waiting lists in referral centers. Basse et al wrote a retrospective analysis of 274 patients with thymic epithelial tumors discussed at the national RYTHMIC MTB focusing on postoperative radiotherapy. Despite MTB recommendations, several patients did not receive treatment, mostly due to excessive delays after surgery, suggesting that MTB decisions should be quicker, avoiding any waste of time30 (Table 1).
 |
Table 1 Pros and Cons of the Multidisciplinary Approach |
Future Perspectives
It has been said that humans make decisions by taking into account five variables at most. The increasing complexity in management of cancer patients has led to the development of computer systems that can help clinicians in choosing the most adequate diagnostic and therapeutic approach. In this context, Walsh et al provided a synopsis of decision-support systems: computer programs integrating all possible data, such as clinical history, imaging, genetics, and costs, to obtain validated predictive models and realize precision medicine.66
Somashekhar et al’s paper was based on the use of AI as a possible new approach to consider in multidisciplinary cancer patients care, too. They compared therapeutic choices made in a breast MTB of an expert panel of specialists in Bangalore, India to that suggested by IBM’s WFO. WFO is a unique system for oncology-therapy selection, deriving most of its knowledge from literature, protocols, and test cases from Memorial Sloan Kettering Cancer Center. The authors found a high level of agreement — up to 93%. According to stage, concordance was higher in stage II and III cancers. Including receptor status, final choices in triple-negative metastatic breast cancer patients showed less agreement than nonmetastatic HER2-positive cases. Different choices were adopted for patients aged 75 years older also. Nonconcordance could have derived from different drug availability in India and the US and differences in demographic characteristics, such as patient choice, comorbidities, and presence of caregivers. This study demonstrated how AI can help clinicians’ decisions in breast cancer treatment, notably if expert opinions are not easily achievable.31
Krupinski et al provided an overview of the use of a software platform — Navify Tumor Board — helping specialists improve workflow and preparation for MTBs. The authors reported on the experience of the breast cancer multidisciplinary team in Hospital del Mar, Barcelona. Navify is an oncology-informatics platform facilitating the coordination, preparation, scheduling, presentation, and extraction of clinical, biological, radiological, and other significant information during preparation of patient cases. Oncologists, surgeons, radiologists, and pathologists took part in this survey, revealing that using health-information technology can reduce time to provide recommendations for cases compared to current methods, rather pathologists take the same time. Moreover, it is undoubtedly a way of standardizing the presentation of cancer cases to be discussed in a multidisciplinary context.67
Finally, Gallagher et al proposed the realization of a clinical database to improve patient care and research, describing all phases requested in constituting the Genitourinary Oncology Database, created by the University of North Carolina. This project needed attention by all members of the MTB, accounting for their personal experience and reviewing literature. Indeed, there were several critical features, such as the security policy for patient data and reducing errors, in insertion of baselines and updating them. The authors hoped that their experience could mark the way for similar skills.68
Conclusion
Since its introduction in clinical oncology, multidisciplinary management and specifically MTBs have met with increasing enthusiasm as ways to improve the quality of patient care. Moreover, MTB implementation in everyday clinical practice should lead theoretically to increased knowledge, awareness, and reduction of anxiety for members who participate in discussions of MTBs. Although several guidelines suggest that MTBs are crucial in different settings (particularly in rare cancer types), there is a lack of general consensus on what can be done to assess properly whether MTB determines a real improvement in cancer survival and which methods can be used to prove MTB effectiveness. Our review has shown that almost all published papers agree on the fact that adherence to guidelines is one of the main factors that is encouraged by the implementation of MTB discussions. Since we believe that adherence to guidelines is the factor that is more strongly associated with quality of treatment (ie, offering what is generally considered as must for each patient), we believe that this factor should be the one used to check whether MTBs are working in an adequate (or not) fashion.
Interestingly, though adherence of guidelines was maintained, benefit in overall survival was usually less described, and sometimes adherence to guidelines did not determine any change whatsoever in survival outcomes between patients discussed in MTBs vs those who were managed outside the setting of MTBs. This can be partly explained by the fact that MTBs take into account decisions based on data that are actually presented in the discussions. There are a few factors (patient preference, social and financial status, and presence/lack of adequate caregiver) that are rarely discussed in the meetings (owing also to the lack of studies inquiring about the real weight of these factors in influencing treatment decisions). These factors can lead to changes in the proposed treatment plan, particularly when the disease that is treated is not a rare cancer type, thus reducing the impact of a multidisciplinary meeting recommendation.
Our review has also highlighted that though published papers do support a benefit in implementation of MTB discussion, there are a few limitations that should be taken into account to optimize this treatment modality. First of all, MTBs are not a substitute for expertise, and it is required that experience in the management of that specific disease is proven for all members who participate in the MTB. For some instances, such as in the case of rare cancer types, this means that MTBs for the management of these tumor types should only be present in high-volume centers where such cases are concentrated.
Furthermore, there is a common misconception concerning MTBs regarding costs. It is usually hypothesized that sharing knowledge during the course of MTBs should lead to improvement in patient survival just for the sake of improvement of management of the patient, with no additional cost. All papers focusing on this matter highlighted that MTB implementation leads to an increase in costs, due to more efficiency (and thus better access to diagnostic resources or treatment options, with an increase in costs). Moreover, there is also a cost in terms of additional hours spent in MTB meetings, a cost that is usually outside that considered necessary for everyday patient care. Finally, MTBs occupy a gray area concerning their role in the patient–physician relationship. Strictly speaking, from a legal point of view there are a few unsolved issues in terms of responsibility. When a treatment decision that was issued by the MTB (and was “wrong”) and was supported by the treating physician results in damage to the patient, who is to blame? Is it the responsibility of the primary treating physician or of the MTB itself?
These issues will have to be resolved, particularly in the setting of medical oncology, where owing to the increasing complexity of the disease, it is foolish to believe that the oncologist by themselves is able to make all the adequate treatment choices for each patient. Nonetheless, as supported by the data that we have reported, MTBs are also improving with the times, and we believe that with the implementation of novel methods of computational analysis, they could offer a wider range of possibilities and more evidence-based treatment choices for patients who come to ask for our help.
Disclosure
Rossana Berardi reports grants from Astra Zeneca, Novartis, Merck Sharp & Dohme, and Lilly and personal fees from Otsuka, Boehringer, Merck Sharp & Dohme, and Lilly outside the submitted work. The other authors report no conflicts of interest in this work.
References
1. El Saghir NS, Keating NL, Carlson RW, Khoury KE, Fallowfield L. Tumor boards: optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am Soc Clin Oncol Educ Book. 2014;e461–6. doi:10.14694/EdBook_AM.2014.34.e461
2. Blamey RW, Cataliotti L. EUSOMA accreditation of breast units. Eur J Cancer. 2006;42(10):1331–1337. doi:10.1016/j.ejca.2006.04.003
3. National Cancer Institute. Defınition of tumor board review. Available from: http://www.cancer.gov/dictionary?cdrid=322893.
4. Dickhoff C, Dahele M. The multidisciplinary lung cancer team meeting: increasing evidence that it should be considered a medical intervention in its own right. J Thorac Dis. 2019;11(3):311–314. doi:10.21037/jtd.2019.01.14
5. Vlasto Milligan. Further reports on cases exhibited before the section at previous meetings, session 1921–1922. Proc R Soc Med. 1922;15:
6. O’Brien JC. History of tumor site conferences at Baylor University Medical Center. Proc (Bayl Univ Med Cent). 2006;19(2):130–131. doi:10.1080/08998280.2006.11928145
7. Munro AJ, Swartzman S. What is a virtual multidisciplinary team (vMDT)? Br J Cancer. 2013;108(12):2433–2441. doi:10.1038/bjc.2013.231
8. Hermes-Moll K, Dengler R, Riese C, Baumann W. Tumor boards from the perspective of ambulant oncological care. Oncol Res Treat. 2016;39:377–383. doi:10.1159/000446311
9. Horlait M, Baes S, Dhaene S, Van Belle S, Leys M. How multidisciplinary are multidisciplinary team meetings in cancer care? An observational study in oncology departments in Flanders, Belgium. J Multidiscip Healthc. 2019;12:159–167. doi:10.2147/JMDH.S196660
10. Takeda T, Takeda S, Uryu K, et al. Multidisciplinary lung cancer tumor board connecting eight general hospitals in Japan via a high-security communication line. JCO Clin Cancer Inform. 2019;3:1–7. doi:10.1200/CCI.18.00115
11. El Saghir NS, Charara RN, Kreidieh FY, et al. Global practice and efficiency of multidisciplinary tumor boards: results of an American Society of Clinical Oncology International Survey. J Glob Oncol. 2015;1(2):57–64. doi:10.1200/JGO.2015.000158
12. El Saghir NS, El-Asmar N, Hajj C. Survey of utilization of multidisciplinary management tumor boards in Arab countries. Breast. 2011;20(Suppl 2):70–74. doi:10.1016/j.breast.2011.01.011
13. Lumenta DB, Sendlhofer G, Pregartner G, et al. Quality of teamwork in multidisciplinary cancer team meetings: a feasibility study. PLoS One. 2019;14(2):e0212556. doi:10.1371/journal.pone.0212556
14. Choy E, Chiu A, Butow P, Young J, Spillane A. A pilot study to evaluate the impact of involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Breast. 2007;16:178–189. doi:10.1016/j.breast.2006.10.002
15. Butow P, Harrison J, Choy E, Young J, Spillane A, Evans A. Health professional and consumer views on involving breast cancer patients in the multidisciplinary discussion of their disease and treatment plan. Cancer. 2007;110:1937–1944. doi:10.1002/cncr.23007
16. Siddique O, Yoo ER, Perumpail RB, et al. The importance of a multidisciplinary approach to hepatocellular carcinoma. J Multidiscip Healthc. 2017;Volume 10(10):95–100. doi:10.2147/JMDH.S128629
17. Rainone F. Treating adult cancer pain in primary care. J Am Board Fam Pract. 2004;17(Suppl 1):S48–56. doi:10.3122/jabfm.17.suppl_1.S48
18. Johnston MJ, King D, Arora S, et al. Smartphones let surgeons know WhatsApp: an analysis of communication in emergency surgical teams. Am J Surg. 2015;209(1):45–51. doi:10.1016/j.amjsurg.2014.08.030
19. Mascia D, Rinninella E, Pennacchio NW, Cerrito L, Gasbarrini A. It’s how we communicate! Exploring face-to-face versus electronic communication networks in multidisciplinary teams. Health Care Manage Rev. 2019. doi:10.1097/HMR.0000000000000246
20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–431. doi:10.1016/j.ijsu.2010.02.007
21. Croke JM, El-Sayed S. Multidisciplinary management of cancer patients: chasing a shadow or real value? An overview of the literature. Curr Oncol. 2012;19(4):e232–8. doi:10.3747/co.19.944
22. Kabat GC, Matthews CE, Kamensky V, Hollenbeck AR, Rohan TE. Adherence to cancer prevention guidelines and cancer incidence, cancer mortality, and total mortality: a prospective cohort study. Am J Clin Nutr. 2015;101(3):558–569. doi:10.3945/ajcn.114.094854
23. Jury RP, Nadeau L, Wasvary H, Levine R, Robertson J. Implementing a multidisciplinary open-access clinic at a private practice-based community hospital. J Oncol Pract. 2010;6(6):e38–41. doi:10.1200/JOP.2010.000029
24. Gross GE. The role of the tumor board in a community hospital. CA Cancer J Clin. 1987;37(2):88–92. doi:10.3322/canjclin.37.2.88
25. Riquet M, Mordant P, Henni M, et al. Should all cases of lung cancer be presented at tumor board conferences? Thorac Surg Clin. 2013;23(2):123–128. doi:10.1016/j.thorsurg.2013.01.003
26. Hollunder S, Herrlinger U, Zipfel M, et al. Cross-sectional increase of adherence to multidisciplinary tumor board decisions. BMC Cancer. 2018;18(1):936. doi:10.1186/s12885-018-4841-4
27. Shah BA, Qureshi MM, Jalisi S, et al. Analysis of decision making at a multidisciplinary head and neck tumor board incorporating evidence-based National Cancer Comprehensive Network (NCCN) guidelines. Pract Radiat Oncol. 2016;6(4):248–254. doi:10.1016/j.prro.2015.11.006
28. Brauer DG, Strand MS, Sanford DE, et al. Utility of a multidisciplinary tumor board in the management of pancreatic and upper gastrointestinal diseases: an observational study. HPB. 2017;19(2):133–139. doi:10.1016/j.hpb.2016.11.002
29. Ioannidis A, Konstantinidis M, Apostolakis S, Koutserimpas C, Machairas N, Konstantinidis KM. Impact of multidisciplinary tumor boards on patients with rectal cancer. Mol Clin Oncol. 2018;9(2):135–137. doi:10.3892/mco.2018.1658
30. Basse C, Thureau S, Bota S, et al. Multidisciplinary tumor board decision making for postoperative radiotherapy in thymic epithelial tumors: insights from the RYTHMIC prospective cohort. J Thorac Oncol. 2017;12(11):1715–1722. doi:10.1016/j.jtho.2017.07.023
31. Somashekhar SP, Sepúlveda MJ, Puglielli S, et al. Watson for Oncology and breast cancer treatment recommendations: agreement with an expert multidisciplinary tumor board. Ann Oncol. 2018;29(2):418–423. doi:10.1093/annonc/mdx781
32. Lamb BW, Green JS, Benn J, Brown KF, Vincent CA, Sevdalis N. Improving decision making in multidisciplinary tumor boards: prospective longitudinal evaluation of a multicomponent intervention for 1,421 patients. J Am Coll Surg. 2013;217(3):412–420. doi:10.1016/j.jamcollsurg.2013.04.035
33. Shah S, Arora S, Atkin G, et al. Decision-making in colorectal cancer tumor board meetings: results of a prospective observational assessment. Surg Endosc. 2014;28(10):2783–2788. doi:10.1007/s00464-014-3545-3
34. Jalil R, Akhter W, Lamb BW, et al. Validation of team performance assessment of multidisciplinary tumor boards. J Urol. 2014;192(3):891–898. doi:10.1016/j.juro.2014.03.002
35. Serper M, Taddei TH, Mehta R, et al.; VOCAL Study Group. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152(8):1954–1964. doi:10.1053/j.gastro.2017.02.040.
36. Agarwal PD, Phillips P, Hillman L, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51(9):845–849. doi:10.1097/MCG.0000000000000825
37. Liu JC, Kaplon A, Blackman E, Miyamoto C, Savior D, Ragin C. The impact of the multidisciplinary tumor board on head and neck cancer outcomes. Laryngoscope. 2019;130:946–950.
38. Blay JY, Soibinet P, Penel N, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28(11):2852–2859. doi:10.1093/annonc/mdx484
39. Kesson EM, Allardice GM, George WD, Burns HJ, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. 2012;344:e2718. doi:10.1136/bmj.e2718
40. Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107(10):2346–2351. doi:10.1002/cncr.22266
41. Lee B, Kim K, Choi JY, et al. Efficacy of the multidisciplinary tumor board conference in gynecologic oncology: a prospective study. Medicine. 2017;96(48):e8089. doi:10.1097/MD.0000000000008089
42. Wheless SA, McKinney KA, Zanation AM. A prospective study of the clinical impact of a multidisciplinary head and neck tumor board. Otolaryngol Head Neck Surg. 2010;143:650–654. doi:10.1016/j.otohns.2010.07.020
43. AlFarhan HA, Algwaiz GF, Alzahrani HA, et al. Impact of GI tumor board on patient management and adherence to guidelines. J Glob Oncol. 2018;4:1–8. doi:10.1200/JGO.17.00164
44. Lowes M, Kleiss M, Lueck R, et al. The utilization of multidisciplinary tumor boards (MDT) in clinical routine: results of a health care research study focusing on patients with metastasized colorectal cancer. Int J Colorectal Dis. 2017;32(10):1463–1469. doi:10.1007/s00384-017-2871-z
45. Pawlik TM, Laheru D, Hruban RH, et al.; Johns Hopkins Multidisciplinary Pancreas Clinic Team. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15(8):2081–2088. doi:10.1245/s10434-008-9929-7.
46. Deressa BT, Cihoric N, Tefesse E, Assefa M, Zemenfes D. Multidisciplinary Cancer Management of Colorectal Cancer in Tikur Anbessa Specialized Hospital, Ethiopia. J Glob Oncol. 2019;5:1–7. doi:10.1200/JGO.19.00014
47. Solomon D, DeNicola N, Feferman Y, et al. Assessing the Implementation of American College of Surgeons Quality Indicators for Pancreatic Cancer Across an Integrated Health System. J Oncol Pract. 2019;15(8):e739–e745. doi:10.1200/JOP.18.00587
48. Wotman M, Ghaly M, Massaro L, et al. Management of the neck after definitive chemoradiation in patients with HPV-associated oropharyngeal cancer: an institutional experience. Am J Otolaryngol. 2019;40(5):684–690. doi:10.1016/j.amjoto.2019.06.003
49. Herlemann A, Washington SL, Cooperberg MR. Health care delivery for metastatic hormone-sensitive prostate cancer across the globe. Eur Urol Focus. 2019;5(2):155–158. doi:10.1016/j.euf.2018.12.003
50. Fazio N, Ungaro A, Spada F, et al. The role of multimodal treatment in patients with advanced lung neuroendocrine tumors. J Thorac Dis. 2017;9(Suppl 15):S1501–S1510. doi:10.21037/jtd.2017.06.14
51. Rubins J, Unger M, Colice GL; American College of Chest Physicians. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest. 2007;132(3):355S–367S. doi:10.1378/chest.07-1390
52. Gambazzi F, Frey LD, Bruehlmeier M, et al. Image analysis in posttreatment non-small cell lung cancer surveillance: specialists’ interpretations reviewed by the thoracic multidisciplinary tumor board. Multidiscip Respir Med. 2019;14:34. doi:10.1186/s40248-019-0198-z
53. Kuroki L, Stuckey A, Hirway P, et al. Addressing clinical trials: can the multidisciplinary tumor board improve participation? A study from an academic women’s cancer program. Gynecol Oncol. 2010;116(3):295–300. doi:10.1016/j.ygyno.2009.12.005
54. van de Velde CJ, Boelens PG, Borras JM, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50(1):1–e1. doi:10.1016/j.ejca.2013.06.048
55. Mañós M, Giralt J, Rueda A, et al. Multidisciplinary management of head and neck cancer: first expert consensus using Delphi methodology from the Spanish Society for Head and Neck Cancer (part 1). Oral Oncol. 2017;70:58–64. doi:10.1016/j.oraloncology.2017.04.004
56. Rueda A, Giralt J, Mañós M, et al. Multidisciplinary management of head and neck cancer: first expert consensus using Delphi methodology from the Spanish Society for Head and Neck Cancer (part 2). Oral Oncol. 2017;70:65–72. doi:10.1016/j.oraloncology.2017.04.005
57. Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16(11):2985–2993. doi:10.1245/s10434-009-0615-1
58. Taddei TH. A multidisciplinary approach: group dynamics. J Clin Gastroenterol. 2013;47:S27–9. doi:10.1097/MCG.0b013e31829331de
59. Asghar AH, Abbasi AN, Jamal A, Haider G, Rizvi S. City tumour board Karachi: an innovative step in multidisciplinary consensus meeting and its two years audit. J Pak Med Assoc. 2013;63(12):1534–1535.
60. Sandrucci S, Naredi P, Bonvalot S. Centers of excellence or excellence networks: the surgical challenge and quality issues in rare cancers. Eur J Surg Oncol. 2019;45(1):19–21. doi:10.1016/j.ejso.2017.12.012
61. Bui NQ, Wang DS, Hiniker SM. Contemporary management of metastatic soft tissue sarcoma. Curr Probl Cancer. 2019;43(4):289–299. doi:10.1016/j.currproblcancer.2019.06.005
62. Hahlweg P, Didi S, Kriston L, Härter M, Nestoriuc Y, Scholl I. Process quality of decision-making in multidisciplinary cancer team meetings: a structured observational study. BMC Cancer. 2017;17(1):772. doi:10.1186/s12885-017-3768-5
63. Ung KA, Campbell BA, Duplan D, Ball D, David S. Impact of the lung oncology multidisciplinary team meetings on the management of patients with cancer. Asia Pac J Clin Oncol. 2016;12(2):e298–304. doi:10.1111/ajco.12192
64. Zekri J, Dreosti LM, Ghosn M, et al. Multidisciplinary management of clear-cell renal cell carcinoma in Africa and the Middle East: current practice and recommendations for improvement. J Multidiscip Healthc. 2015;27(8):335–344. doi:10.2147/JMDH.S85538
65. Fayet Y, Coindre JM, Dalban C, et al. Geographical accessibility of the referral networks in France. Intermediate results from the IGéAS research program. Int J Environ Res Public Health. 2018;15(10):2204. doi:10.3390/ijerph15102204
66. Walsh S, de Jong EEC, van Timmeren JE, et al. Decision support systems in oncology. JCO Clin Cancer Inform. 2019;3:1–9. doi:10.1200/CCI.18.00001
67. Krupinski EA, Comas M; Gallego LG on behalf of the GISMAR Group. A new software platform to improve multidisciplinary tumor board workflows and user satisfaction: a pilot study. J Pathol Inform. 2018;9:26. doi:10.4103/jpi.jpi_16_18
68. Gallagher SA, Smith AB, Matthews JE, et al. Roadmap for the development of the University of North Carolina at Chapel Hill Genitourinary OncoLogy Database–UNC GOLD. Urol Oncol. 2014;32(1):32–e1. doi:10.1016/j.urolonc.2012.11.019
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
