Back to Journals » Cancer Management and Research » Volume 10
Benefit from the inclusion of surgery in the treatment of patients with stage III pancreatic cancer: a propensity-adjusted, population-based SEER analysis
Authors Wang L , Cheng CS , Chen L, Chen Z
Received 3 March 2018
Accepted for publication 26 April 2018
Published 5 July 2018 Volume 2018:10 Pages 1907—1918
DOI https://doi.org/10.2147/CMAR.S167103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Lai Wang,1,2,* Chien-Shan Cheng,1,2,* Lianyu Chen,1,2 Zhen Chen1,2
1Department of Integrative Oncology, Fudan University Shanghai Cancer Center, Shanghai, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
*These authors contributed equally to this work
Purpose: In the past 20 years, surgical resection has been a secure and applicable procedure for pancreatic cancer (PC), but it remains controversial for stage III PC with data evaluating its efficacy mostly derived from small randomized trials. Hence, we designed this study to further evaluate its benefit using surveillance, epidemiology, and end results dataset.
Patients and methods: Patients with stage III PC were identified in the surveillance, epidemiology, and end results registries from 2004 to 2014. The effect of surgery on cancer-specific survival was assessed by risk-adjusted Cox proportional hazard regression modeling and propensity score matching.
Results: Overall, 6,138 patients with stage III PC were included. Of these, 608 patients underwent primary tumor surgery. On multivariable analyses, surgery was independently associated with improved cancer-specific survival (HR=0.580; 95% CI=0.523–0.643, p<0.001). The survival benefit with surgery was also observed in the propensity score-matched cohort (HR=0.501; 95% CI=0.438–0.573, p<0.001).
Conclusion: Primary tumor surgery is associated with improved survival in stage III PC. Prospective randomized trials are needed to confirm these results, and further efforts are required to address patient selection.
Keywords: stage III pancreatic cancer, propensity score matching, surgery, surveillance, epidemiology, and end results (SEER) database
Introduction
Pancreatic cancer (PC) is the fourth main cause of cancer-related mortality in the USA, with its mortality rate (n=44,330) estimated to approach its incidence rate (n=55,440) in 2018.1 In contrast to the steady improvements on survival rate in various types of malignancies, overall survival gains from technological advancements remain challenging for PC, as reflected by dismal 5-year survival rates of 5%.1 Presently, margin-negative resection (R0) remains the only curative therapy for PC, but only about 20% of patients are considered to be initially resectable. Even for those undergoing complete resection, 5-year overall survival rate is just 20%.
Unfortunately, owing to its unspecific early symptoms, >80% of new cases are diagnosed at locally advanced unresectable PC (LAPC) or metastatic PC.2 About 30% of patients present with locally advanced disease (stage III, T4 any N M0, AJCC 7th edition).2,3 This group of stage III PC patients is often divided into borderline resectable PC (BRPC) and LAPC, depending on the extent of the tumor encasement of major vessels (<180° versus >180°). In the past, stage III PC patients were considered inoperable due to the complexity of the operative procedure and the high risk of increased postoperative complications.4,5 However, with the ongoing development and advancement in chemoradiotherapy and surgical techniques in the last 20 years, more and more patients become operable after a prolonged course of chemoradiotherapy.6 Those patients presenting with singular measurable lesion are often sent for surgical consultation to consider resecting the only remaining tumor. Nevertheless, existing evidence regarding any potential survival benefit of surgery for stage III PC remains paradoxical. Denecke et al reported a poor median overall survival of 12.4 months in six patients who were operated on by distal celiacopancreatectomy, and five of them had tumor recurrence after surgery.7 Another research paper documented distal pancreatectomy with en bloc resection of the celiac artery in 13 patients with LAPC, and the median survival was just 12.2 months.8 In contrast, conclusions from some studies were much more inspiring, with median overall survival ranging from 18 to 35 months.9,10 The difference for survival in these studies could be attributed to the small size of cohorts and various neoadjuvant regimens.
Given that the studies mentioned above showed varying results, we designed this one to more clearly establish the association between surgery and cancer-specific survival (CSS) in patients with stage III PC by analyzing data from the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and methods
Data collection and patient selection
This study was conducted using the 2017 data of the SEER Program of the US National Cancer Institute. The SEER program is a population-based database covering about 28% of the US population. We get data on patient demographics, years of diagnosis, cancer site, histologic type, stage, cause of death, and treatment by SEER ID 16018-Nov 2016.
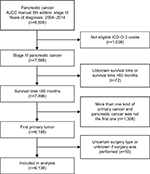
We included only PC patients at TNM stage III (T4 any N M0) based on the American Joint Committee on Cancer Staging Manual, 6th edition. We limited our study from 2004 to 2014 as the sixth edition of the AJCC staging system was published in 2004. Although the 8th edition of the AJCC staging manual is expected to be used in 2018,11 the 7th edition is still being used widely and it is the same as the AJCC 6th edition for PC. The behavior item 3 was used to determine the malignant tumor. The following codes were selected to confirm pancreatic adenocarcinoma according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3): 8140, 8141, 8255, 8260, 8470, 8471, 8480, 8481, 8490, 8500, 8550, 8560. Only patients who met the following criteria were included in the formal analysis: 1) survival time ≤60 months, 2) with one primary cancer only or with PC as the first one if there were two or more, and 3) clear surgery procedure excluding irreversible electroporation (IRE). Of the 8,606 adults with stage III PC diagnosed between 2004 and 2014 in the SEER Program, 6,138 patients were eligible for the study (Figure 1). These eligible patients were divided into the surgery group and the nonsurgery group. We have obtained the approval for the study from the Institutional Review Board of Fudan University Shanghai Cancer Center.
Statistical analysis
The endpoint of this study was CSS, which was determined from the year of diagnosis to the year of death for PC. Other causes of death were considered as censors. Chi-square test was used to compare the categorical data to evaluate differences in the distribution of baseline characteristics between the two groups. Univariate analysis was conducted using Kaplan–Meier method and compared by the log-rank test, while multivariable analysis was carried out by the Cox proportional hazards model. To further adjust for potential baseline confounders, we used the ‘‘MatchIt’’ R packages to perform a 1:1 nearest-neighbor propensity score matching (PSM) between the two groups by logistic regression. A propensity score is the probability to be assigned to a specific intervention given baseline covariates, which has been applied to eliminate the selection bias associated with the variables incorporated into the model.12,13 Subsequently, the baseline characteristics of the matched patients were verified to ensure that no important difference between the two groups remained. All p-values were two sided and considered statistically significant at the 0.001 level. All analyses were performed with R version 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Based on the eligibility criteria (Figure 1), a total of 6,138 patients diagnosed with stage III PC between 2004 and 2014 were identified in the study. Of this population, only 608 (9.91%) patients underwent surgery (the surgery group), whereas 5,530 (90.09%) did not (the nonsurgery group). The following surgical procedures were accomplished: local excision of tumor (n=24; 0.39%), partial pancreatectomy (n=67; 1.09%), local or partial pancreatectomy and duodenectomy (n=25; 0.41%), local or partial pancreatectomy and duodenectomy without distal/partial gastrectomy (n=63; 1.03%), local or partial pancreatectomy and duodenectomy with partial gastrectomy (Whipple; n=318; 5.18%), total pancreatectomy (n=22; 0.35%), total pancreatectomy and subtotal gastrectomy or duodenectomy (n=58; 0.94%), extended pancreatoduodenectomy (n=28; 0.45%), and pancreatectomy (n=3; 0.05%). Table 1 summarizes patient demographics across groups. The median age at diagnosis of the study population was 66 years (range 26–99), and a majority of people were >55 years old (82.28%). Compared with the nonsurgery group, the surgery group had more pancreas head cancer (67.76% versus 55.32%; p<0.001) and more regional lymph node metastasis (N1 stage, based on AJCC 6th edition; 60.52% versus 32.98%; p<0.001). Chemotherapy was performed for most patients. Both groups were similar in terms of sex, ethnicity, and years of diagnosis.
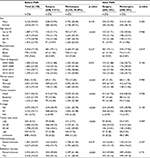  | Table 1 Patient characteristics before and after PSM Abbreviations: NX, unknown N stage; PSM, propensity score matching. |
Adjusting for patient characteristics using PSM
In order to further overcome confusion and selection biases between the two groups, we conducted a PSM procedure. According to the results of Chi-square test, age at diagnosis, marital status, tumor location, N stage, tumor size, chemotherapy, and radiation therapy were enrolled into the propensity model to produce a 1:1 nearest-neighbor matching. Before matching, there were 608 patients in the surgery group and 5,530 patients in the nonsurgery group. After 1:1 matching, both groups had 608 adults, and the propensity score was almost equal to 0.1234 (±0.0532) for both groups. Figure 2 shows the propensity score distribution of the two groups before and after PSM procedure. The covariates distribution is fittingly balanced in the matching data set, as shown in Table 1.
Predictors of CSS
Over a median follow-up of 8 months, mortality occurred in 5,325 (86.75%) patients. On univariate analysis, surgery, age at diagnosis, years of diagnosis, N stage, chemotherapy, radiation therapy, tumor size, and marital status were associated with CSS (p<0.001). Except for N stage and tumor size, other factors maintained statistical significance after PSM (p<0.001). For a more overall approach, all variables were included in the multivariable model by the Cox proportional hazards model. In the unmatched data set, surgery, age at diagnosis, years of diagnosis, marital status, chemotherapy, radiation therapy, and N stage proved to be independent predictors of CSS (p<0.001). After matching, surgery, age at diagnosis, years of diagnosis, and chemotherapy were still independently correlated with CSS (p<0.001). It is worth noting that either before or after PSM, surgery is a significant prognostic factor of CSS (Table 2). The Kaplan–Meier curves for CSS between the two groups before and after PSM are shown in Figure 3.
  | Figure 3 Kaplan–Meier curve of CSS comparing the surgery group with the nonsurgery group before (A) and after (B) PSM. Abbreviations: CSS, cancer-specific survival; PSM, propensity score matching. |
  | Table 2 Prognostic factors for CSS Abbreviations: CSS, cancer-specific survival; HR, hazard ratio; NX, unknown N stage; PSM, propensity score matching. |
Primary tumor surgery and clinical outcome
Before PSM, the median CSS time of the whole cohort was 9 months (range 0–60 months). The median CSS time for the surgery group was 16 months, while it was 9 months for the nonsurgery group. The 1- and 3-year CSS rates of the surgery group were 59.64% and 13.88%, respectively, which were significantly better than those of the nonsurgery group, namely 34.03% and 4.22%. After PSM, the 1- and 3-year CSS rates of the nonsurgery group changed slightly (35.46% and 3.66%) and were still prominently lower than those of the surgery group, as shown in Table 3.
  | Table 3 CSS rate and median CSS before and after PSM Abbreviations: CSS, cancer-specific survival; PSM, propensity score matching. |
Subgroup analysis
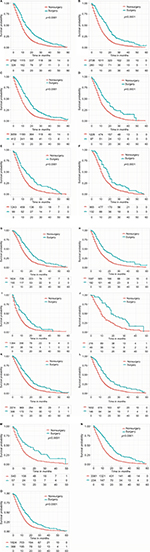
We also performed a subgroup analysis to confirm which patients can benefit from the surgery (Figure S1). Improved survival outcomes can be noticed for the surgery group regardless of sex, tumor location, tumor size, N stage, and age at diagnosis.
Discussion
Nowadays, systemic therapy is the first step in treating stage III PC.14,15 However, before 1997, fluorouracil (5-FU) was the only option and had no obvious superiority over best-supportive care.16 In the year 1997, Burris et al performed a pivotal study to compare gemcitabine with 5-FU in advanced PC. As a milestone event, the study demonstrated the powerful efficacy of gemcitabine and established gemcitabine monotherapy as the new standard option for advanced PC.16,17 Motivated by this study, a lot of clinical trials have been carried out trying to find better gemcitabine-based combination options, but none succeeded. In 2007, PA.3 trial showed important enhanced survival comparing gemcitabine plus erlotinib versus gemcitabine monotherapy (p=0.023) in patients with stage III PC. Even so, this combination has not replaced gemcitabine as it contributes higher costs, more side effects, and selected improvement in survival.18
In the past, contradictory opinions existed regarding surgical intervention for stage III PC among different national or institutional standards. However, with the improvement of aggressive chemoradiation and surgical procedure, a substantial number of patients can achieve better local tumor control or tumor downstaging after 4–6 months of systemic therapy.19 Therefore, transitioning to perform surgical intervention becomes an alternative in such patients with good performance, especially if side effects from chemoradiotherapy are important obstacles to continued initial therapy.20–25 Some meta-analyses have shown that surgical resection for stage III PC can be performed with safety and got similar benefit in patients without vein infiltration (superior mesenteric vein or portal vein).26–28 A recent observational study included 101 patients with LAPC receiving a median of six cycles of induction FOLFIRINOX (5-FU, oxaliplatin, irinotecan, and leucovorin). Nearly one-third of patients downstaged to resectable tumors and underwent surgery.29 Even with arterial resection, surgery is also feasible from a technological perspective. Hence, patients with stage III PC should not be excluded for surgical resection.
Moreover, surgical intervention is attractive to both patients and their doctors as it removes the single measurable tumor. Many clinical trials have been performed to assess surgical intervention for stage III PC. Dholakia et al identified 50 patients with BRPC who received chemoradiotherapy from 2007 to 2012. Of this population, 29 patients (58%) who had better performance status underwent resection with an increased median overall survival compared with unresected patients (22.9 months versus 13.0 months, p<0.001).30 Several uncontrolled retrospective studies and two meta-analyses also found tumor surgery to have a favorable prognostic impact.31–33 In the first meta-analysis based on data from 26 studies, Mollberg et al reported that pancreatectomy with arterial resection was associated with better survival in comparison to patients who did not have arterial resection.31 The second meta-analysis compared resection versus palliative treatments for LAPC and found that pancreatic resection was associated with increased survival and decreased costs, despite the evidence quality being low.32 Nakamura et al reviewed 80 patients with LAPC who underwent distal pancreatectomy with en bloc celiac axis resection at a single institution. The 1-, 2-, and 5-year overall survivals were, respectively, 81.1%, 56.9%, and 32.7%, and the median survival time was 30.9 months.33
However, conflicting results were reported in a recent study based on data from the American College of Surgeons-National Surgical Quality Improvement Program Pancreatectomy Demonstration Project. In this study, Beane et al found a 10% postoperative acute kidney injury and a 10% operative mortality rate in 20 patients who underwent distal pancreatectomy with celiac axis resection. The authors suggested that the increased risks should be disclosed carefully before considering a modified Appleby procedure for the patients with LAPC.34 The data from Amano et al also suggested that resection is helpful only when margin-negative resection is performed for selected LAPC.35 As these trials were small and poorly accurate, we designed this population-based analysis using PSM. Our SEER data analysis provides evidence of a clear association of surgery in stage III PC with increased CSS in a large patient cohort. Both univariate analysis and multivariable analysis reveal a benefit of surgery, no matter whether before or after PSM. In our study, 1-year CSS rate was larger for the surgery group versus the nonsurgery group (59.64% versus 34.03%), as was the 3-year CSS rate (13.88% versus 4.22%; p<0.001). Surgery improved the median CSS when compared with the nonsurgery group (16 months versus 9 months, p<0.001). Although both 1- and 3-year survival rates and the median survival time in our study were lower than the studies mentioned above, our study is more convincing and reliable as we included patients treated in the “real-world” setting rather than the highly selected patients included in these clinical trials. As far as we know, this is the largest study evaluating the role of surgical resection in patients with stage III PC. Also, we observe that surgery is associated with improved CSS among a variety of subgroups regardless of sex, tumor location, tumor size, N stage, and age at diagnosis.
A limitation of our study is that we excluded patients treated with IRE because of the small size of the cohort. However, more and more studies have been investigating IRE safety and efficacy.36–39 In a 2017 systematic review of 10 studies reporting on the use of IRE to treat 102 patients with advanced PC, the median recurrence-free survival was 2.7–12.4 months and the median overall survival was 7–23 months postoperatively, with less postprocedural complications.40
Our present study is not without limitations. Although we applied for the SEER Radiation Therapy and Chemotherapy Information, the detailed regimens and the timing of chemoradiotherapy are yet unknown. However, given the narrow and contemporary scope of this analysis (2004–2014), patients are more likely to be treated with FOLFIRINOX or gemcitabine-based chemotherapeutic regimens. Furthermore, given the limited resolution of the SEER Registry, we cannot subdivide stage III PC into BRPC and LAPC. However, both multivariable analysis and additional PSM were carried out to reduce potential confounding, with a strict statistical significance level (0.001). Additionally, comorbidity information was not included in the SEER data, which may increase data on surgery-related complications or death, thus changing the survival benefits.
Conclusion
In conclusion, our present SEER data analysis provides evidence that patients with stage III PC who are treated with surgical resection have a survival benefit. Prospective randomized trials are needed to confirm this result and further efforts are required to address patient selection.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 81573752).
Author contributions
All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. | ||
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. | ||
Malvezzi M, Carioli G, Bertuccio P, et al. European cancer mortality predictions for the year 2017, with focus on lung cancer. Ann Oncol. 2017;28(5):1117–1123. | ||
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(8):1028–1061. | ||
Younan G, Tsai S, Evans DB, Christians KK. Techniques of vascular resection and reconstruction in pancreatic cancer. Surg Clin North Am. 2016;96(6):1351–1370. | ||
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. | ||
Denecke T, Andreou A, Podrabsky P, et al. Distal pancreatectomy with en bloc resection of the celiac trunk for extended pancreatic tumor disease: an interdisciplinary approach. Cardiovasc Intervent Radiol. 2011;34(5):1058–1064. | ||
Kondo S, Katoh H, Hirano S, et al. Results of radical distal pancreatectomy with en bloc resection of the celiac artery for locally advanced cancer of the pancreatic body. Langenbecks Arch Surg. 2003;388(2):101–106. | ||
Klompmaker S, de Rooij T, Korteweg JJ, et al. Systematic review of outcomes after distal pancreatectomy with coeliac axis resection for locally advanced pancreatic cancer. Br J Surg. 2016;103(8):941–949. | ||
Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford). 2016;18(10):835–842. | ||
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. | ||
Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. | ||
Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837–2849. | ||
Tsai S, Evans DB. Therapeutic advances in localized pancreatic cancer. JAMA Surg. 2016;151(9):862–868. | ||
Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist. 2014;19(3):266–274. | ||
Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326(7):455–465. | ||
Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. | ||
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. | ||
Kleeff J, Friess H, Buchler MW. Neoadjuvant therapy for pancreatic cancer. Br J Surg. 2007;94(3):261–262. | ||
Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18(5):543–548. | ||
Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol. 2013;108(4):236–241. | ||
Sherman WH, Chu K, Chabot J, et al. Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by gemcitabine and capecitabine/radiation therapy and surgery in locally advanced, unresectable pancreatic adenocarcinoma. Cancer. 2015;121(5):673–680. | ||
Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA Surg. 2016;151(8):e161137. | ||
Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–810. | ||
Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol. 2012;19(5):1644–1662. | ||
Yu XZ, Li J, Fu DL, et al. Benefit from synchronous portal-superior mesenteric vein resection during pancreaticoduodenectomy for cancer: a meta-analysis. Eur J Surg Oncol. 2014;40(4):371–378. | ||
Zhou Y, Zhang Z, Liu Y, Li B, Xu D. Pancreatectomy combined with superior mesenteric vein-portal vein resection for pancreatic cancer: a meta-analysis. World J Surg. 2012;36(4):884–891. | ||
Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg. 2016;103(3):179–191. | ||
Sadot E, Doussot A, O’Reilly EM, et al. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22(11):3512–3521. | ||
Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor-vessel relationships. J Radiat Oncol. 2013;2(4):413–425. | ||
Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. 2011;254(6):882–893. | ||
Gurusamy KS, Kumar S, Davidson BR, Fusai G. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev. 2014;2:CD010244. | ||
Nakamura T, Hirano S, Noji T, et al. Distal pancreatectomy with en Bloc Celiac axis resection (Modified Appleby Procedure) for locally advanced pancreatic body cancer: a single-center review of 80 consecutive patients. Ann Surg Oncol. 2016;23(Suppl 5):969–975. | ||
Beane JD, House MG, Pitt SC, et al. Distal pancreatectomy with celiac axis resection: what are the added risks? HPB (Oxford). 2015;17(9):777–784. | ||
Amano H, Miura F, Toyota N, et al. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg. 2009;16(6):850–857. | ||
Orcutt S, Kis B, Malafa M. Case report: irreversible electroporation for locally advanced pancreatic cancer. Int J Surg Case Rep. 2017;40:54–57. | ||
Lin M, Liang S, Wang X, et al. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin Oncol. 2017;143(12):2607–2618. | ||
Moir J, White SA, French JJ, Littler P, Manas DM. Systematic review of irreversible electroporation in the treatment of advanced pancreatic cancer. Eur J Surg Oncol. 2014;40(12):1598–1604. | ||
Martin RC 2nd, McFarland K, Ellis S, Velanovich V. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol. 2013;20(Suppl 3):S443–S449. | ||
Ansari D, Kristoffersson S, Andersson R, Bergenfeldt M. The role of irreversible electroporation (IRE) for locally advanced pancreatic cancer: a systematic review of safety and efficacy. Scand J Gastroenterol. 2017;52(11):1165–1171. |
Supplementary material
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.