Back to Journals » Clinical and Experimental Gastroenterology » Volume 12
Beneficial effects of Saccharomyces boulardii CNCM I-745 on clinical disorders associated with intestinal barrier disruption
Authors Terciolo C, Dapoigny M, Andre F
Received 27 July 2018
Accepted for publication 13 November 2018
Published 11 February 2019 Volume 2019:12 Pages 67—82
DOI https://doi.org/10.2147/CEG.S181590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Andreas M. Kaiser
Chloe Terciolo,1,2 Michel Dapoigny,3 Frederic Andre4
1INRA, UMR 1331 Toxalim, Research Center in Food Toxicology, F-31027 Toulouse, France; 2Aix-Marseille Université, INSERM, UMR 911, CRO2, Marseille, France; 3Médecine Digestive, CHU Estaing, CHU Clermont-Ferrand, Université Clermont Auvergne, INSERM UMR 1107, Neuro-Dol, Clermont-Ferrand, France; 4Centre de Recherche en Cancérologie de Marseille (CRCM), INSERM U1068, CNRS UMR 7258, Aix-Marseille Université and Institut Paoli-Calmettes, Parc scientifique et technologique de Luminy, Marseille, France
Abstract: Intestinal barrier defects lead to “leaky gut syndrome”, defined as an increase in intestinal permeability that allows the passage of luminal content into intestinal tissue and the bloodstream. Such a compromised intestinal barrier is the main factor underlying the pathogenesis of inflammatory bowel disease, but also commonly occurs in various systemic diseases such as viral infections and metabolic syndrome. The non-pathogenic yeast Saccharomyces boulardii CNCM I-745 has demonstrated its effectiveness as a probiotic in the prevention and treatment of antibiotic-associated, infectious and functional diarrhea. Via multiple mechanisms of action implicated in intestinal barrier function, S. boulardii has beneficial effects on altered intestinal microbiota and epithelial barrier defects in different pathologies. The well-studied probiotic yeast S. boulardii plays a crucial role in the preservation and/or restoration of intestinal barrier function in multiple disorders. This could be of major interest in diseases characterized by alterations in intestinal barrier function.
Keywords: Saccharomyces boulardii CNCM I-745, apical junctional complex, leaky gut syndrome, intestinal barrier function, intestinal permeability, tight junctions
Introduction
In the human body, the gastrointestinal tract represents the largest surface area exposed to the external environment. The intestinal epithelium has a dual function, acting on one hand as an exchange surface between luminal nutrients, molecules produced by the intestinal microbiota and intestinal tissue, and on the other hand as a barrier to prevent the entry of and protect the tissue from external harmful substances such as pathogenic toxins and antigens.1 This barrier is formed by the interconnection of epithelial cells via the apical junctional complex and desmosomes.2 Its disruption leads to an increased intestinal permeability that facilitates translocation of luminal contents into the intestinal tissue and bloodstream, a situation referred to as “leaky gut syndrome”.3 A significant body of evidence indicates that such disruption plays a crucial role in intestinal diseases such as inflammatory bowel diseases (IBDs) and irritable bowel syndrome (IBS), but more research evidence highlights that it also occurs in certain systemic diseases, including type 2 diabetes, obesity and HIV infection. The maintenance of intestinal barrier integrity is essential to the preservation of gastrointestinal homeostasis and could be of major importance in the treatment of various diseases and in the prevention of severe complications.3,4 The lack of published studies on the beneficial effects of other strains of Saccharomyces boulardii prompted us to focus on a specific strain of Saccharomyces boulardii, CNCM I-745 (S. boulardii). We summarize the clinical effects of S. boulardii on intestinal barrier function in gastrointestinal and systemic diseases, followed by a discussion of the mechanisms by which S. boulardii modulates intestinal permeability.
Intestinal barrier function
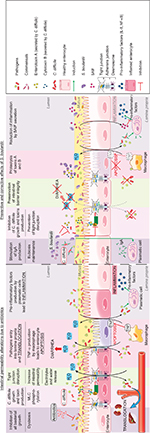
The intestinal epithelium functions as a barrier, preventing and controlling the penetration of food and bacterial antigens into the tissue. At the same time, it has to be permeable to allow the translocation of nutrients, electrolytes and water. This intestinal permeability allows the exchange of solutes and fluids between the intestinal lumen and tissue5 and is mediated by two pathways: the transcellular pathway, which is generally associated with the transport of solutes by specific transporters present in the cell membrane, and the paracellular pathway, which is associated with the transport of small molecules in the space between epithelial cells.6 Permeability can be assessed by different techniques in vitro and in vivo, in animal and human studies, respectively. In vitro assessments include the measurement of transepithelial resistance (TER) or macromolecular flux in Ussing chambers, morphological measurements of tight junction (TJ) components, and measurement of the polyethylene glycol profile to characterize pore pathways. In vivo approaches consist of the oral ingestion of probes (lactulose/mannitol) followed by their measurement in urinary excretion.5 The integrity of the intestinal barrier is essential for intestinal homeostasis and is maintained by the presence and correct functioning of several components (Figure 1A).
Intestinal microbiota
Bacteria, fungi, archaea, viruses and protozoa compose the gut microbiota and inhabit the gastrointestinal tract. Bacteria are the major component of the human microbiota, with more than 400 species hosted by the human gut.7 Gene sequencing data show that three phyla predominate in the human microbiota (Bacteroidetes, Firmicutes and Actinobacteria), with a large diversity in bacterial species but functional homogeneity.8 Freter et al showed that bacterial competition for nutrients and adhesion sites limits the colonization by external bacteria. Indeed, exogenous bacteria must have an ecological advantage, compared to residing bacteria, to occupy an ecological niche,9 which reveals a barrier effect induced by microbiota. Bacteria also act as modulators of intestinal permeability. Escherichia coli Nissle 1917 increases the TER by stimulating the TJ protein zonula occludens-2 (ZO-2). By contrast, E. coli C25 increases intestinal permeability.10,11 In addition, the microbiota protects intestinal tissue by secreting into the intestinal lumen bacterial toxins, known as bacteriocins, that inhibit the growth of other bacteria, thus reducing bacterial invasion.12 In addition, some bacteria transform primary into secondary biliary acids, which protect against some pathogens.13 Short-chain fatty acids (SCFAs), produced by microbial fermentation, promote intestinal barrier function by enhancing TJ assembly and the establishment of the TER.14 Together, these findings demonstrate the active role played by commensal bacteria in intestinal barrier integrity.
Mucus
The mucus is both a physical and a chemical barrier which spatially delineates the lumen from the epithelium by the presence of antimicrobial peptides and trefoil peptides.15 The gastrointestinal mucus is mainly composed of highly glycosylated proteins called mucins, synthesized and secreted by goblet cells into the lumen, but also of carbohydrates, lipids and water. In humans, the major component is mucin-2, followed by the trefoil factor-3.15,16 These components confer the viscoelastic properties displayed by mucus, and in humans allow the formation of a two-layered mucus coat several hundreds of micrometers thick.16,17
Epithelium
Although single layered, the epithelium is the physical living boundary between exterior and interior. The passage of molecules and ions across the epithelium and the cohesion between cells are regulated by the presence of two types of junctional complexes: apical TJs and below them adherens junctions (AJs).2 The TJs are multiprotein complexes that seal cells together, maintaining membrane polarity and regulating the selective paracellular permeability of ions, nutrients and water. Transmembrane proteins (occludins, claudins, junctional adhesion molecules and tricellulin) link to actin microfilaments by cytoplasmic proteins called zonula occludens (ZO-1, ZO-2 and ZO-3). Every component contributes towards the assembly and/or the maintenance of the TJ and plays a role in the regulation of intestinal barrier function.6,18,19 AJs are localized below the TJs and participate in both the integrity of the epithelial layer and cell–cell communication. AJs consist of calcium-dependent transmembrane proteins, called cadherins, which interact via their C-terminal domain with scaffold proteins, p120-catenin and β-catenin. In the intestinal epithelium, the major component is E-cadherin. The anchorage of the E-cadherin/catenins complex with actin cytoskeleton is mediated by α-catenin.20 Within the epithelial cells, the tightness of both TJs and AJs is regulated through signal transduction proteins, such as myosin light chain kinase (MLCK), RhoGTPases, protein kinase C (PKC) and mitogen-activated protein kinase (MAPK), in response to various stimuli.21 The expression level of junctional proteins is also controlled by mechanisms including transcriptional and post-transcriptional regulation, transport or recycling at the cell membrane.22
Lamina propria
Below the intestinal epithelium, the lamina propria contains immune cells and contributes toward protection against potentially harmful molecules or pathogens while tolerating the presence of commensal bacteria. One immune response is demonstrated by intestine-specific IgA, which is secreted by B cells and binds to microorganisms, forming a complex, which is cleared by bowel movement.3 In case of infections, kinase signaling cascades are activated, which induce nuclear factor-κB (NF-κB), leading to the secretion of pro-inflammatory mediators including IL-6, IL-8, IL-12 and TNF-α.23 The secretion of anti-inflammatory cytokines, such as IL-10, by T lymphocytes is crucial to maintain the balance between tolerance and immune response.6
Disruption of intestinal barrier function
The entry of bacteria, food contaminants and luminal antigens through damaged intestinal epithelial cells (IECs), dendritic cells or microfold cells poses a risk to the maintenance of intestinal integrity (Figure 1B).24 Intestinal barrier disruption induces a systemic inflammatory response and causes increased permeability, functional impairment and disease.2 Intestinal alkaline phosphatases (IAPs) protect intestinal tissue against luminal endotoxins such as lipopolysaccharide (LPS) by dephosphorylation, resulting in a reduction of the inflammatory response.25 Loss of intestinal integrity with increased permeability plays a major role in the pathogenesis of multiple intestinal and extraintestinal disorders (eg, IBD, IBS, viral or bacterial infections, obesity, type 2 diabetes and non-alcoholic steatohepatitis). Increased intestinal permeability can also be iatrogenic following treatment with antibiotics or NSAIDs, leading to leaky flux diarrhea. Different factors can increase intestinal permeability, including genetic alterations or abnormal regulation of TJ function, dysbiosis of microbiota and chronic inflammation.26 Such TJ alterations can lead to the relocalization of TJ proteins or to their disruption by phosphorylation state regulation. For example, the phosphorylation of occludin is essential for a functional TJ complex.27 TJ alterations also include indirect mechanisms implicating actin cytoskeleton reorganization.22 Such actin cytoskeleton alterations occur through myosin light chain (MLC) phosphorylation by MLCK, alteration in RhoGTPase activity or PKC activation. In physiological conditions, MLCK regulates TJ dynamics but in pathological conditions, secretion of pro-inflammatory cytokines can induce MLCK activation and disruption of TJs.21 For example, interferon-γ or TNF-α was found to induce protein relocation and MLC phosphorylation, which facilitated actin contraction and caused the opening of TJs and extension of the intercellular space.28
Modulation of intestinal epithelial barrier function by the probiotic Saccharomyces boulardii CNCM I-745
Definition and properties of S. boulardii CNCM I-745
Saccharomyces boulardii CNCM I-745 (S. boulardii) is a non-pathogenic yeast discovered in 1923 by a French microbiologist in Indochina. It has since been widely used as probiotic in the prevention and treatment of gastrointestinal disorders. As a yeast, S. boulardii is distinct from bacterial probiotics, in particular because of its intrinsic resistance to antibiotic treatment.29 In addition, S. boulardii can adapt to and survive in the gastrointestinal tract owing to its ability to grow at 37°C, and its resistance to low gastric pH30,31 and to bile acids.30,32,33 Once in the gastrointestinal tract, S. boulardii reaches a maximum concentration within 2 days and is cleared in the stools within 3–5 days after oral administration.32
Intestinal permeability alterations due to enteric pathogens and corrective effects of S. boulardii
The IECs act as a physical barrier via TJs to protect tissue from invasion by pathogens. The bacterial recognition occurs by pattern recognition receptors such as Toll-like receptors (TLRs).34 The TLRs are type I membrane proteins that protect mucosal and commensal homeostasis but also induce adaptive immune signaling in response to bacterial invasion.35 They bind a variety of bacterial lipid structures and bacterial cell wall components. For example, TLR-2 signaling enhances the TJ-associated barrier through activation of the PI3K/Akt pathway.34,36 During invasion, enteric pathogens (bacteria and viruses) use TJs as receptors to attach to the cell membrane in order to become internalized, or they disrupt the TJ first before penetrating into the tissue. Alterations in TJs are implicated in diarrhea through a “leaky flux” mechanism, which allows the passage of ions and water toward the lumen after the impairment of intestinal barrier integrity. The S. boulardii CNCM I-745 strain is registered in many countries for the treatment of diarrhea in adults and children. Its efficacy has been proven through many randomized clinical trials and its use recommended by numerous scientific societies.37 S. boulardii owes its clinical efficacy to a wide variety of actions counteracting numerous pathogen-induced deleterious effects. The modes of action of S. boulardii can be summarized as follows:38 1) luminal action, referring to the action of S. boulardii within the lumen: antitoxinic effect, notably against cholera toxin and E. coli LPS, antimicrobial activity, modulation of intestinal flora and metabolic activity; 2) trophic action at the villi, ie, secretion of digestion-enhancing enzymes and induction of host digestive enzymes39 and 3) mucosal action, referring to the action of S. boulardii deeper within the mucosa, including anti-inflammatory activity. Altogether, the various modes of action of S. boulardii act in concert to counteract infections and to support barrier function and regeneration of damaged intestinal tissue. A broad spectrum of non-clinical data supports the beneficial effects of S. boulardii counteracting the pathogenicity of various pathogens (Table 1).
E. coli
Enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC) are pathogenic strains of attaching and effacing bacteria. The interaction between bacterial toxins and IECs leads to intestinal barrier disruption and intestinal permeability alterations.40 This type of invasion (for E. coli strains HB101 and LF82) is followed by alterations in electrolyte transport, chloride secretion and diarrhea.41,42 In various intestinal cell models, pathogenic effector proteins delivered into enterocytes have caused TJ disruption by MLCK and PKC activation, and increased intestinal permeability.21 EPEC and EHEC cause gastroenteritis, with different mechanisms being responsible for the increased intestinal permeability. More precisely, EPEC infection stimulates phosphorylation of MLC by MLCK, inducing TJ disruption and the stimulation of cytoskeletal contraction. This event causes a drop in TER and thus increased intestinal permeability. EPEC infection is also associated with an alteration of occludin distribution (shift from TJ to cytosol) by a dephosphorylation of this protein, essential to this localization in the TJ complex.40 In contrast to EPEC, EHEC infection is promoted by an alteration in ZO-1 distribution.43 In EHEC O157:H7, the regulation of the dynamic of the actin cytoskeleton is also impaired in the paracellular permeability via a PKC-dependent mechanism which inactivates the RhoA/Rac/Cdc42 pathway and in turn increases actomyosin contractility.44 Furthermore, E. coli O157:H7 strain has been shown to induce secretion of pro-inflammatory cytokines (IL-8) mediated by MAPK and NF-κB activation.45 IECs treated with S. boulardii before infection by EPEC strain E2348/69 display a reduced level of secreted pro-inflammatory cytokines and a preserved TJ structure due to the abolition of MLC phosphorylation.4 Administration of S. boulardii in mice reduced C. rodentium strain DBS100-induced colitis by a decrease in intestinal permeability and a reduction in chloride secretion and mannitol flow.46 S. boulardii also modulates bacterial attachment of 055B5 E. coli to enterocytes by secreting a 63 kDa alkaline phosphatase.47 Endotoxins such as LPS are dephosphorylated by this phosphatase, causing a reduction in bacterial attachment and a 60% decrease in the TNF-α level in the bloodstream.47
Shigella
Invasion by Shigella requires two events that decrease barrier function: TJ disruption and E-cadherin intracellular domain cleavage.48 An increased intestinal permeability facilitates Shigella invasion into IECs, leading to bacterial dysentery. In T84 cells, modification of the phosphorylation status of occludin and reduction in claudin-1 expression mediated TJ disruption.27 In both in vitro and in vivo models, S. boulardii has shown to restore partially this claudin-1 expression and the barrier integrity. It also exerted anti-inflammatory effects by inhibiting IL-8 secretion mediated by NF-κB and ERK1/2 phosphorylation.49
Salmonella
Salmonella species belong to the family of proteobacteria. The subtype Salmonella enterica serovar Typhimurium is a major cause of gastroenteritis. Translocation of effector proteins is a key event in S. enterica Typhimurium invasion. Translocation is caused by decreased expression of ZO-1, modified phosphorylation status of occludin and activation of RhoGTPases. The resulting alteration in TJ and AJ localization causes a drop in TER as well as an increase in intestinal permeability, allowing bacterial invasion and amplified diarrhea.28 Invasion by S. enterica Typhimurium also induces IL-8 secretion by NF-κB activation. S. boulardii protects mice during S. enterica Typhimurium infection and preserves epithelial barrier function in IECs. It was found to interfere with signaling pathways implicated in the invasion, in particular by reducing activation of RhoGTPases, which are implicated in actin cytoskeleton rearrangement and modulation of TJ proteins. It also decreased activation of MAPKs and reduced the level of inflammatory cytokines while up-regulating the secretion of anti-inflammatory cytokines such as IL-10 and TGF-β.50,51 In addition, S. boulardii adheres to S. enterica Typhimurium and, thus, prevents contact of the pathogen with the epithelial surface, decreasing the bacterial translocation to almost 50%.51 Adhesion of S. enterica Typhimurium to yeast cells is mediated by type I fimbriae and this interaction uses mannose from yeast cell walls.52 Furthermore, S. boulardii trapped S. enterica Typhimurium within the intestinal lumen, thereby deviating its trajectory and modifying its motility, thus increasing the fecal elimination of the pathogenic bacteria. These two mechanisms limit bacterial invasion.53,54 Intestinal epithelial barrier alterations have only been studied in animal models and need to be confirmed in humans.
Vibrio cholerae
V. cholerae is an enteropathogen responsible for cholera epidemics. Two toxins are produced: zonula occludens toxin, which alters the barrier function of TJs, and the 84 kDa enterotoxin cholera toxin, which modulates membrane channels. Cyclic adenosine monophosphate (cAMP) stimulation resulting from toxin catalytic activity induces chloride secretion into the intestinal lumen and causes hydroelectrolytic diarrhea.55 Cell adhesion leakage allows the diffusion of water and ions into the lumen, causing diarrhea. A pre-incubation with S. boulardii was shown to dose-dependently decrease the cAMP level by up to 50% in male rats and rat epithelial intestinal cell lines (IRD 98 and IEC 17). The preventive role of S. boulardii is mediated by secretion of a 120 kDa protein which interferes with the cAMP-dependent induction of chloride secretion and decreases diarrhea caused by V. cholerae.56,57 Pretreatment by S. boulardii was shown to reduce intestinal damage and lesions in a rat model of V. cholerae infection.58
Rotavirus
Rotavirus infection is associated with a drastic reduction in TER (from 325 to 22 Ω.cm2 in 24 hours).34 An increase in intestinal permeability stems from a deregulated secretion and absorption of ions. Thus, noxious substances can penetrate into the intestinal tissue, inducing diarrhea and morbidity.35 Rotavirus produces the enterotoxin non-structural protein-4 (NSP-4), which alters the localization of TJ proteins (ZO-1, occludin and claudin-3), leading to the disruption of intestinal barrier integrity and blockade of epithelial barrier formation in MDCK cells.59 In addition, NSP-4 alters redox balance by enhancing ROS production in the mitochondrial compartment of enterocytes, with the potential to cause damage to intestinal barrier integrity. The generation of ROS also induces chloride secretion and diarrhea. In a study with infected IECs, S. boulardii restored glutathione levels, which in turn inhibited ROS production, leading to a decrease in intestinal permeability.60
Intestinal permeability alterations due to antibiotics and preventive and corrective effects of S. boulardii
While antibiotic treatment does result in the destruction of pathogens, it also destroys commensal bacteria, leading to osmotic diarrhea and the promotion of intestinal permeability, associated with potential bacterial and viral translocation. The S. boulardii CNCM I-745 strain has proven efficient in the prevention of such antibiotic-induced diarrhea, independent of the specific antibiotic (Figure 2).39,61 In addition, by reducing the occurrence of diarrhea, S. boulardii is an effective adjunctive therapy for H. pylori eradication during antibiotic treatment.62 Owing to its multiple modes of action, it is also effective in the treatment of Clostridium difficile infection and in the reduction of recurrence.39 Finally, concomitant treatment by S. boulardii was recently shown to prevent the huge shifts in the microbiome composition observed following use of antibiotics alone.63 Infection by C. difficile, responsible for diarrhea or even pseudomembranous colitis, can occur further to antibiotic treatment (Figure 2). It is associated with frequent stools of liquid consistency during or after the antibiotic treatment, and sometimes a fever. Some evidence suggests a higher incidence of C. difficile infections among older adults, in particular those with multiple associated pathologies.64 This kind of infectious diarrhea has a high risk of relapse even in the absence of antibiotic retreatment. C. difficile produces two toxins (A and B; enterotoxin and cytotoxin, respectively) which alter the assembly and maturation of TJs.65 Such TJ alterations lead to a reduction in TER and an increase in intestinal permeability, a key element in the pathological progression, as reflected by antibiotic-associated diarrhea (30% of cases) or pseudomembranous diarrhea (95%).66,67 In hamsters and gnotobiotic mice, S. boulardii reduced the rate of clindamycin-induced mortality caused by pseudomembranous colitis or C. difficile infection. The reduction in mortality was highest when S. boulardii was administered preventively.68–70 These effects were correlated with a reduction in the number of C. difficile bacteria as well as a lowered toxin expression.71 Moreover, S. boulardii was found to increase the concentration of IgAs directed against bacteria by 57%,72 induce proteolysis of toxins A and B, and inhibit the interaction with enterocytes by secretion of a 54 kDa serine protease.73,74 This serine protease blocks JNK and ERK1/2 kinase activation, inhibits MLC phosphorylation and prevents TJ disruption. S. boulardii secretes another factor, named S. boulardii anti-inflammatory factor (SAIF; <1 kDa), which exerts anti-inflammatory effects by blocking NF-κB activation and IL-8 secretion mediated by IL-1β.75 Altogether, S. boulardii-secreted factors exert anti-inflammatory and barrier-protective/restorative effects.
Gut response during childhood
Children’s gut responses to noxious agents are very different from adults’ because of the immaturity of the intestinal epithelial barrier in children. The development of the intestinal epithelial barrier occurs in utero and postnatally with the formation of AJ complexes, and then with the development of physical and chemical barriers (eg, defensins, lysozymes and mucins). The development in utero and the maturation postnatally are under the control of multiple factors, especially the development of the microbiota.76 The first colonizers create a new environment that promotes colonization, such as Bacteroides, Clostridium and Bifidobacterium.77 For many years, it was thought that uterine life was sterile and that newborns acquired a microbiota during delivery. In the past decade, investigations have shown bacterial transmission through the placental barrier and have detected bacteria in the placenta, umbilical cord blood, amniotic fluid and fetal membranes.78–81 These findings suggest that the placenta is not sterile and that mother-to-child efflux of commensal bacteria exists, which influences both the microbiota and the immune system in utero. It is currently held that the maturation of microbiota begins during delivery and ends at 3 years old, at which timepoint it achieves the adult characteristics. During the maturation stage, several environmental factors may influence and affect the establishment and diversity of intestinal microbiota, including gestational age, mode of delivery (vaginal or cesarean), diet (breast milk or formula) and antibiotic treatment.82 It has been reported that birth mode influences the level of bacterial species in the first 6 months until complete maturation of the microbiota.83 Yassour et al described the influence of birth mode on the diversity and maturation of microbiota in 39 children aged 3–36 months. They observed multiple similarities in the composition of microbiota over time between birth modes (succession of bacterial populations in the gut communities); they also found a distinct microbial signature of Bacteroides in the first 6 months in babies born by cesarean section compared to vaginal delivery.83 This can be explained by the fact that cesarean delivery transfers a large part of the commensal bacteria from the skin microbiota instead of the vaginal and fecal microbiota. Cesarean delivery increases by 20% the risk of acute gastroenteritis, and this risk is increased by 62–78% when cesarean section is combined with preterm delivery and exclusive formula feeding.84
Diet is another major factor that influences the development of the microbiota. Investigations have shown that breastfeeding influences the growth of microbes and improves the intestinal barrier.85 Components of the breast milk may improve the epithelial intestinal barrier and stimulate the immune system.86
Other substances, such as prebiotic and probiotic compounds, may stimulate the production of various immunoglobulins (IgG, IgM and secretory IgA).84 The development of a mature immune system is correlated with the development of the microbiota.87 The SCFAs produced by microbes can affect the inflammatory response by binding to and stimulating G-protein coupled receptor-43 (GPR43).88 The presence of Bacteroides fragilis in commensal bacteria is capable of suppressing inflammation by down-regulation of IL-17. It can also convert CD4+ T cells in regulatory T cells, which produce an anti-inflammatory cytokine (IL-10).89 Exposure to postnatal antibiotics, total parental nutrition and delay in breastfeeding are factors responsible for the impairment of intestinal colonization and favor the overgrowth of pathological microorganisms.90–93 Disruption of the gut barrier accompanied by altered microbiota and/or intestinal inflammation with impaired immune-regulatory mechanisms has been shown in many gastrointestinal diseases with onset in childhood (eg, celiac disease,94 IBD and infectious diarrhea) but also in numerous extraintestinal pathological, non-communicable diseases (eg, asthma, allergy, eczema, diabetes and cystic fibrosis with cirrhosis).80,95–100 Furthermore, several studies have shown that the gut microbiota has a role in the development of non-communicable diseases. Some bacteria are correlated with the onset of allergy, eczema or type 1 diabetes while others protect against these pathologies. For instance, Lactobacilli, Bacteroidetes, Bifidobacteria and Proteobacteria taxa are decreased in children who develop allergy.88,96,101 With regard to non-communicable diseases, meta-analyses of clinical trials using probiotics for allergy prevention demonstrate a reduced incidence of eczema, but mechanistic pathways to understand how probiotics mediate these beneficial effects are still to be investigated. Furthermore, no studies have analyzed the effectiveness of S. boulardii in non-communicable diseases.95
Only a few studies have specifically focused on the effects of S. boulardii on gut permeability in children. One important difference between bacteria and yeast is their cell wall composition, which is responsible for the modulation of the mucosal immune response.29 In children with acute gastroenteritis, Ozkan et al showed that blood levels of IgA and of CD8 lymphocytes increased at day 7 while both C-reactive protein and complement C4 decreased in reaction to S. boulardii treatment.102 These modifications in blood immune parameters are in line with the findings of Caetano et al in adults.103
Leaky gut in gastrointestinal diseases
Luminal contents, diarrhea, dysbiosis and allergy can cause damage to IECs and lead to alterations in cell–cell junctions and finally to impaired intestinal permeability, known as the “leaky gut”. The leaky gut allows molecules, drugs or toxins to penetrate into the bloodstream and create chronic inflammation and further increases in intestinal permeability. This condition is responsible for many complications in gastrointestinal disorders, such as diarrhea or IBS, but also in non-gastrointestinal diseases. For example, the leaky gut observed in HIV patients can lead to cardiovascular disease or chronic kidney disease. In obesity, intestinal permeability can induce visceral obesity and/or type 2 diabetes.27 More precisely, alterations in intestinal function are associated with the pathogenicity of type 2 diabetes. Several causes are responsible for the decrease in intestinal barrier in type 2 diabetes. Among them, changes in the dietary composition (from high carbohydrates to western diet) are important factors which allow a rapid modification of the microbiota community.104 The nutritional imbalance along with changes in microbiota composition/activity contributes to the increase in intestinal permeability. The paracellular permeability allows the passage of bacterial lipoproteins such as LPS and their binding to TLR-4. Secretion of pro-inflammatory cytokines is induced after the activation of TLRs. The inflammatory cascade promotes the phosphorylation of the receptor insulin substrate and impairs insulin signaling.105
Intestinal permeability is also believed to play a crucial role in some liver diseases, such as alcoholic or non-alcoholic steatohepatitis, cirrhosis and hepatocellular carcinoma.106,107
IBDs
IBDs are multifactorial diseases with two main subtypes: Crohn’s disease (CD) and ulcerative colitis (UC). The etiology is still unknown but IBDs are driven by an immune activation against the host microbiota in genetically predisposed patients.108 IBDs have a prevalence of 1/1,000 and clinical morphological features that differ between CD and UC.109 The microbiota (composition and diversity) and environmental factors are important in the pathogenesis of either IBD subtype.110 While smoking increases the frequency of CD relapse, it shows no effect in UC patients. While all UC patients display AJ abnormalities, only 50% of patients with CD show such abnormalities. Impairment of intestinal permeability causes “leaky flux” diarrhea and an easier uptake of noxious substances, which could be of importance in IBD patients suffering from transit disorders and abdominal pain despite complete mucosal healing of their intestinal disease.111 Alterations in intestinal barrier integrity cause a deregulation in the immune response with secretion of pro-inflammatory cytokines.112 The impact of probiotics, including S. boulardii, in IBDs has been rigorously studied, with controversial results to date. A clinical study with 159 IBD patients treated with steroids or salicylates showed no significant effects of S. boulardii regarding CD relapse rate;113 however, S. boulardii was effective on intestinal permeability, as shown by the decrease in the lactulose/mannitol ratio (33.33%).114 Experimental studies on the effect of S. boulardii supernatant on mucosal lesions found that it accelerates the migration of enterocytes compared to control conditions by modulating α2β1- and αVβ5-integrin activation states.115,116 Another study showed, both ex vivo and in vitro, that S. boulardii accelerates the expression of E-cadherin at the cell membrane by inducing E-cadherin recycling in Rab11A-dependent recycling endosomes.20 These experimental studies indicated that S. boulardii could have beneficial effects in the treatment and prevention of intestinal permeability in patients with IBDs.
IBS
IBS is a functional gastrointestinal disorder affecting more than 10% of the population across the world.117 It is defined by the Rome IV criteria and characterized by intermittent abdominal pain, transit disorders such as diarrhea, constipation or alternating diarrhea/constipation, and is associated with bloating during the symptomatic periods. While no detectable structural or biochemical abnormalities have been found and despite many studies on motility, psychology, diet, visceral hypersensitivity and microbiota in IBS patients, the physiopathology remains unclear.118 Nevertheless, some evidence does suggest the involvement of interactions between the gut, immune system and nervous system in the pathogenesis. In addition, increased intestinal permeability has been noted in patients with IBS, associated with a lower expression of ZO-1 and a decrease in TER.119,120 Increased intestinal permeability can contribute to more severe symptoms and hypersensitivity. In a mouse model, TJ alterations were found to originate from MLCK-dependent activation. While IBS is not lethal, patients do suffer from an altered quality of life and have increased health care costs. Clinical trials on probiotic efficiency have given controversial results. Some have suggested that S. boulardii can restore intestinal epithelial barrier function in IBS patients by modulating the intestinal immune response.121 This could affect IBS symptoms and the hypersensitivity associated with an impaired intestinal barrier. These results need to be validated in larger studies.
The gut microbiota plays a major role in the pathogenesis of IBD and IBS. A dysbiosis characterized by an decrease in protective species and correlated with an increase in inflammatory species has been found in the mucosa and feces of IBD patients. Even if probiotics, and especially S. boulardii, do represent a plausible treatment, further studies are necessary to better characterize the exact role of S. boulardii in IBD and IBS and its specific mechanisms of action, including the modulation of microbiota. More evidence on the beneficial effects of S. boulardii in IBD and IBS needs to be validated with further placebo-controlled clinical studies.
New pathways to prevent intestinal permeability alterations in obesity and metabolic syndrome
The composition of the diet has a critical role in the colonization, maturation and stability of the microbial community. Dietary changes induce changes in the microbiota and lead to negative effects such as dysbiosis and low-grade inflammation. In non-obese humans, overfeeding leads to rapid changes in the composition of the gut microbiota and decreases nutrient absorption.122 High-fat or high-sugar diets modify the composition of the microbiota by increasing the proportion of Firmicutes and reducing the proportion of Bacteroidetes.123 Furthermore, a western diet can have long-term consequences and can lead to the permanent loss of bacteria and induce inheritable metabolic changes.124 Obesity is associated with chronic diseases such as diabetes, cardiovascular disorders and liver diseases. These pathologies are induced by a high-fat, high-sugar diet and are characterized by low-grade inflammation and the secretion of pro-inflammatory factors such as TNF-α or IL-1β, which in turn enhance the dysbiosis associated with intestinal permeability.
Impairments of epithelial barrier function are correlated with alterations in the structure and localization of TJ proteins, ZO-1 and occludin.125 In addition, an imbalance in the Firmicutes/Bacteroidetes ratio contributes to alterations in the expression of TJ proteins.126 In “leaky gut” patients, modifications of the intestinal microbiota composition lead to metabolic endotoxemia and to the translocation of commensal bacteria or bacterial products such as LPS into the circulation, contributing to a chronic systemic low-grade inflammation.127
In a preclinical study evaluating the impact of S. boulardii on obesity, S. boulardii administered daily to 6-week-old obese, type 2 diabetic mice (db/db) for 4 weeks led to reductions in body weight, fat mass, hepatic steatosis and secreted pro-inflammatory cytokines (IL-1β, -4 and -6). In addition, S. boulardii was found to alter the microbial composition of the gut-affecting bacterial species known to be associated with diabetes, inflammation and intestinal permeability. This may have contributed to restoring intestinal barrier function.128 However, in humans results are rare and inconsistent. Further investigations are required to determine whether S. boulardii has beneficial effects in the treatment of obesity and type 2 diabetes.
Excessive alcohol consumption is associated with alcoholic liver diseases (steatosis, acute alcoholic hepatitis and cirrhosis) and can lead to hepatocellular carcinoma. Alcohol was found to decrease the level of defensins, the expression of TJs and the number of immune cells within the intestinal wall.129 Such defects associated with hepatic “micro-inflammation” play a role in the pathogenicity of liver diseases and bacterial translocation. In addition, a newly identified regulator of intestinal permeability (FoxO4) is increased by alcohol.130 The relationship between intestinal permeability and liver disease is still speculative, and any factor found to restore intestinal barrier function could be of interest. Methionine–choline-deficient diet-induced non-alcoholic steatohepatitis in a mouse model highlights that gut microbiota and overnutrition are other major factors involved in the pathogenicity of non-alcoholic fatty liver disease. The use of probiotics in that case prevents liver steatosis and inflammation induced by the diet and improves high-fat diet-induced insulin resistance and steatosis.131
Leaky gut due to enteral feeding
Enteral nutrition is responsible for major changes in intestinal nutrient uptake. These changes produce a deficiency in SCFAs, which are known to participate in TJ assembly, and thus SCFA deficiency contributes to a loss of epithelial barrier function and alterations in intestinal permeability.132 In a mouse model, enteral nutrition was associated with reduced expression of E-cadherin and β-catenin.133 In addition, reduced SCFA levels caused diarrhea, the most frequent complication observed in ill, tube-fed patients. Schneider et al showed that treatment with S. boulardii increased the total fecal content of SCFAs, mostly in terms of butyrate level, in patients receiving long-term enteral nutrition (150.2±27.2 vs 107.5±18.2 mmol/kg, P=0.02) but not in controls (129±28.6 vs 113±15.2 mmol/kg, not significant).132 An absence of luminal nutrition is associated with deleterious consequences to the intestinal barrier. In human intestinal Caco-2 cells, butyrate promoted barrier function by increasing AMP-activated protein kinase activity, accelerating TJ assembly and inhibiting NF-κB activation.14 By increasing SCFA concentrations in patients with enteral nutrition, S. boulardii was shown to contribute to the restoration of the intestinal epithelial barrier, thereby preventing diarrhea in these patients.
Leaky gut in immunocompromised HIV patients
About 50–60% of HIV patients in western countries suffer from diarrhea. In HIV patients, the HIV envelope glycoprotein gp120 induces a reduction in intestinal TER. In addition, HIV caused alterations in TJ protein expression (claudin-1, occludin and ZO-1) and induced a pro-inflammatory response (IL-1, IL-6 and TNF-α), resulting in increased intestinal permeability.134 Impairment of intestinal barrier function causes increased entry of LPS into the bloodstream along with a systemic immune activation, but also contributes to the translocation of HIV into tissue, both leading to faster disease progression.134 Recent studies have focused on developing therapies that control bacterial translocation by decreasing intestinal permeability. In a double-blind, randomized, placebo-controlled trial, Villar-García et al assessed the impact of S. boulardii on microbial translocation. Among 44 antiretroviral therapy-treated patients receiving S. boulardii or placebo for 12 weeks, supplementation with S. boulardii significantly decreased serum levels of LPS and IL-6, which are responsible for pore-forming claudin-2 expression.135,136 After 12 weeks of treatment, patients receiving S. boulardii showed increased levels of Megamonas and Desulfovibrionales species and decreased levels of certain pathogenic species (Clostridiales and Catenibacterium) known to correlate with IL-6 and TNF-α secretion.135,137 Analysis of the microbiota profile by 16s rDNA did not establish the mechanisms underlying the modulation of gut microbiota by S. boulardii, but these data suggest that rather than acting directly on TJ barrier function, S. boulardii instead modulated the intestinal microbiota composition. More precisely, it is known that TNF-α and Il-6 secretion increases intestinal permeability through mechanisms involving TJs. IL-6 impairs ZO-1 apical localization and increases actin cytoskeleton contraction.138 TNF-α increases MLCK protein levels and RhoA activation, correlated with an increase in intestinal permeability.138 The change in microbial community after S. boulardii treatment restores a homeostatic microbiota and reduces pathogenic-related species.139 Moreover, S. boulardii restored the production of certain SCFAs (acetate and propionate) to normal levels in an ex vivo model of the pig fecal microbial ecosystem disturbed by clindamycin.140 We can speculate that S. boulardii has similar effects on the microbiota in HIV patients. Further studies are needed to explore the role of S. boulardii in TJ function in HIV infection, while taking into account the safety warning regarding the use of probiotics in patients with central venous catheters and in immunocompromised or critically ill patients. Modifications in bacterial diversity and the reduction of pro-inflammatory cytokines by S. boulardii may restore intestinal barrier and prevent microbial translocation in HIV patients.
Conclusion
The intestinal epithelial barrier plays a major role in tissue defense and intestinal homeostasis. Disruption of this barrier is a key element in various gastrointestinal disorders and systemic diseases, and occurs through complex cross-talk between signaling pathways and TJ/AJ complex regulation. Understanding the mechanisms implicated in intestinal permeability is essential for the development of new therapeutics that may prevent or restore intestinal epithelial barrier function. Saccharomyces boulardii CNCM I-745, already used in the prevention and treatment of diarrhea of differing etiology (antibiotic-associated or infectious), has proven protective effects on the barrier function in various diseases. In this review, we have summarized the impact of S. boulardii on various gastrointestinal and systemic diseases associated with intestinal epithelial barrier defects. Through anti-inflammatory, anti-secretion, pro-migratory and adhesive effects, S. boulardii preserves and restores intestinal barrier function. Possibly, a yeast-induced general metabolic activation may enhance barrier function by the acceleration of enterocyte turnover.
This review opens new perspectives for various pathologies associated with impaired gut permeability, in which S. boulardii treatment could be investigated.
Abbreviations
AJ, adherens junction; cAMP, cyclic adenosine monophosphate; CD, Crohn’s disease; EHEC, enterohemorrhagic Escherichia coli; EPEC, enteropathogenic Escherichia coli; IAP, intestinal alkaline phosphatase; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IEC, intestinal epithelial cell; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MLC, myosin light chain; MLCK, myosin light chain kinase; NF-κB, nuclear factor-κB; NSP-4, non-structural protein-4; PKC, protein kinase C; SAIF, Saccharomyces boulardii anti-inflammatory factor; SCFA, short-chain fatty acid; TER, transepithelial resistance; TJ, tight junction; TLR, Toll-like receptor; UC, ulcerative colitis; ZO-1, zonula occludens-1.
Acknowledgments
This review has received research funding from Biocodex, France. This manuscript has been edited by an independent scientific language service (Angloscribe, Calvisson, France).
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interests in this work.
References
König J, Wells J, Cani PD, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10):e196. | ||
Lechuga S, Ivanov AI. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim Biophys Acta. 1864;2017(7):1183–1194. | ||
Kato T, Honda Y, Kurita Y, et al. Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: a prospective randomized pilot study in healthy volunteers. PLoS One. 2017;12(4):e0175626. | ||
Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun. 2000;68(10):5998–6004. | ||
Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11(9):1075–1083. | ||
Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20; quiz 21–22. | ||
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–8803. | ||
Rajilić-Stojanović M, Smidt H, de Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol. 2007;9(9):2125–2136. | ||
Freter R, Brickner H, Fekete J, Vickerman MM, Carey KE. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983;39(2):686–703. | ||
Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14(7):685–690. | ||
Ukena SN, Singh A, Dringenberg U, et al. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One. 2007;2(12):e1308. | ||
Garcia-Gutierrez E, Mayer MJ, Cotter PD, Narbad A. Gut microbiota as a source of novel antimicrobials. Gut Microbes. 2018;60:1–21. | ||
Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. 2008;190(7):2505–2512. | ||
Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. | ||
Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–361. | ||
Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two MUC2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–15069. | ||
Merga Y, Campbell BJ, Rhodes JM. Mucosal barrier, bacteria and inflammatory bowel disease: possibilities for therapy. Dig Dis. 2014;32(4):475–483. | ||
Tamura A, Hayashi H, Imasato M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140(3):913–923. | ||
Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13(1):11–18. | ||
Terciolo C, Dobric A, Ouaissi M, et al. Saccharomyces boulardii CNCM I-745 restores intestinal barrier integrity by regulation of E-cadherin recycling. J Crohns Colitis. 2017;11(8):999–1010. | ||
Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C. Bacterial interactions with the host epithelium. Cell Host Microbe. 2010;8(1):20–35. | ||
Awad W, Hess C, Hess M, Pathogens E. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel). 2017;9(2):E60. | ||
Stier H, Bischoff S. Influence of Saccharomyces boulardii CNCM I-745 on the gut-associated immune system. Clin Exp Gastroenterol. 2016;9:269–279. | ||
Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–667. | ||
Lallès JP. Intestinal alkaline phosphatase: novel functions and protective effects. Nutr Rev. 2014;72(2):82–94. | ||
Förster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130(1):55–70. | ||
Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 1788;2009(4):832–841. | ||
Köhler H, Sakaguchi T, Hurley BP, Kase BA, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol. 2007;293(1): G178–G187. | ||
Buts JP, De Keyser N. Effects of Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci. 2006;51(8):1485–1492. | ||
Graff S, Chaumeil JC, Boy P, Lai-Kuen R, Charrueau C. Influence of pH conditions on the viability of Saccharomyces boulardii yeast. J Gen Appl Microbiol. 2008;54(4):221–227. | ||
Edwards-Ingram L, Gitsham P, Burton N, et al. Genotypic and physiological characterization of Saccharomyces boulardii, the Probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 2007;73(8):2458–2467. | ||
Fietto JL, Araújo RS, Valadão FN, et al. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can J Microbiol. 2004;50(8):615–621. | ||
Pardo S, Galvagno MA, Cerrutti P. [Studies of viability and vitality after freezing of the probiotic yeast Saccharomyces boulardii: physiological preconditioning effect]. Rev Iberoam Micol. 2009;26(2):155–160. Spanish | ||
Cario E. Barrier-protective function of intestinal epithelial Toll-like receptor 2. Mucosal Immunol. 2008;(Suppl 1):S62–S66. | ||
Yu S, Gao N. Compartmentalizing intestinal epithelial cell Toll-like receptors for immune surveillance. Cell Mol Life Sci. 2015;72(17):3343–3353. | ||
Oppong GO, Rapsinski GJ, Newman TN, Nishimori JH, Biesecker SG, Tükel Ç. Epithelial cells augment barrier function via activation of the Toll-like receptor 2/phosphatidylinositol 3-kinase pathway upon recognition of Salmonella enterica serovar typhimurium curli fibrils in the gut. Infect Immun. 2013;81(2):478–486. | ||
Szajewska H, Guarino A, Hojsak I, et al; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr. 2014;58(4):531–539. | ||
McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16(18):2202–2222. | ||
Moré MI, Swidsinski A. Saccharomyces boulardii CNCM I-745 supports regeneration of the intestinal microbiota after diarrheic dysbiosis – a review. Clin Exp Gastroenterol. 2015;8:237–255. | ||
Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52(3):439–451. | ||
Ngendahayo Mukiza C, Dubreuil JD. Escherichia coli heat-stable toxin B impairs intestinal epithelial barrier function by altering tight junction proteins. Infect Immun. 2013;81(8):2819–2827. | ||
Shawki A, McCole DF. Mechanisms of intestinal epithelial barrier dysfunction by adherent-invasive Escherichia coli. Cell Mol Gastroenterol Hepatol. 2017;3(1):41–50. | ||
Ugalde-Silva P, Gonzalez-Lugo O, Navarro-Garcia F. Tight junction disruption induced by type 3 secretion system effectors injected by enteropathogenic and enterohemorrhagic Escherichia coli. Front Cell Infect Microbiol. 2016;6:87. | ||
Shen-Tu G, Kim H, Liu M, Johnson-Henry KC, Sherman PM. Protein kinase C mediates enterohemorrhagic Escherichia coli O157:H7-induced attaching and effacing lesions. Infect Immun. 2014;82(4):1648–1656. | ||
Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T84 cells. Infect Immun. 2003;71(2):766–773. | ||
Wu X, Vallance BA, Boyer L, et al. Saccharomyces boulardii ameliorates Citrobacter rodentium-induced colitis through actions on bacterial virulence factors. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G295–G306. | ||
Buts JP, Dekeyser N, Stilmant C, Delem E, Smets F, Sokal E. Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr Res. 2006;60(1):24–29. | ||
Hoy B, Geppert T, Boehm M, et al. Distinct roles of secreted HtrA proteases from gram-negative pathogens in cleaving the junctional protein and tumor suppressor E-cadherin. J Biol Chem. 2012;287(13):10115–10120. | ||
Mumy KL, Chen X, Kelly CP, McCormick BA. Saccharomyces boulardii interferes with Shigella pathogenesis by postinvasion signaling events. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G599–G609. | ||
Martins FS, Dalmasso G, Arantes RM, et al. Interaction of Saccharomyces boulardii with Salmonella enterica serovar typhimurium protects mice and modifies T84 cell response to the infection. PLoS One. 2010;5(1):e8925. | ||
Martins FS, Vieira AT, Elian SD, et al. Inhibition of tissue inflammation and bacterial translocation as one of the protective mechanisms of Saccharomyces boulardii against Salmonella infection in mice. Microbes Infect. 2013;15(4):270–279. | ||
Tiago FC, Martins FS, Souza EL, et al. Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J Med Microbiol. 2012;61(Pt 9):1194–1207. | ||
Pontier-Bres R, Prodon F, Munro P, et al. Modification of Salmonella typhimurium motility by the probiotic yeast strain Saccharomyces boulardii. PLoS One. 2012;7(3):e33796. | ||
Pontier-Bres R, Munro P, Boyer L, et al. Saccharomyces boulardii modifies Salmonella typhimurium traffic and host immune responses along the intestinal tract. PLoS One. 2014;9(8):e103069. | ||
Schmidt E, Kelly SM, van der Walle CF. Tight junction modulation and biochemical characterisation of the zonula occludens toxin C-and N-termini. FEBS Lett. 2007;581(16):2974–2980. | ||
Czerucka D, Rampal P. Effect of Saccharomyces boulardii on cAMP- and Ca2+ -dependent Cl- secretion in T84 cells. Dig Dis Sci. 1999;44(11):2359–2368. | ||
Czerucka D, Nano JL, Bernasconi P, Rampal P. [Response to cholera toxin of 2 epithelial intestinal cell lines. Effect of Saccharomyces boulardii]. Gastroenterol Clin Biol. 1989;13(4):383–387. French. | ||
Brandão RL, Castro IM, Bambirra EA, et al. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol. 1998;64(2):564–568. | ||
Tafazoli F, Zeng CQ, Estes MK, Magnusson KE, Svensson L. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J Virol. 2001;75(3):1540–1546. | ||
Buccigrossi V, Laudiero G, Russo C, et al. Chloride secretion induced by rotavirus is oxidative stress-dependent and inhibited by Saccharomyces boulardii in human enterocytes. PLoS One. 2014;9(6):e99830. | ||
Swidsinski A, Loening-Baucke V, Kirsch S, Doerffel Y. Functional biostructure of colonic microbiota (Central fermenting area, germinal stock area and separating mucus layer) in healthy subjects and patients with diarrhea treated with Saccharomyces boulardii. Gastroentérologie Clinique et Biologique. 2010;34(Suppl 1):S79–S92. French. | ||
Bin Z, Ya-Zheng X, Zhao-Hui D, Bo C, Li-Rong J, Vandenplas Y. The efficacy of Saccharomyces boulardii CNCM I-745 in addition to standard Helicobacter pylori eradication treatment in children. Pediatr Gastroenterol Hepatol Nutr. 2015;18(1):17–22. | ||
Kabbani TA, Pallav K, Dowd SE, et al. Prospective randomized controlled study on the effects of Saccharomyces boulardii CNCM I-745 and amoxicillin-clavulanate or the combination on the gut microbiota of healthy volunteers. Gut Microbes. 2017;8(1):17–32. | ||
Jump RL. Clostridium difficile infection in older adults. Aging Health. 2013;9(4):403–414. | ||
Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69(3):1329–1336. | ||
Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82(5):1516–1524. | ||
Beaugerie L, Flahault A, Barbut F, et al; Study Group. Antibiotic-associated diarrhoea and Clostridium difficile in the community. Aliment Pharmacol Ther. 2003;17(7):905–912. | ||
Massot J, Sanchez O, Couchy R, Astoin J, Parodi AL. Bacterio-pharmacological activity of Saccharomyces boulardii in clindamycin-induced colitis in the hamster. Arzneimittelforschung. 1984;34(7):794–797. | ||
Toothaker RD, Elmer GW. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984;26(4):552–556. | ||
Corthier G, Dubos F, Ducluzeau R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can J Microbiol. 1986;32(11):894–896. | ||
Elmer GW, McFarland LV. Suppression by Saccharomyces boulardii of toxigenic Clostridium difficile overgrowth after vancomycin treatment in hamsters. Antimicrob Agents Chemother. 1987;31(1):129–131. | ||
Buts JP, Bernasconi P, Vaerman JP, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35(2):251–256. | ||
Castagliuolo I, LaMont JT, Nikulasson ST, Pothoulakis C. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in the rat ileum. Infect Immun. 1996;64(12):5225–5232. | ||
Castagliuolo I, Riegler MF, Valenick L, LaMont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxins A and B in human colonic mucosa. Infect Immun. 1999;67(1):302–307. | ||
Sougioultzis S, Simeonidis S, Bhaskar KR, et al. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-kappaB-mediated IL-8 gene expression. Biochem Biophys Res Commun. 2006;343(1):69–76. | ||
Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. 2015;3(1–2):e1000707. | ||
Rodríguez JM, Murphy K, Stanton C, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. | ||
Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. | ||
Digiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. | ||
Flass T, Tong S, Frank DN, et al. Intestinal lesions are associated with altered intestinal microbiome and are more frequent in children and young adults with cystic fibrosis and cirrhosis. PLoS One. 2015;10(2):e0116967. | ||
Jiménez E, Fernández L, Marín ML, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51(4):270–274. | ||
Ajslev TA, Andersen CS, Gamborg M, Sørensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond). 2011;35(4):522–529. | ||
Yassour M, Vatanen T, Siljander H, et al; DIABIMMUNE Study Group, Xavier RJ. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81. | ||
Johnson CC, Ownby DR. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl Res. 2017;179:60–70. | ||
Berding K, Donovan SM. Diet can impact microbiota composition in children with autism spectrum disorder. Frontiers in neuroscience. 2018;12. | ||
Jakaitis BM, Denning PW. Human breast milk and the gastrointestinal innate immune system. Clin Perinatol. 2014;41(2):423–435. | ||
West CE, Renz H, Jenmalm MC et al. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. Journal of Allergy and Clinical Immunology. 2015 Jan 1;135(1):3–13. | ||
Sjögren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39(12):1842–1851. | ||
Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107(27):12204–12209. | ||
Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatrica. 2013;102(12):e560–e565. | ||
Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000;30(11):1548–1553. | ||
Ong M-S, Umetsu DT, Mandl KD. Consequences of antibiotics and infections in infancy: bugs, drugs, and wheezing. Ann Allergy Asthma Immunol. 2014;112(5):441–445.e1. | ||
Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168(11):1063–1069. | ||
Cukrowska B, Sowińska A, Bierła JB, Czarnowska E, Rybak A, Grzybowska-Chlebowczyk U. Intestinal epithelium, intraepithelial lymphocytes and the gut microbiota - Key players in the pathogenesis of celiac disease. World J Gastroenterol. 2017;23(42):7505–7518. | ||
West CE, Renz H, Jenmalm MC, et al; in-FLAME Microbiome Interest Group. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. 2015;135(1):3–13; quiz 14. | ||
Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108(4):516–520. | ||
Harbison JE, Roth-Schulze AJ, Barry SC, et al. Gut microbiome dysbiosis and increased intestinal permeability in Australian children with islet autoimmunity and type 1 diabetes. Diabetes. 2018;67(Suppl 1):230-OR–2300. | ||
Leiva-Gea I, Sánchez-Alcoholado L, Martín-Tejedor B, et al. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care. 2018;41(11):2385–2395. | ||
Li X, Atkinson MA. The role for gut permeability in the pathogenesis of type 1 diabetes--a solid or leaky concept? Pediatr Diabetes. 2015;16(7):485–492. | ||
Maffeis C, Martina A, Corradi M, et al. Association between intestinal permeability and faecal microbiota composition in Italian children with beta cell autoimmunity at risk for type 1 diabetes. Diabetes Metab Res Rev. 2016;32(7):700–709. | ||
Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–440e1–e2. | ||
Ozkan TB, Sahin E, Erdemir G, Budak F. Effect of Saccharomyces boulardii in children with acute gastroenteritis and its relationship to the immune response. J Int Med Res. 2007;35(2):201–212. | ||
Caetano JA, Paramés MT, Babo MJ, et al. Immunopharmacological effects of Saccharomyces boulardii in healthy human volunteers. Int J Immunopharmacol. 1986;8(3):245–259. | ||
Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr. 2013;109(Suppl 2):S81–S85. | ||
Liu L, Steinle JJ. Toll-like receptor 4 regulates insulin signal transduction in retinal Müller cells. Growth Factors. 2017;35(6):234–238. | ||
Szabo G. Gut–Liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. | ||
Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49(6):1877–1887. | ||
Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–1657. | ||
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. | ||
Rajca S, Grondin V, Louis E, et al. Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm Bowel Dis. 2014;20(6):978–986. | ||
Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology. 2017;153(3):723–731.e1. | ||
Zbar AP, Simopoulos C, Karayiannakis AJ. Cadherins: an integral role in inflammatory bowel disease and mucosal restitution. J Gastroenterol. 2004;39(5):413–421. | ||
Bourreille A, Cadiot G, Le Dreau G, et al; FLORABEST Study Group. Saccharomyces boulardii does not prevent relapse of Crohn’s disease. Clin Gastroenterol Hepatol. 2013;11(8):982–987. | ||
Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo da Gama Torres H, et al. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand J Gastroenterol. 2008;43(7):842–848. | ||
Canonici A, Siret C, Pellegrino E, et al. Saccharomyces boulardii improves intestinal cell restitution through activation of the α2β1 integrin collagen receptor. PLoS One. 2011;6(3):e18427. | ||
Canonici A, Pellegrino E, Siret C, et al. Saccharomyces boulardii improves intestinal epithelial cell restitution by inhibiting αvβ5 integrin activation state. PLoS One. 2012;7(9):e45047. | ||
Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(7):712–721.e4. | ||
Mearin F, Be L, Chang L. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. | ||
Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201. | ||
Bertiaux-Vandaële N, Youmba SB, Belmonte L, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106(12):2165–2173. | ||
Abbas Z, Yakoob J, Jafri W, et al. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: a randomized trial. Eur J Gastroenterol Hepatol. 2014;26(6):630–639. | ||
Jumpertz R, Le DS, Turnbaugh PJ, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58–65. | ||
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015;16(4):7493–7519. | ||
Zinöcker M, Lindseth I. The western diet–microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3):E365. | ||
Genser L, Poitou C, É B-L. Alteration of intestinal permeability: the missing link between gut microbiota modifications and inflammation in obesity? Med Sci (Paris). 2016;32(5):461–469. | ||
Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. | ||
Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol. 2015;308(10):G840–G851. | ||
Everard A, Matamoros S, Geurts L, Delzenne NM, Cani PD. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio. 2014;5(3):e01011–01014. | ||
Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–1524. | ||
Chang B, Sang L, Wang Y, Tong J, Wang B. The role of FOXO4 in the relationship between alcohol-induced intestinal barrier dysfunction and liver injury. Int J Mol Med. 2013;31(3):569–576. | ||
Karahan N, Işler M, Koyu A, et al. Effects of probiotics on methionine choline deficient diet-induced steatohepatitis in rats. Turk J Gastroenterol. 2012;23(2):110–121. | ||
Schneider SM, Girard-Pipau F, Filippi J, et al. Effects of Saccharomyces boulardii on fecal short-chain fatty acids and microflora in patients on long-term total enteral nutrition. World J Gastroenterol. 2005;11(39):6165–6169. | ||
Feng Y, Sun X, Yang H, Teitelbaum DH. Dissociation of E-cadherin and beta-catenin in a mouse model of total parenteral nutrition: a mechanism for the loss of epithelial cell proliferation and villus atrophy. J Physiol. 2009;587(Pt 3):641–654. | ||
Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):e1000852. | ||
Villar-García J, Hernández JJ, Güerri-Fernández R, et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr. 2015;68(3):256–263. | ||
Al-Sadi R, Ye D, Boivin M, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS One. 2014;9(3):e85345. | ||
Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829. | ||
Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 1788;2009(4):864–871. | ||
Villar-García J, Güerri-Fernández R, Moya A, et al. Impact of probiotic Saccharomyces boulardii on the gut microbiome composition in HIV-treated patients: a double-blind, randomised, placebo-controlled trial. PLoS One. 2017;12(4):e0173802. | ||
Breves G, Faul K, Schröder B, Holst H, Caspary WF, Stein J. Application of the colon-simulation technique for studying the effects of Saccharomyces boulardii on basic parameters of porcine cecal microbial metabolism disturbed by clindamycin. Digestion. 2000;61(3):193–200. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.