Back to Journals » Drug Design, Development and Therapy » Volume 13
Beneficial effects of resveratrol and exercise training on cardiac and aortic function and structure in the 3xTg mouse model of Alzheimer’s disease
Authors Esfandiarei M , Hoxha B, Talley NA, Anderson MR , Alkhouli MF, Squire MA, Eckman DM , Babu JR, Lopaschuk GD, Broderick TL
Received 27 November 2018
Accepted for publication 4 March 2019
Published 17 April 2019 Volume 2019:13 Pages 1197—1211
DOI https://doi.org/10.2147/DDDT.S196119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Mitra Esfandiarei,1 Brikena Hoxha,1 Nicholas A Talley,1 Miranda R Anderson,2 Mustafa F Alkhouli,2 Michaela A Squire,2 Delrae M Eckman,1 Jeganathan Ramesh Babu,3 Gary D Lopaschuk,4 Tom L Broderick2
1Department of Biomedical Sciences, College of Graduate Studies, Midwestern University, Glendale, AZ, USA; 2Department of Physiology, Laboratory of Diabetes and Exercise Metabolism, College of Graduate Studies, Midwestern University, Glendale, AZ, USA; 3Department of Nutrition, Dietetics, and Hospitality Management, Auburn University, Auburn, AL, USA; 4Cardiovascular Research Centre, Mazankowski Alberta Heart Institute University of Alberta, Edmonton, AB, Canada
Background: Studies have indicated an association between Alzheimer’s disease (AD) and increased risk of developing cardiovascular complications. Lifestyle modifiable factors, such as exercise and diet, are known to prevent cardio-cerebral disease. Recent studies demonstrate that hearts from early onset triple-transgenic AD mice exhibit pathologies, but it is not clear whether cardiovascular function is altered in this model.
Methods: In this study, we measured in vivo cardiovascular function in 7-month-old male 3xTg mice and age-matched wild-type (WT) mice using high-frequency high-resolution ultrasound imaging.
Results: Our findings indicated that aortic root measurements and interventricular septal dimensions were similar in 3xTg and wild-type mice. Systolic function, expressed as ejection fraction and fractional shortening, were decreased in 3xTg mice. Late (A) ventricular filling velocities, the early/atrial (E/A) ratio, and mitral valve deceleration time, all indices of diastolic function, were increased in 3xTg mice compared to WT mice. Treadmill exercise training and resveratrol supplementation in the diet for 5 months improved ejection fraction, fractional shortening, and restored diastolic deceleration times. Pulse wave velocity was ∼33% higher in 3xTg, and accompanied by a significant increase in elastin fiber fragmentation within the aortic wall, which was associated with decrease in elastin content and fiber length. Aortic wall and adventitia thickness were increased in 3xTg mice compared to the WT group. Exercise training and resveratrol supplementation, or both, improved overall aortic morphology with no change in pulse wave velocity.
Conclusion: Taken together, the results indicate that the aberrations in cardiac function and aortic elastin morphology observed in the 3xTg mouse model of AD can be prevented with exercise training and treatment with resveratrol. The benefits of regular exercise training and resveratrol supplementation of heart and aortic structure in the 3xTg mouse support the value of healthy lifestyle factors on cardiovascular health.
Keywords: Alzheimer’s disease, exercise, resveratrol, heart, aorta, ultrasound imaging, pulse wave velocity, aortic wall elasticity
Introduction
Alzheimer’s disease (AD) is the most common form of dementia in the United States. This debilitating age-related neurodegenerative disease, with an etiology unknown, affects one in seven Americans aged 65 years and older and its prevalence is expected to increase as the aging population continues to grow.1 AD is characterized by disruption of the blood–brain barrier, neuroinflammation from amyloid-beta (Aβ) accumulation, misfolded proteins, mitochondrial dysfunction, and oxidative stress.2–7 Because of these pathological changes, patients with AD experience loss of multiple cognitive functions as well as behavioral and psychiatric manifestations.8,9
Patients with AD are at increased risk of developing cardiovascular diseases.10–12 In fact, it is now well established that AD and cardiovascular diseases share common risk factors.13–15 Patients with AD exhibit ischemic damage to the left ventricle (LV), frequent valvular dysfunction, increased prevalence of silent ischemia,10,16 and diastolic dysfunction.17 Disturbances in LV function have also been reported in transgenic mouse models of AD. Cardiomyocytes isolated from 10-month-old APP/PS1 mice demonstrate impaired mechanics, including decreased maximal velocity of shortening and relengthening properties, which reflect contraction and relaxation, respectively.18 Additionally, recent studies indicate that cardiovascular disease and AD share a common pathogenesis involving Aβ regulation.17 With both diseases sharing significant medical, social, and economic burdens due to prolonged life expectancy, current treatment options remain inadequate despite the expanded research in the development of therapeutic drugs. However, epidemiological studies indicate that non-pharmacological approaches, such as regular physical activity and dietary intervention, can prevent or delay the progression of these diseases. In fact, exercise training is a safe, inexpensive, and effective intervention known to improve the quality of life in patients with neurodegenerative and/or cardiovascular diseases. Regular exercise has been shown to improve brain function and reduce the incidence of cognitive defects, thus slowing the progression of AD.19–24 The decreased exercise capacity and endothelial dysfunction in patients with chronic heart failure can be improved by regular physical exercise.25 Further, aerobic exercise training improves both oxygen utilization and ejection fraction in elderly patients with heart failure26,27 and reduces arterial stiffness in older adults.28
Promoting a diet rich in polyphenols is an effective strategy aimed at preventing the progression of a variety of human diseases. Within the polyphenol family, the stilbene resveratrol (3,5,4-trihydroxy-trans-stilbene) is found in relatively high concentrations in grapes and wine. Resveratrol exerts significant anti-oxidant and anti-inflammatory effects,29–31 and recent studies indicate that this polyphenol is both cardio- and neuroprotective in models of obesity and AD.32–34 Considering the benefits of exercise training and daily resveratrol supplementation on cardiovascular function, and the relationship between cardiovascular diseases and AD, the aim of this study was to examine the effects of the AD state on cardiac and vascular function in a well-established murine model of familial AD. Additionally, we determined whether exercise training or resveratrol treatment could reverse the cardiac- and vascular-related disturbances in AD.
Materials and methods
Experimental animals and treatment protocols
All protocols involving mice were approved by the Midwestern University Institutional Animal Care and Use Committee. All animals received humane care in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (8th edition; revised 2011). Eight-week-old male triple-transgenic mice (3xTg) harboring three mutant genes, amyloid-beta (Aβ) precursor protein, presenilin-1, and tau (Jackson Laboratories, Bar Harbor, ME, USA) were used in this study. Transgenic mice were randomly assigned to the following groups: 3xTg, 3xTg and exercise (3xTg+Ex), 3xTg and resveratrol (3xTg+Resv), and 3xTg exercise and resveratrol (3xTg+Ex+Resv). Non-transgenic, age-matched wild-type (WT) littermates (B6129SS2/J) mice were used as controls. The duration of treatment was 5 months.
Exercise training was performed on a treadmill designed for mice (Exer 3/6, Columbus Instruments, Columbus, OH, USA) using an incremental training protocol. Before the start of the exercise protocol, mice were initially acclimated to daily 10-min exercise sessions at 10 m/min for 2 weeks. After this period, training consisted of a 3-week graded increase in both exercise duration and intensity: week 1, 10 mins at 10 m/min; week 2, 20 mins at 10 m/min; and week 3, 30 mins at 12 m/min. Duration was increased to 45 mins and intensity to 15 m/min corresponding to ~80% maximal oxygen carrying capacity.35 Mice were able to run for the entire 45-min session at this intensity without reluctance and all mice completed the exercise protocol during the study.
Resveratrol (Lalilab Inc., Durham, NC, USA) was incorporated in the diet (4g/kg, AIN-93G, Dyets Inc., Bethlehem, PA, USA). This concentration was selected based on previous studies showing accumulation in heart.36 Mice in the WT, 3xTg, and 3xTg+Ex groups received the same diet (Dyets Inc., Bethlehem, PA, USA) without the addition of resveratrol. Mice were housed two per cage and maintained in a room with a 12:12 hrs light-dark cycle and given food and water ad libitum.
Vivo ultrasound imaging
Study of cardiac and aortic function and structure was performed using Vevo® 2100 ultrasound imaging system (FUJIFILM, VisualSonics, Toronto, ON, Canada). Measurements were completed in random order from mice that were labeled with numbers only. The high-frequency high-resolution ultrasound system is equipped with a 40 MHz transducer (MS-5505) with a focal length of 7.0 mm, frame rate of 557 fps (single zone, 5.08 mm width, B-mode), and a maximum two-dimensional field of view of 14.1×15.0 mm with a spatial resolution of 90 μm (lateral) by 40 μm (axial). Mice were anesthetized in an induction chamber with 3% isoflurane and 1 L/min flow of 100% oxygen for 1–2 mins prior to being laid supine on a heated platform with a constant flow of 1.5–2% isoflurane to keep the mouse anesthetized during the measurements. Heart rate, electrocardiogram (ECG), and respiratory rate were measured by the four ECG electrode embedded in the platform. Body temperature was maintained at 36–38°C and monitored by a rectal probe throughout the protocol.
Aortic diameters at the annulus [L1], sinuses of Valsalva [L2], and sinotubular junctions [L3] were measured from the B-mode aortic arch view as described previously.37 The ascending and descending aortic peak velocities were measured from the pulse wave (PW) Doppler-mode aortic arch view. Pulse wave velocity (PWV) was obtained from the B-mode and Doppler-mode aortic arch view, calculated as PWV (mm·s−1)=aortic arch distance (d2-d1)/transit time (T1-T2). The PW Doppler mode sample volume was placed in the ascending aorta to verify the time from the onset of the QRS complex to the onset of the ascending aortic Doppler waveform (T1). Using the same image plane, the time from the onset of the QRS complex to the onset of the descending aortic Doppler waveform (T2) was also measured, and the average values for T1 and T2 over 10 cardiac cycles were calculated. Furthermore, the aortic arch distance was measured between the two sample volume positions along the central axis of aortic arch on the B-mode image.
The left ventricular (LV) structural and functional parameters were calculated from the LV parasternal short-axis M-mode view, which was recorded at the level of two papillary muscles. An M-mode cursor was positioned perpendicular to the anterior and posterior walls in the middle of the LV for measuring wall thickness. LV functional parameters (stroke volume, ejection fraction , fractional shortening, and cardiac output) were obtained from this M-mode view. Interventricular septal wall (IVS) thickness during diastole (IVSd) and systole (IVSs) were also obtained from LV parasternal long-axis M-mode view. Mitral flow velocity, expressed as early (E) and atrial (A) velocities, were acquired from the Doppler-mode apical four chamber view. The isovolumic contraction time (IVCT), isovolumic relaxation time (IVRT), and ejection time (ET) were also measured from this view. The myocardial performance index (MPI=[(IVCT+IVRT)/ET]) was used for evaluating LV systolic function.
Histological studies of the aortic wall
Mice were euthanized using 5% isoflurane inhalation followed by cervical dislocation. Aortic root segments (2–4 mm in length) were dissected from the thoracic cage, placed in ice-cold oxygenated (95% O2 - 5% CO2) HEPES Physiological Salt Solution (HEPES-PSS), cleaned of connective tissue and blood and fixed in 10% buffered formalin for 48 hrs. Formalin-fixed aortic segments were then embedded in paraffin and cut into 10-μm-thick serial cross-sections. Tissue sections were deparaffinized in xylene, rehydrated in graded ethanol, and stained with Accustain® Elastic Stain Kit (Sigma-Aldrich, St. Louis, MO, USA) in accordance with the manufacturer’s instructions as previously described.38 Image acquisition was performed using an Olympus light microscope equipped with an Axiozeiss digital camera. Digital images were obtained at 400× magnification using an Olympus Vanox AH-3 microscope and AxioVision v4.8.2 imaging software. The length and counts of elastin fiber fragments within the aortic wall were determined using the line profiling tool in National Institutes of Health Image J 1.43j software, while the elastin thickness was determined utilizing the count and measure objects tool in Image-Pro Premier 9.1 (Media Cybernetics, Bethesda, MD, USA). Elastin fibers were traced or outlined and their length or thickness measured in pixels followed by a unit conversion to either micrometers (for fiber length) or micrometers squared (for fiber area). To measure elastin total area, the mean intensity of positive signal after subtracting the background noise was measured in minimum of four regions of interest in each aortic section. The percentage area of elastic fibers within the aortic wall was determined by using a commercial three-dimensional image processing and analysis program (Amira, FEI, France). By tracing the elastic fibers for the whole area of the aortic ring (transverse section), pixel size was counted and converted to the actual size. MATLAB (Mathworks, Natick, MA, USA) was used for calculating the area of elastic fibers within the aortic wall area.
Statistical analysis
Data using ultrasound imaging were generated using the VisualSonics cardiac package software, and all measurements were repeated over five cardiac cycles to reduce potential bias. All echocardiographic image acquisitions were performed by a single investigator. Data analyses were conducted by two independent observers who were blinded to animal genotypes or treatment plans. Statistical significance was determined by a one-way ANOVA followed by a Tukey-Kramer post hoc test. All values are reported as mean±SEM. Significance was set at P<0.05.
Results
Measurements of physical characteristics in experimental mice
Basic physical characteristics are presented in Table 1. There were no significant differences in body weight between the groups at the end of the study. However, an estimated 10–15% decrease in body weight was observed in mice subjected to exercise training, resveratrol treatment, and exercise combined with resveratrol treatment. This resulted in significant increases in the heart weight to body weight ratio compared with WT mice.
 | Table 1 Physical characteristic of mice after resveratrol treatment and exercise training in 3xTg mice |
Measurements of aortic root diameters using high-resolution ultrasound imaging
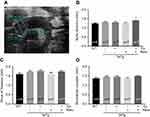
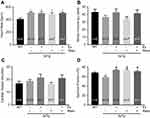
In order to compare the rate of aortic root growth among different experimental groups, we utilized ultrasound imaging to measure root diameters of the aortic annulus, sinus of Valsalva, and sinotubular junction (Figure 1A). Our data revealed no significant differences in aortic root diameters at the annulus (Figure 1B), sinus of Valsalva (Figure 1C), and sinotubular junction (Figure 1D) between groups, with the exception of an increase in root diameter at the aortic annulus in 3xTg mice subjected to the combination of exercise and resveratrol (Figure 1B).
Measurements of aortic PWV using high-resolution ultrasound imaging
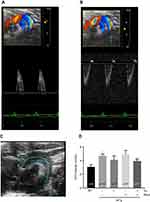
Aortic PWV, a strong indicator of aortic wall stiffness, was measured using ultrasound imaging (Figure 2A, B, and C). Our data showed that PWV was significantly higher in 3xTg mice when compared to age-matched WT mice (Figure 2D). However, no significant differences were observed following treatment with exercise, resveratrol, or in combination when compared to WT mice (Figure 2D).
Evaluation of aortic wall structural integrity
Significant increases in adventitial (Figure 3A), medial (Figure 3B), and overall aortic wall (Figure 3C) thickness were observed in 3xTg mice when compared to age-matched WT mice. Interventions with exercise, resveratrol, and combination of both were effective in normalizing adventitial, medial, and overall aortic wall thickness. However, no detectable effects on medial thickness in 3xTg mice subjected to the combination of exercise and resveratrol were observed (Figure 3B).
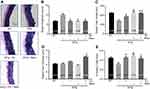
Changes in elastin fiber structure and organization have a direct impact on aortic wall elasticity and stiffness. We used van Gieson staining to determine elastin fiber structural integrity in aortic segments isolated from mice. As shown in Figure 4A, elastin fibers in the WT mouse aorta demonstrated the normal sigmoid shape with a highly organized and parallel zigzag structure. This is in contrast to the fragmentation and disorganization of elastin fibers in aortic rings isolated from 3xTg mice (Figure 4A). Analysis of elastin fiber length and fragment counts revealed an increase in elastin fragmentation within the aortic wall of 3xTg mice, as evident by the increased elastin fragment counts (Figure 4B) and reduced fiber length within the medial layer of the aortic wall (Figure 4C). All treatment protocols normalized elastin fiber structure in the aortic wall of 3xTg mice (Figure 4B and C). The average thickness of elastin fibers was not different between groups. However, compared to WT mice elastin thickness was increased in 3xTg mice subjected to combination of exercise and resveratrol treatment (Figure 4D), suggesting that the overall elastogenesis and elastin fiber turnover might not have been affected in 3xTg mice. Interestingly, the percentage of total area for elastin fibers within the medial layer was significantly reduced in 3xTg mice aorta when compared to WT mice, reconfirming the aortic elastin degradation and fragmentation in 3xTg mice (Figure 4E). Treatment increased and normalized the total area measured for elastin in the aortic wall of 3xTg mice (Figure 4E), highlighting the beneficial effects of exercise and resveratrol (or in combination) on elastin fiber fragmentation and disorganization.
Measurement of cardiac function and structure using high-resolution ultrasound imaging
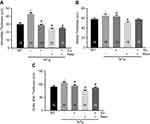
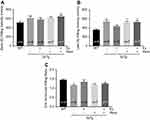
Cardiac function and structure were evaluated by ultrasound imaging. Heart rate was significantly increased in all 3xTg mice when compared to WT mice (Figure 5A). None of the treatment protocols had any effect on heart rate (Figure 5A). With respect to systolic function, there were no significant differences in stroke volume (Figure 5B) and cardiac output (Figure 5C) between groups. However, ejection fraction was significantly decreased in 3xTg mice when compared to WT mice (Figure 5D). Treatment protocols were beneficial and improved ejection fraction in 3xTg mice (Figure 5D).
We used tissue Doppler echocardiography to evaluate ventricular inflow pattern as a reliable measure for diastolic function and ventricular relaxation (Figure 6). Peak velocity flow in early diastole (the E wave velocity) was not different between groups, with the exception of a significant difference between WT and 3xTg mice subjected to the combination of exercise and resveratrol (Figure 6A). However, further analysis revealed that peak velocity flow in late diastole (the A wave velocity) was significantly higher in 3xTg mice when compared to WT mice, indicating an increase in ventricular wall stiffness in 3xTg mice (Figure 6B). Exercise training reduced the A wave velocity in 3xTg mice to a level that was not significantly different from WT mice. However, resveratrol alone or in combination with exercise did not have any effect on the A wave velocity in 3xTg mice (Figure 6B). In addition, because of the increase in the A wave velocity, a significant decrease in E/A ventricular filling ratio was observed in the 3xTg group, further confirming the presence of diastolic dysfunction in these mice (Figure 6C). Only hearts from the exercise training group displayed an E/A ratio that was similar to WT mice (Figure 6C).
To further investigate LV function, we measured fractional shortening, which is the degree of shortening of the LV diameter between end-diastole and end-systole. Fractional shortening was significantly reduced in 3xTg mice when compared to WT mice (Figure 7A). All treatment protocols were effective in correcting fractional shortening in 3xTg mice (Figure 7A).
Since our data indicated an abnormality in LV diastolic filling, we utilized mitral valve Doppler tracings to evaluate mitral deceleration time as a measure of LV compliance and filling. As expected, deceleration time was markedly increased in 3xTg mice (Figure 7B), further confirming the abnormal LV filling pattern and diastolic dysfunction in 3xTg mice. All treatment protocols corrected mitral valve deceleration time to the level observed in WT mice (Figure 7B).
MPI was measured as an index that incorporates both systolic and diastolic time intervals in expressing global ventricular function. As shown in Figure 7C, no significant differences in MPI were observed between groups of mice. To determine whether the observed changes in cardiac function in 3xTg mice were associated with cardiac hypertrophy, ultrasound imaging was used to measure the thickness of the IVS, left ventricular anterior wall, and left ventricular posterior wall during diastole and systole (Table 2). Only IVS during systole was significantly decreased in 3xTg mice when compared to WT mice (Table 2).
 | Table 2 Echocardiography measurements of cardiac dimensions |
Discussion
The non-pharmacological management of cardiovascular and neurodegenerative diseases focuses on a lifestyle that includes physical activity and healthful eating. Clinical and experimental evidence indicates that regular exercise and a diet containing polyphenols are protective against the development of these age-related diseases. With recent studies suggesting common pathologies between AD and the development of cardiovascular dysfunction,17,39–41 we examined cardiac and vascular function in the 3xTg mouse model of AD. We also determined whether any aberrations in cardiovascular function observed in this mouse model could be attenuated with either exercise training or resveratrol supplementation, or with combination therapy. The 3xTg mouse model was selected because of its resemblance to human familial AD in terms of etiology and progression of disease. This model exhibits cognitive impairment by 3 months of age42 and Aβ deposits in the frontal cortex by 6 months.43 Seven-month-old mice were chosen to reflect the early stages associated with AD in the absence of tau pathology, which typically appears when mice are ~1 year of age.43
Our results indicate that hearts from 7-month-old 3xTg mice demonstrate deficiencies in LV function. In vivo assessment of indices of systolic function, expressed as ejection fraction and fractional shortening, were reduced in the 3xTg mouse. Diastolic dysfunction was also evident in hearts from 3xTg mice as highlighted by an increase in late ventricular filling, resulting in a decrease in the E/A ratio. Further, mitral valve deceleration time, also indicative of relaxation properties of the heart, was reduced in the 3xTg mouse. Both exercise training and resveratrol treatment reversed the systolic dysfunction of hearts, although combination treatment had no synergistic effects on function. Diastolic function, expressed as mitral valve deceleration time, was also improved with exercise training and resveratrol as well as in the presence of combination treatment. However, only exercise had a beneficial effect of increasing late ventricular filling and reversing the decrease in the E/A ratio.
In addition to these alterations in cardiac function, histological analysis revealed significant aberrations in the aortic root structure. These include increased fragmentation of elastin fibers, decreased elastin fiber length as well as increased aortic and adventitial wall thickness. Exercise training and resveratrol treatment reversed these histological consequences in the aorta resulting from the AD state of mice. Interestingly, combined exercise training and resveratrol treatment significantly increased elastin thickness within the aorta of mice. Taken together, our results indicate that deficiencies in both systolic function and relaxation properties observed in hearts from 3xTg mice can be largely prevented with either exercise training or treatment with resveratrol, suggesting that these interventions may prevent LV remodeling in this model. Furthermore, the observation that exercises training and resveratrol improved the structural defects in aorta of the AD mouse also indicates a potential role of these interventions in vascular wall remodeling.
The improvement in LV function with resveratrol is consistent with the benefits reported in mouse models of ventricular dysfunction, including heart failure and diabetes.34,44–49 Several beneficial properties and mechanisms of action of resveratrol have been demonstrated. One study reported that resveratrol increased the expression of sarcoplasmic/endoplasmic reticulum calcium ATPase 2a (SERCA2a) and restored SERCA2a promoter activity. These effects are dependent on silent information regulators (SIRT) 1 and 3 expression.44 These observations suggest that resveratrol might enhance systolic and diastolic function in the 3xTg mouse by improving calcium handling via the SIRT1/3 pathways and restoring SERCA2a expression. Interestingly, these same pathways are regulated in response to chronic exercise training. In fact, both resveratrol treatment and chronic exercise training share molecular pathways associated with mitochondrial biogenesis, oxidative metabolism, regulation of blood pressure, and vascular function.50–55 Although the metabolic or physiological basis for the observed diastolic dysfunction observed in hearts of 3xTg mice is not well defined, recent emerging studies indicate that diastolic dysfunction in patients with AD is related to disturbances in calcium homeostasis. Indeed, LV cardiomyocytes obtained from patients with AD displayed slower relaxation velocities and prolonged calcium transients. These changes are correlated with a decline in myocardial compliance,17 which could also explain the diastolic dysfunction recently reported in these patients.56 A defect in relaxation, expressed as maximum velocity of relengthening time, has been reported in cardiomyocytes obtained from the APP/PS1 mouse model of AD.18 This mechanical disturbance is related to reduced expression of phospholamban,18 a key protein involved in the regulation of SERC2a activity. Based on these observations, it is possible that alterations in calcium transients are contributing to impaired diastolic function in the 3xTg mouse, although this needs to be confirmed. Further, whether impaired diastolic function is linked to disturbances in systolic function is also unclear. Decrease in myocardial compliance and filling volumes from impaired relaxation could translate into reduced ejection fraction and fractional shortening. Because the pathology of myocardial tissue in AD is multi-faceted, disturbances in calcium homeostasis accompanied with Aβ inclusions39 are known to contribute to LV dysfunction. Increased interstitial space, cardiomyocyte disarray, decreased anti-apoptosis proteins, and elevated cytochrome C and caspase-9 expression recently reported in 3xTg mice could also lead to impaired function.57
Compliance of the aorta is necessary to buffer the pressures generated by the heart during systole and to maintain arterial blood flow by acting as an elastic reservoir during diastole. As the aortic wall stiffens, compliance of the aorta is reduced resulting in increased cardiac afterload with consequences on diastolic function of the heart and peripheral perfusion. While an increase in arterial wall stiffening, measured by an increase in aortic PWV, is predictive of hypertension, myocardial infarct, and failure,58–60 increased PWV of carotid arteries has been associated with accelerated brain aging and cognitive impairment.61 Aortic wall stiffening is associated with inflammation, oxidative stress, reduced endothelial function, and disorganization of elastin in the aortic wall,62 and can potentially be involved in the aortic pathology of 3xTg mice.
Elastin is a key protein component of elastic fibers in the aorta and provides reversible elasticity allowing it to expand during a hemodynamic load. Aortic segments isolated from 3xTg mice exhibited significant elastin breaks and reduced elastin fiber length, confirmed by histological analysis. Our observations indicate that the increased aortic wall stiffness in 3xTg mice is related to loss of elastin structural integrity. Increases in PWV and wall stiffness have been reported in other models of cardiovascular diseases, including Marfan syndrome,63 chronic heart failure,64 diet-induced obesity,65 and aging.66,67 Further, increased PWV was demonstrated in the common carotid artery of 9-month-old 3xTg mice, although elastin content and aortic wall thickness were not different compared to representative control mice used in the study.68 The reasons explaining the differences in elastin content between the common carotid artery and aorta of 3xTg mice are not known. An elevated heart rate is a risk factor for cardiovascular mortality69 and known to increase PWV and aortic wall stiffness.70 Thus, it seems plausible that the higher heart rates, indicative of increased sympathetic tone,71 may induce a greater hemodynamic stress and accelerate the progression of elastin loss and fragmentation in 3xTg mice.
To date, no studies have addressed the effects of exercise training and resveratrol treatment on PWV and elastin properties in aorta from 3xTg mice. The results of our study indicate that either exercise training or resveratrol treatment, or combination of both, exerted beneficial effects on elastin content and organization in aorta of 3xTg mice. Despite these favorable changes in aortic wall structure, in vivo measurements of PWV remained elevated in these mice after treatment, although it is noteworthy that a non-significant decrease of ~20% in PWV was observed with combination treatment. Our results are in contrast with a recent study demonstrating that chronic exercise training improved elastin content and decreased PWV in aorta of aged rats.67,72 Similar results on elastin morphology using circumferential aortic wall tension as index of aortic stiffness have been reported in the spontaneously hypertension rat following 20 weeks of treadmill running.73 Similarly, resveratrol treatment prevented the increase in both PWV and arterial wall stiffening induced by a Western diet in nonhuman primates.74 In a mouse model of Marfan syndrome, which exhibits increased PWV from senescence,37 resveratrol decreased the number of elastin breaks in aorta.63 Taken together, this suggests that PWV and elastin organization are improved in aorta with exercise training and resveratrol treatment in these models of cardiovascular dysfunction. However, the improvement in elastin morphology was not accompanied by a decrease in PWV in 3xTg mouse following treatment. The reasons why this perturbation in PWV was not prevented are not known, but may relate to differences in pathology exhibited by the experimental models, the duration and severity of disease, and age of mice. One possible explanation for the observed beneficial effects of resveratrol and exercise on elastic fiber structure within the aortic wall of 3xTg mice is that both interventions can regulate elastase expression and activity in aorta. Studies have demonstrated that human neutrophil elastase is involved in the degradation of the extracellular matrix, and that polyphenols can inhibit elastase activity.75,76 Similar effects on adipose tissue elastase expression have been reported following 16 weeks of exercise training in mice fed a diet high in fat.77 On the other hand, protein levels of elastin and elastase activity in aorta of healthy mice were not influenced after 6 weeks of endurance training.78
Conclusion
In conclusion, no studies have examined cardiovascular function in the 3xTg model of AD as well as the roles of polyphenol treatment and exercise training on basic indices of cardiac and aortic function. Here, we have reported that hearts from 3xTg mice exhibit functional abnormalities in systolic and diastolic properties that are similar to those observed in patients diagnosed with dementia and AD,56,79 suggesting that the 3xTg model may be relevant for mechanistic-based investigation of AD. Further, our data show that consumption of the polyphenol resveratrol and exercise training are protective against the development of certain indices of LV dysfunction and aortic wall structure in this model. Resveratrol and exercise training increased ejection fraction and fractional shortening, and prevented the increase in mitral valve deceleration time from occurring. Resveratrol and exercise training also restored elastin and aortic wall integrity, despite no improvement in aortic PWV velocity. Although the mechanisms for these benefits were not determined, the purpose of his study was to characterize cardiovascular function and establish the proof-of-concept for the beneficial effects on resveratrol and exercise training on cardiovascular function and structure in a well-established mouse model of familial AD. Our preliminary observations also warrant further mechanistic studies to investigate the underlying mechanisms responsible for the observed beneficial effects as well as to expand the study to aged AD mice that present with more severe AD-like brain pathology. Finally, our data are consistent with the hypothesis that preventative medicine in the form of nutrition and exercise promote cardiovascular health and may delay age-related diseases.
Acknowledgments
This work was funded by the Diabetes Action and Research Education Foundation (TLB), and the Midwestern University Office of Research and Sponsored Programs (TLB, ME).
Author contributions
TLB, ME, and JRB conceived and designed the experiments. BK and NAT performed the echocardiography measurements. MRA, MFA, and MAD performed the exercise and feeding studies. MRA performed the aortic staining experiments. ME and TLB wrote the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MRA was a recipient of a Midwestern University Summer Research Fellowship. The authors report no other conflicts of interest in this work.
References
1. Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262(18):2551–2556.
2. Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer’s beta-amyloid peptide (1-40) in cultured hippocampal neurons. Exp Neurol. 1995;131(2):193–202.
3. Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3(1):75–80.
4. Selkoe DJ. Deciphering the genesis and fate of amyloid beta-protein yields novel therapies for Alzheimer disease. J Clin Invest. 2002;110(10):1375–1381. doi:10.1172/JCI16783
5. Eckert A, Hauptmann S, Scherping I, et al. Soluble beta-amyloid leads to mitochondrial defects in amyloid precursor protein and tau transgenic mice. Neurodegener Dis. 2008;5(3–4):157–159. doi:10.1159/000113689
6. Hauptmann S, Scherping I, Drose S, et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30(10):1574–1586. doi:10.1016/j.neurobiolaging.2007.12.005
7. Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526.
8. Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi:10.1016/j.nlm.2013.07.002
9. Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12(1):27–43. doi:10.1007/s12017-009-8109-7
10. Zulli R, Nicosia F, Borroni B, et al. Increased prevalence of silent myocardial ischaemia and severe ventricular arrhythmias in untreated patients with Alzheimer’s disease and mild cognitive impairment without overt coronary artery disease. Clin Neurol Neurosurg. 2008;110(8):791–796. doi:10.1016/j.clineuro.2008.05.002
11. de Bruijn RF, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12:130. doi:10.1186/s12916-014-0141-2
12. Almahmoud MF, Gonzalez HM, Swett K, et al. Association of cardiac structure and function with neurocognition in hispanics/latinos: the echocardiographic study of latinos. Mayo Clin Proc Innov Qual Outcomes. 2018;2(2):165–175. doi:10.1016/j.mayocpiqo.2018.02.003
13. Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322(7300):1447–1451.
14. Cowppli-Bony P, Dartigues JF, Orgogozo JM. [Vascular risk factors and Alzheimer disease risk: epidemiological studies review]. Psychol Neuropsychiatr Vieil. 2006;4(1):47–60.
15. de Toledo Ferraz Alves TC, Ferreira LK, Wajngarten M, Busatto GF. Cardiac disorders as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2010;20(3):749–763. doi:10.3233/JAD-2010-091561
16. Sposato LA, Ruiz Vargas E, Riccio PM, et al. Milder Alzheimer’s disease pathology in heart failure and atrial fibrillation. Alzheimers Dement. 2017;13(7):770–777. doi:10.1016/j.jalz.2016.12.002
17. Troncone L, Luciani M, Coggins M, et al. Abeta amyloid pathology affects the hearts of patients with Alzheimer’s disease: mind the heart. J Am Coll Cardiol. 2016;68(22):2395–2407. doi:10.1016/j.jacc.2016.08.073
18. Turdi S, Guo R, Huff AF, Wolf EM, Culver B, Ren J. Cardiomyocyte contractile dysfunction in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. PLoS One. 2009;4(6):e6033. doi:10.1371/journal.pone.0006033
19. Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. doi:10.1001/jama.2009.1144
20. Morris MC. The role of nutrition in Alzheimer’s disease: epidemiological evidence. Eur J Neurol. 2009;16 Suppl 1:1–7. doi:10.1111/j.1468-1331.2009.02735.x
21. Radak Z, Hart N, Sarga L, et al. Exercise plays a preventive role against Alzheimer’s disease. J Alzheimers Dis. 2010;20(3):777–783. doi:10.3233/JAD-2010-091531
22. Stranahan AM, Martin B, Maudsley S. Anti-inflammatory effects of physical activity in relationship to improved cognitive status in humans and mouse models of Alzheimer’s disease. Curr Alzheimer Res. 2012;9(1):86–92.
23. Souza LC, Filho CB, Goes AT, et al. Neuroprotective effect of physical exercise in a mouse model of Alzheimer’s disease induced by beta-amyloid(1)(-)(4)(0) peptide. Neurotox Res. 2013;24(2):148–163. doi:10.1007/s12640-012-9373-0
24. Rege SD, Geetha T, Broderick TL, Babu JR, Diet C. Physical activity limit Alzheimer’s disease risk? Curr Alzheimer Res. 2017;14(1):76–93.
25. Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98(24):2709–2715.
26. Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62(7):584–592. doi:10.1016/j.jacc.2013.04.033
27. Tucker WJ, Beaudry RI, Liang Y, et al. Meta-analysis of exercise training on left ventricular ejection fraction in heart failure with reduced ejection fraction: a 10-year update. Prog Cardiovasc Dis. 2018. doi:10.1016/j.pcad.2018.08.006
28. Okamoto T, Hashimoto Y, Kobayashi R. Effects of interval walking training compared to normal walking training on cognitive function and arterial function in older adults: a randomized controlled trial. Aging Clin Exp Res. 2018. doi:10.1007/s40520-018-1093-8
29. Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526.
30. Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17(14):1975–1985. doi:10.1096/fj.03-0168rev
31. de Sa Coutinho D, Pacheco MT, Frozza RL, Bernardi A. Anti-inflammatory effects of resveratrol: mechanistic insights. Int J Mol Sci. 2018;19(6):1812. doi:10.3390/ijms19061812
32. Breen DM, Dolinsky VW, Zhang H, et al. Resveratrol inhibits neointimal formation after arterial injury through an endothelial nitric oxide synthase-dependent mechanism. Atherosclerosis. 2012;222(2):375–381. doi:10.1016/j.atherosclerosis.2012.03.021
33. Dolinsky VW, Soltys CL, Rogan KJ, et al. Resveratrol prevents pathological but not physiological cardiac hypertrophy. J Mol Med (Berl). 2015;93(4):413–425. doi:10.1007/s00109-014-1220-8
34. Sung MM, Das SK, Levasseur J, et al. Resveratrol treatment of mice with pressure-overload-induced heart failure improves diastolic function and cardiac energy metabolism. Circ Heart Fail. 2015;8(1):128–137. doi:10.1161/CIRCHEARTFAILURE.114.001677
35. Fernando P, Bonen A, Hoffman-Goetz L. Predicting submaximal oxygen consumption during treadmill running in mice. Can J Physiol Pharmacol. 1993;71(10–11):854–857.
36. Dolinsky VW, Jones KE, Sidhu RS, et al. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590(11):2783–2799.
37. Lee L, Cui JZ, Cua M, et al. Aortic and cardiac structure and function using high-resolution echocardiography and optical coherence tomography in a mouse model of Marfan syndrome. PLoS One. 2016;11(11):e0164778. doi:10.1371/journal.pone.0164778
38. Gibson C, Nielsen C, Alex R, et al. Mild aerobic exercise blocks elastin fiber fragmentation and aortic dilatation in a mouse model of Marfan syndrome associated aortic aneurysm. J Appl Physiol (1985). 2017;123(1):147–160. doi:10.1152/japplphysiol.00132.2017
39. Gianni D, Li A, Tesco G, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121(10):1216–1226. doi:10.1161/CIRCULATIONAHA.109.879510
40. Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction. N Engl J Med. 2013;368(18):1755. doi:10.1056/NEJMra1106180
41. Willis MS, Patterson C. Proteotoxicity and cardiac dysfunction–Alzheimer’s disease of the heart? N Engl J Med. 2013;368(5):455–464. doi:10.1056/NEJMra1106180
42. Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi:10.1016/j.neuron.2005.01.040
43. Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421.
44. Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298(3):H833–H843. doi:10.1152/ajpheart.00418.2009
45. Rimbaud S, Ruiz M, Piquereau J, et al. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6(10):e26391. doi:10.1371/journal.pone.0026391
46. Zhang F, Wang H, Wu Q, et al. Resveratrol protects cortical neurons against microglia-mediated neuroinflammation. Phytother Res. 2013;27(3):344–349. doi:10.1002/ptr.4734
47. Dolinsky VW, Chakrabarti S, Pereira TJ, et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832(10):1723–1733. doi:10.1016/j.bbadis.2013.05.018
48. Gupta PK, DiPette DJ, Supowit SC. Protective effect of resveratrol against pressure overload-induced heart failure. Food Sci Nutr. 2014;2(3):218–229. doi:10.1002/fsn3.92
49. Riba A, Deres L, Sumegi B, Toth K, Szabados E, Halmosi R. Cardioprotective effect of resveratrol in a postinfarction heart failure model. Oxid Med Cell Longev. 2017;2017:6819281. doi:10.1155/2017/6819281
50. Wallerath T, Deckert G, Ternes T, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106(13):1652–1658.
51. Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. doi:10.1113/jphysiol.2004.068197
52. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi:10.1038/nature05354
53. Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. doi:10.1016/j.cmet.2011.10.002
54. Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi:10.1016/j.cmet.2012.04.003
55. Serpiello FR, McKenna MJ, Bishop DJ, et al. Repeated sprints alter signaling related to mitochondrial biogenesis in humans. Med Sci Sports Exerc. 2012;44(5):827–834. doi:10.1249/MSS.0b013e318240067e
56. Calik AN, Ozcan KS, Yuksel G, et al. Altered diastolic function and aortic stiffness in Alzheimer’s disease. Clin Interv Aging. 2014;9:1115–1121. doi:10.2147/CIA.S63337
57. Lin KH, Chiu CH, Kuo WW, et al. The preventive effects of edible folic acid on cardiomyocyte apoptosis and survival in early onset triple-transgenic Alzheimer’s disease model mice. Environ Toxicol. 2018;33(1):83–92. doi:10.1002/tox.22498
58. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–511. doi:10.1161/CIRCULATIONAHA.109.886655
59. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–881. doi:10.1001/2012.jama.10503
60. Tsao CW, Lyass A, Larson MG, et al. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:11. doi:10.1161/JAHA.115.002189
61. Pase MP, Himali JJ, Mitchell GF, et al. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: the framingham third generation cohort study. Hypertension. 2016;67(3):513–519. doi:10.1161/HYPERTENSIONAHA.115.06610
62. Cocciolone AJ, Hawes JZ, Staiculescu MC, Johnson EO, Murshed M, Wagenseil JE. Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;315(2):H189–H205. doi:10.1152/ajpheart.00087.2018
63. Hibender S, Franken R, van Roomen C, et al. Resveratrol inhibits aortic root dilatation in the Fbn1C1039G/+ Marfan mouse model. Arterioscler Thromb Vasc Biol. 2016;36(8):1618–1626. doi:10.1161/ATVBAHA.116.307841
64. Ahmet I, Tae HJ, Lakatta EG, Talan M. Long-term low dose dietary resveratrol supplement reduces cardiovascular structural and functional deterioration in chronic heart failure in rats. Can J Physiol Pharmacol. 2017;95(3):268–274. doi:10.1139/cjpp-2016-0512
65. Fry JL, Al Sayah L, Weisbrod RM, et al. Vascular smooth muscle sirtuin-1 protects against diet-induced aortic stiffness. Hypertension. 2016;68(3):775–784. doi:10.1161/HYPERTENSIONAHA.116.07622
66. Kroner ES, Lamb HJ, Siebelink HM, et al. Pulse wave velocity and flow in the carotid artery versus the aortic arch: effects of aging. J Magn Reson Imaging. 2014;40(2):287–293. doi:10.1002/jmri.24470
67. Gu Q, Wang B, Zhang XF, Ma YP, Liu JD, Wang XZ. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol. 2014;56:37–44. doi:10.1016/j.exger.2014.02.014
68. Huang CC, Cheng HF, Zhu BP, et al. Studying arterial stiffness using high-frequency ultrasound in mice with Alzheimer disease. Ultrasound Med Biol. 2017;43(9):2054–2064. doi:10.1016/j.ultrasmedbio.2017.04.029
69. Palatini P, Julius S. Elevated heart rate: a major risk factor for cardiovascular disease. Clin Exp Hypertens. 2004;26(7–8):637–644.
70. Tan I, Butlin M, Liu YY, Ng K, Avolio AP. Heart rate dependence of aortic pulse wave velocity at different arterial pressures in rats. Hypertension. 2012;60(2):528–533. doi:10.1161/HYPERTENSIONAHA.112.194225
71. Perpetuini D, Cardone D, Chiarelli AM, et al. Autonomic impairment in Alzheimer’s disease is revealed by complexity analysis of functional thermal imaging signals during cognitive tasks. Physiol Meas. 2019. doi:10.1088/1361-6579/ab057d
72. Nosaka T, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28(2):204–212.
73. Moraes-Teixeira Jde A, Felix A, Fernandes-Santos C, Moura AS, Mandarim-de-Lacerda CA, de Carvalho JJ. Exercise training enhances elastin, fibrillin and nitric oxide in the aorta wall of spontaneously hypertensive rats. Exp Mol Pathol. 2010;89(3):351–357. doi:10.1016/j.yexmp.2010.08.004
74. Mattison JA, Wang M, Bernier M, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20(1):183–190. doi:10.1016/j.cmet.2014.04.018
75. Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118(Pt 10):2325–2340. doi:10.1242/jcs.02360
76. Wittenauer J, Mackle S, Sussmann D, Schweiggert-Weisz U, Carle R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia. 2015;101:179–187. doi:10.1016/j.fitote.2015.01.005
77. Kawanishi N, Niihara H, Mizokami T, Yada K, Suzuki K. Exercise training attenuates neutrophil infiltration and elastase expression in adipose tissue of high-fat-diet-induced obese mice. Physiol Rep. 2015;3:9. doi:10.14814/phy2.12534
78. Gilbert A, Wyczalkowska-Tomasik A, Zendzian-Piotrowska M, Czarkowska-Paczek B. Training differentially regulates elastin level and proteolysis in skeletal and heart muscles and aorta in healthy rats. Biol Open. 2016;5(5):556–562. doi:10.1242/bio.017459
79. Reitz C, Brickman AM, Luchsinger JA, Wu WE, Small SA, Tang MX. Frequency of subclinical heart disease in elderly persons with dementia. Am J Geriatr Cardiol. 2007;16(3):183–188.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.