Back to Journals » International Journal of Nanomedicine » Volume 15
Beneficial Effect of TaON-Ag Nanocomposite Titanium on Antibacterial Capacity in Orthopedic Application
Authors Hu CC , Chang CH , Chang Y, Hsieh JH, Ueng SWN
Received 15 June 2020
Accepted for publication 16 September 2020
Published 13 October 2020 Volume 2020:15 Pages 7889—7900
DOI https://doi.org/10.2147/IJN.S264303
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
Chih-Chien Hu,1– 4 Chih-Hsiang Chang,1– 3 Yuhan Chang,1– 3 Jang-Hsing Hsieh,5,6 Steve Wen-Neng Ueng1– 3
1Bone and Joint Research Center, Chang Gung Memorial Hospital, Kweishan, Taoyuan 33305, Taiwan; 2Department of Orthopedic Surgery, Chang Gung Memorial Hospital, Kweishan, Taoyuan 33305, Taiwan; 3College of Medicine, Chang Gung University, Kweishan, Taoyuan 33302, Taiwan; 4Department of Orthopedic Surgery, Xiamen Chang Gung Hospital, Xiamen, Fujian, People’s Republic of China; 5Department of Materials Engineering, Ming Chi University of Technology, Taishan, Taipei 24301, Taiwan; 6Center for Thin Film Technologies and Applications, Ming Chi University of Technology, Taishan, Taipei 24301, Taiwan
Correspondence: Steve Wen-Neng Ueng
Department of Orthopedic Surgery, Chang Gung Memorial Hospital, No. 5, Fu-Hsin St, Kweishan, Taoyuan 33305, Taiwan
Tel +88633281200-2420
Fax +886 33278113
Email [email protected]
Purpose: In this study, a novel oxygenated nanocomposite thin film, TaON-Ag, was investigated in vitro and in vivo to evaluate its biocompatibility and antibacterial ability.
Material and Methods: The antibacterial ability of TaON-Ag nanocomposite-coated titanium (Ti) was evaluated using the Kirby-Bauer disk diffusion susceptibility test. The effects of TaON-Ag nanocomposite-coated metal on osteogenesis were further evaluated in an in vitro osteogenic culture model with rat marrow-derived mesenchymal stem cells (rMSCs). Furthermore, titanium rods coated with TaON-Ag were implanted into a rat femur fracture model either with or without osteomyelitis to investigate the effects of TaON-Ag in osteogenesis.
Results: The TaON-Ag-coated Ti exhibited an effective antibacterial effect against Staphylococcus aureus, coagulase-negative Staphylococcus, and the Gram-negative strains Escherichia coli and Pseudomonas aeruginosa. Using an osteogenic culture with rMSCs and a rat femoral fracture model, the TaON-Ag-coated Ti did not interfere with the ossification of rMSCs in vitro or during fracture healing in vivo. Field-emission scanning electron microscopy (FE-SEM) revealed that coating with TaON-Ag could inhibit pathogen adhesion and biofilm formation in both Staphylococcus aureus and Escherichia coli.
Conclusion: Using the proposed novel oxygenation process, TaON-Ag nanocomposite-coated Ti yielded robust biocompatibility and antibacterial ability against common microorganisms in orthopedic infections, thereby demonstrating potential for use in clinical applications.
Keywords: silver nanoparticles, ossification, antibacterial ability, biofilm
Introduction
Silver nanoparticles (AgNPs) are increasingly being used in various fields, including medical, health care, consumer, and industrial purposes, because of their unique physical and chemical properties. Their applications include use as antibacterial agents; in industrial household- and healthcare-related products, consumer products, medical device coatings, orthopedics, and drug delivery; and even as anticancer agents.1,2 The antibacterial activity of the majority of metal coatings is closely linked to their ionic or nano form, rather than the bulk material. Despite extensive research, coating implants with a thin layer of metal is still not a standard practice.3,4 This has been reported in devices that are predominantly temporary, permanent orthopedic devices, and vascular prostheses. Antimicrobial effects have been observed when AgNPs are used in trauma implants, tumor prostheses, bone cement, and when combined with hydroxyapatite coatings. Although promising results have been obtained for in vitro and in vivo studies, there are a limited number of clinical studies.5,6 Based on the results of in vitro studies, it was revealed that a Ag plasma-modified hierarchical TiO2 film can be fabricated on the surface of Ti via acid etching to produce micropits, followed by hydrothermal treatment to generate TiO2 nanorods, and subsequent plasma immersion ion implantation to impregnate Ag onto the surface of TiO2. The antimicrobial activity, bioactivity, and cytocompatibility were systematically evaluated, demonstrating enhanced bioactivity and bacteriostatic effects.7,8 The addition of Ag demonstrated a minor effect on the bioactivity. It had no adverse effect on and even promoted the MG63 cell function, including adhesion, spread, and proliferation. Devlin-Mullin et al reported 3D Ti structures fabricated using selective laser melting and Ag nanolayer coating using atomic layer deposition on either methicillin-resistant Staphylococcus aureus (MRSA) or Staphylococcus epidermidis. The results revealed significant reductions in both bacterial recovery and biofilm formation. Ag nanolayer coatings on Ti implants significantly reduce pathogenic in vitro biofilm formation, facilitating in vivo vascularization and osseointegration.9 As such, it is a promising technique for clinical orthopedic applications. However, most of these reported materials are not yet appropriate for clinical application. The majority of the limited clinical usage of these Ag nanoparticle materials has the potential for adverse health effects as a result of prolonged exposure at varying ion concentration levels in humans, and their environment effects have not yet been established.5,10
In our previous study, TaN-(Ag,Cu) thin films prepared using reactive co-sputtering with a Ta target in plasma formed using Ar, N, and O exhibited in vitro antibacterial activity.11–13 Theoretically, even at the doping level, the incorporation of oxygen into a nitrogen framework causes specific changes in the physical and chemical properties, which can affect the release of Ag and Cu ions. Therefore, variation in the O/N atomic ratio affects the mechanical, electrochemical, and bio-related properties.14 The current study was designed to evaluate the in vitro and in vivo applications of this proposed oxygenated nanocomposite thin-film metal with respect to biocompatibility and antibacterial ability.
Materials and Methods
Preparation of Nanocomposite Thin Films
TaN thin films were prepared using reactive co-sputtering with a Ta target in plasma formed using argon and nitrogen. Previously, TaN thin films doped with soft metals (ie, TaN–Cu, TaN–Ag, and TaN–(Ag,Cu)) were prepared using a hybrid process that starts with reactive co-sputtering, followed by rapid thermal annealing (RTA) at temperatures higher than 350 °C. Ta metals and their alloys exhibit excellent biocompatibility. Therefore, TaN is an excellent choice for protective coatings in bio-related applications. Cu and Ag as doping elements are immiscible with TaN, which facilitates the synthesis of TaN–Ag or TaN–(Ag,Cu) nanocomposite thin films.13
Moreover, the oxynitrides of transition metals exhibit tunable optical, mechanical, electrical, and bio-related properties by varying the ratio of oxygen to nitrogen. Theoretically, even at a doping level, the incorporation of oxygen into a nitrogen framework causes specific changes in the physical and chemical properties. The oxygen flow rate can be varied from 0 sccm to 15 sccm.14 The sample with oxygen deposited at a rate of 1 sccm exhibited the highest roughness, resulting in high biocompatibility. According to our unpublished data, the sample with oxygen deposited at a rate of 1–3 sccm exhibited the best antibacterial efficiency against Escherichia coli. Under these process parameters, TaON-Ag thin films were prepared using reactive co-sputtering with an oxygen flow rate of 1 sccm. The material characteristics of the TaON-Ag-coated thin films, including those obtained using XRD, SEM, EDS, and AFM surface analysis, are presented in Supplement Figures 1-4.
In vitro Antibacterial Ability Assay
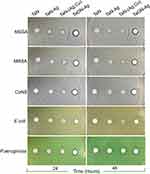
To evaluate the antibacterial ability of various metal coatings on common pathogenic bacteria in muscle-skeletal infections, the antibacterial ability of TaN-Ag, TaN-(Ag,Cu), TaON-Ag, and TaN-coated Ti alloys on various common pathogenic bacteria was investigated using the Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. The antibacterial effects of various coating samples were evaluated using selected strains, including methicillin-sensitive Staphylococcus aureus ATCC 25923 (MSSA), methicillin-resistant Staphylococcus aureus ATCC 43300 (MRSA), coagulase-negative Staphylococcus ATCC 12228 (CoNS), Pseudomonas aeruginosa ATCC 27853, and Escherichia coli ATCC 25922. Metal specimens were sterilized in an autoclave at 121 °C for 40 min. Bacteria at a concentration of McFarland 0.5 (1 × 108) were inoculated onto a culture medium on a Mueller-Hinton (M-H) agar plate by the streaking method. Different coated samples were manually placed on an agar plate using forceps, and the M-Hagar plates were incubated at 37 °C for 24 and 48 h. After incubation, bacterial activity was recorded according to the inhibition zone.
In vitro Osteogenic Assay
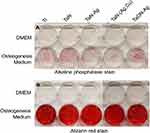
In the development of new materials for orthopedic applications, the osteogenetic properties should not be compromised. Thus, the various coated metals were co-cultured using rat-marrow-derived mesenchymal stem cells (rMSCs), and the effect of Ag ions on the ossification process was observed. The ossification capacities were evaluated by measuring the alkaline phosphatase (ALP) activity and the degree of calcification. rMSCs were obtained from the bone marrow of six-week-old male Sprague-Dawley (SD) rats (BioLASCO Taiwan Co., Ltd). The cells were suspended in MSC medium containing 10% FBS (Gibco) and 1% Pen-Strep, and seeded into 10-cm culture dishes. The dishes were maintained at 37 °C in a 5% CO2 incubator for 1–2 weeks. (5 × 107 cells/dish). After 4 days at 37 °C in the incubator, the medium was replaced to remove non-adherent cells. rMSCs were subcultured to passage 3 (P3) for transduction and transplantation.15 The P3 cells were then cultured in an osteogenic induction medium (DMEM-LG with 10% FBS, 0.1 mM dexamethasone, 0.05 mM ascorbic acid-2-phosphate, and 2 mM β-glycerophosphate) for up to 21 days.16
The ALP profile in the culture medium was measured using an ALP substrate (p-nitrophenyl phosphate). The assay was performed according to the manufacturer’s instructions. An adequate amount of a 1 mg mL−1 solution of ALP substrate in a 50 mmol L−1 glycine buffer and 1 mmol L−1 MgCl2·6H2O was mixed with the waste culture medium collected from each microbioreactor. After 3 min, the solution was removed and transferred to a tube containing an equal volume of 1 mol L−1 NaOH. The absorbance of the resulting solution was determined at 405 nm and compared with a standard curve generated from a series of dilutions of p-nitrophenol.16
In the calcium assay, calcium was extracted using 0.6 M HCl on day 20 of the osteogenic induction culture. Aliquots of the extract were mixed with a reagent from a commercial calcium assay kit (Biotron, Hemet, CA, USA), and the absorbance was determined at 575 nm. The calcium concentration was determined using the standard curve generated from a series of dilutions of CaCl2.16 Calcium deposition was further evaluated using Alizarin red stain.
In vivo Osteogenic Assay Using a Rat Femoral Fracture Model
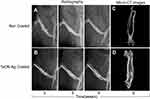
Twelve SD rats obtained from BioLASCO Taiwan Co. Ltd were used in this study, which was approved by the Institutional Animal Care and Use Committee (IACUC) at Chang Gung University, which follows the Animal Protection Law of the Council of Agriculture, Executive Yuan, R.O.C. After the administration of anesthesia following an approved protocol, the fur overlying the anterior distal femur was shaved and the skin was prepped with betadine prior to performing a 10-mm incision. A 1-mm-diameter Ti needle with or without a coating of TaON-Ag was inserted into the intercondylar notch after exposing the knee joint using the trephine technique. The nail was advanced through the intramedullary canal into the proximal femur. Following insertion of the needle, a closed mid-shaft fracture was generated using the method described by Nyman et al.17 The wound was then irrigated with sterile saline and closed with an absorbable suture. The fractures and needle positions were confirmed using anterior-posterior (AP) radiographs. Radiographic examination of the SD rats was performed at two-week intervals between 3 and 9 weeks post-fracture. Both the fractured and un-fractured contralateral femur specimens were harvested by performing joint disarticulations and clearing soft tissue without disturbing the fracture callus at nine weeks post-operation. The specimens were stored in a phosphate buffer solution-soaked gauze at 20 °C. The bones from different time points were investigated using radiography, micro-computed tomography (µCT), and histological examinations.17,18
In vivo Antibacterial Assay
The in vivo antibacterial assay of the Ti needle with or without coating with TaON-Ag was evaluated using the rat osteomyelitis model.19 Twelve Balb/c mice (BioLASCO Taiwan Co., Ltd) were used in this study, which was approved by the Institutional Animal Care and Use Committee (IACUC) at Chang Gung University. To evaluate the antibacterial activity of the test materials, the femur osteomyelitis model with MSSA ATCC 25923 and Escherichia coli ATCC 25922 infection was used. In summary, the needle was inserted into the right distal femoral bone cavity of mice simultaneously loaded with 5 × 105 CFU micro-organisms. To determine the time interval for which the surface of the TaON-Ag can hinder the adhesion of bacteria, animals were sacrificed on the 3rd, 7th, and 14th-day post-needle insertion. The adhesion status of the bacterial biofilm on the surface of the implant was examined using field-emission scanning electron microscopy (FE-SEM, JSM-6701F, JEOL, USA).20
Results
Our data show that a TaON-Ag coating on the surface of titanium has an antibacterial effect on MSSA and MRSA, CoNS, E. coli, and Pseudomonas in vitro, as well as Staphylococcus aureus and E. coli in vivo, without fracture healing interference.
Antibacterial Ability
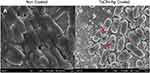
Based on the Kirby-Bauer Disk Diffusion Susceptibility Test Protocol, it was determined that the TaON-Ag-coated Ti exhibited the best antibacterial effect on MSSA, MRSA, CoNS, Pseudomonas a., and E. coli, in comparison with TaN-Ag, TaN-(Ag,Cu), and the control TaN coating (Figure 1). Further examination of the surface of the sample using FE-SEM with high magnification (15,000X) revealed the formation of a biofilm by E. coli cells two days post in vitro co-culture with Ti alloy (Figure 2A). In contrast, abnormal geometry E. coli cell distortion and cytoplasmic membrane wrinkles were observed 24 h post-co-culture for the TaON-Ag-coated sample, leading to cyto-plasma leakage and cell lysis (Figure 2B).
In vitro Osteogenic Assay
Osteogenetic differentiation of rMSCs under various TaN coating profiles was assayed based on staining of the calcium deposition using alizarin red on day 20. Calcium mineralization was observed in all the study groups, and the density of calcium deposition did not differ significantly among coatings. To further confirm the osteogenetic induction effect of TaNs on rMSC differentiation, the ALP activity of rMSCs cultured with various types of TaN was assayed on day 10 (Figure 3). Although over-expression of ALP activity was observed in the TaN study group, a significant decrease in ALP expression for the TaN-(Ag,Cu) coating was also observed. Thus, by integrating the results for both the antibacterial disk diffusion test and the examination of osteogenetic ability, TaON-Ag was selected for additional in vivo studies, considering the optimal broad-spectrum antimicrobial ability and non-inferior osteogenesis potential.
In vivo Osteogenic Assay
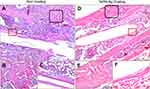
Fractured rat femurs underwent sequential radiography at 3, 5, 7, and 9 weeks post-operation. Both the callus area and bone remodeling of the fracture were observed during follow-up. The TaON-Ag coating did not have an adverse effect on the fracture healing process. Thus, all fractures achieved union (Figure 4). Histological examination of fracture healing based on hematoxylin and eosin (H&E) staining revealed the presence of newly generated callus and bone in both the pure Ti and TaON-Ag study groups. In comparison with the control group (Ti alloy), TaON-Ag did not trigger significant immune reactions (Figure 5). After the SD rats were sacrificed at 9 weeks post-operation, all specimens were scanned after removal of the needle using an animal CT imaging system (BioScan, Washington, DC). (Figure 4C and D) The µCT images were reconstructed using the accompanying software. Subsequent analysis was performed using third-party software (Amira, Carlsbad, CA) to calculate the bone volume and bone density (averaged Hounsfield units [HU] in the cylindrical volume of interest) in the region 10 mm above to 10 mm below the fracture site of the regenerated bone with 11 cuts in comparison with the un-fractured contralateral femur.17 There was no significant difference in the remodeled bone and contralateral femur between the TaON-Ag-coated and control groups. (Table 1) The augmentation rate of the callus and remodeled bone was 154.8% for the TaON-Ag group, which is almost equal to 154.5% for the Ti control group (augmentation rate: average R/average L).
 |
Table 1 Average Hounsfield Units (HU) of the Cylindrical Bone Volume of the Fractured Rat Femur (R) and the Contralateral Site Femur (L) |
In vivo Antibacterial Assay
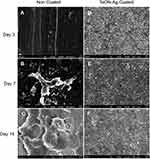
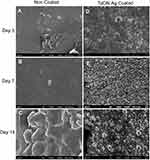
After the rats were sacrificed, the bone needles were removed from the femur of the specimens, and possible bacterial adhesion on the surface of the TaON-Ag-coated and non-coated needles were assessed using FE-SEM. The relatively smooth surface of the Ti needles observed at 5000X magnification for the unstained bacterial control group at 7 and 14 days post-implantation served as the control (Supplement Figure 4). The non-coated Ti needles with MSSA revealed single to multiple cocci adhesion at 3 and 7 days onwards (Figure 6A and B), and biofilm formation after 14 days (Figure 6C). It was also determined that a few E. coli cells (red arrows) were present on the surface after 3 days (Figure 7A), followed by biofilm formation 7 and 14 days after implantation of the non-coated Ti needle (Figure 7B and C). In contrast, SEM imaging did not reveal any bacterial adhesion for the TaON-Ag-coated needle infected with either S. aureus or E. coli until 14 days (Figures 6D–F and 7D–F).
Discussion
Based on the current data, the TaON-Ag nanocomposite thin film-coated Ti exhibits antibacterial activity without interfering with the osteogenic processes in either the in vitroorin vivo models. The commercial application of AgNP materials has been widely accepted for several decades. Ag ions and AgNPs are used as antimicrobials in a variety of industrial, healthcare, and domestic applications. Ag has been used in wound dressings, and as an antimicrobial coating for medical devices to prevent biofilm formation.1,3,5,7,21,22 The clinical potential of Ag nanotechnology is of particular interest in the field of orthopedics, wherein the infection of implanted devices is a persistent threat.5,23–25 The biological activity of AgNPs depends on several factors, including surface chemistry, size, size distribution, shape, particle morphology, particle composition, coating/capping, agglomeration, dissolution rate, particle reactivity in solution, and efficiency of ion release. In addition, the type of reducing agents used for the synthesis of AgNPs is a crucial factor for the determination of cytotoxicity.1,26 Dissolved Ag cations are biochemically active agents that interfere with bacterial cell membrane permeability and cellular metabolism. Ag also contributes to the formation of reactive oxygen species and other mechanisms that potentially affect prokaryotic cells. There is concern regarding the toxicity of Ag ions. However, most reports have documented acceptably low, and even an absence of, cytotoxicity based on in vitro and in vivo investigations with variable control release. A high concentration of Ag-containing coating was used, which revealed the dose-dependent manner of inferior biocompatibility.27,28 The development of green methods for nanomaterial synthesis is another important aspect of research in the field of nanotechnology. The reported biosynthesis of Fe3O4@Ag nanocomposite by Spirulinaplatensis cyanobacterium impacts the expression of efflux pump genes and also shows effective antibacterial activity against antibiotic-resistant ciprofloxacin-resistant S. aureus (CRSA) in medicine.29
Our data may serve as the basis for a new fabrication process for orthopedic materials. AgNPs seem to be alternative antibacterial agents, in comparison with traditional antibiotics, and they demonstrate the potential to address the problem of bacterial resistance. Among several promising nanomaterials, AgNPs may potentially be used as antibacterial agents because of their large surface-to-volume ratios and crystallographic surface structure.1 In comparison with previously published reports on Ag ion materials, the proposed material exhibits relatively stable mechanical characteristics that are more suitable for orthopedic applications because of the controlled release of metal ions and high wear resistance.11 If the antibacterial Ag or Cu nanoparticles are formed on a ceramic matrix, the constructed nanocomposite thin films exhibit varying antibacterial behaviors, depending on the metal particle size and the total exposed amount.7,22 According to our previous reports, it was determined that TaN-Ag is more effective against E. coli, whereas TaN-Cu is more effective against S. aureus. Therefore, Ag–Cu nanocomposite thin films have been proposed and used because of their potential for a wider spectrum of bactericidal effects.13 However, the results of the comparison of the manufacture of the different TaN-Ag, TaN-(Ag,Cu), and TaON-Ag coatings using the Kirby-Bauer Disk Diffusion Susceptibility Test Protocol to evaluate the broad-spectrum antibacterial ability confirm that the best effectiveness as well as the widest inhibition zone were achieved using the TaON-Ag nanocomposite thin film.
The improvement of the antibacterial capacity of TaN nanocomposite thin films using an appropriate oxygen/nitrogen atomic ratio has been investigated in only a few articles.8,30 Li et al reported Ag-embedded hierarchical TiO2 films with excellent antimicrobial activity. TiO2 can inhibit microbial adhesion because of its excellent photocatalytic activity. When excited by photons with a minimum energy equal to its bandgap, electron/hole (e−/h+) pairs are produced and promote strong reduction-oxidation (redox) reactions that kill the microbe cells. The release of Ag ions was a result of the silver oxide in the aqueous solution. These Ag ions react with the hydroxide radicals to form unstable silver hydroxide, thereby decomposing the Ag ions in the aqueous solution. In this experiment, Ag-O ionic bonds were detected on the surface of the coated film. Once the Ag-O is in contact with the aqueous solution, a redox reaction releases Ag ions and free radicals. This results in the release of a large amount of Ag ions, demonstrating an excellent antibacterial effect. The greater inhibition zone revealed that Gram-positive or Gram-negative pathogens were not inhibited by the TaON-Ag coating material. This significant finding is consistent with the results presented in our previous reports: oxygen induction during fabrication results in the exchange of mechanical, electrochemical, and bio-related properties of the nanocomposite thin film.14 The biocompatibility of oxygen induction TaON-Ag nanocomposite thin films has been confirmed using an MTT assay, according to previous publications.14,31 However, the osteogenic ability of Ag composite thin-film materials has been investigated in only a few studies. Li et al reported a Ag-embedded hierarchical TiO2 film, which exhibited excellent bioactivity and cytocompatibility, as a promising candidate for orthopedic and dental implants.8 Their data revealed that the embedded Ag caused minor degradation of the bioactivity and exhibited insignificant toxic side effects on MG63 cell function, based on an examination of the adhesion/morphology, proliferation, and vitality. Using immunofluorescence microscopy, it was determined that all cell functions were maintained or even promoted. Qing et al reported that AgNPs demonstrate the capacity to enhance mineralization and ALP expression in MC3T3-E1 cells at a concentration of 20 μg/mL.23 When AgNPs were incorporated into TiO2nanotubes and cultured with MC3T3-E1 cells, favorable effects were observed for the promotion of cell spreading based on cell morphology assays. The current study using various metal coatings co-cultured with rMSCs under osteogenic induction revealed variable ALP staining performance. TaON-Ag exhibited non-inferior performance compared with the Ti material; however, its performance was much better than that of TaN-Ag and TaN-(Ag,Cu). The current data confirm that oxygen induction TaON-Ag results in improved biocompatibility, particularly for osteogenic induction.
To confirm the in vivo osteogenic induction ability for orthopedic applications, the femoral fracture healing model of the SD rat was adopted following the methodology described by Kevin R. O’Neill.18 The fracture healing process was evaluated by performing serial radiography, quantitative µCT, and histological examinations. The results of our SD rat fracture model revealed no significant difference in callus formation and the fracture healing bone augmentation ratio between the TaON-Ag-coated and control groups, which is consistent with our in vitro data. In our literature review, we found that only a few articles reported animal fracture model results for AgNPs. Zhang et al reported that AgNPs promote the proliferation and osteogenesis of MSCs, which improved fracture healing in mice.6 AgNPs are also considered to facilitate the promotion of the formation of fracture callus and the early closure of the fracture gap in vivo, based on the findings of Qing et al.23 Moreover, we investigated an implant infection model based on a method described by Guan et al.20 Their imaging and histopathology results revealed that the injected MRSA was killed by the Ag-TiO2 coating. FE-SEM examination was utilized to identify the morphology and membrane integrity of both E. coli and S. aureus on AgNPs.8,20,32 These studies examined the in vitro antibacterial ability of these particles, and morphological changes in AgNP-treated bacteria were observed. These findings are highly correlated with the present results.
Conclusion
TaON-Ag-coated titanium exhibits broad-spectrum antibacterial ability without interfering with in vitro and in vivo fracture healing. Further validation of its superior antibacterial durability, as well as its low cytotoxicity, may lead to additional clinical applications of this TaON-Ag nanocomposite thin film.
Funding
This work was supported by grants from CMRPG3C1041, CMRPG3C1042, CMRPG3C1043, and CMRPG3G0491 from the Chang Gung Medical Foundation and MOST 107-2314-B-182A-031 of the Ministry of Science and Technology, Taiwan, Republic of China. In addition, we would like to thank Dr. Mei-Feng Chen for helpful discussions related to this study and the Center for Advanced Molecular Imaging and Translation, Chang Gung Memorial Hospital, Linkou.
Disclosure
Dr Chih-Chien Hu reports grants from Chang Gung Medical foundation, grants from Ministry of Science and Technology, Taiwan, during the conduct of the study. Professor Jang-Hsing Hsieh reports grants from Ministry of Science and Technology ROC, during the conduct of the study. The authors declare no other potential conflicts of interest for this work.
References
1. Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534. doi:10.3390/ijms17091534
2. Qin H, Cao H, Zhao Y, et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials. 2014;35(33):9114–9125. doi:10.1016/j.biomaterials.2014.07.040
3. Gallo J, Holinka M, Moucha CS. Antibacterial surface treatment for orthopaedic implants. Int J Mol Sci. 2014;15(8):13849–13880. doi:10.3390/ijms150813849
4. Liu X, Tian A, You J, et al. Antibacterial abilities and biocompatibilities of Ti-Ag alloys with nanotubular coatings. Int J Nanomedicine. 2016;11:5743–5755. doi:10.2147/IJN.S113674
5. Brennan SA, Ni Fhoghlu C, Devitt BM, O’Mahony FJ, Brabazon D, Walsh A. Silver nanoparticles and their orthopaedic applications. Bone Joint J. 2015;97–B(5):582–589. doi:10.1302/0301-620X.97B5.33336
6. Zhang RZ, Lee PY, Lui VCH, et al. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed-Nanotechnol. 2015;11(8):1949–1959. doi:10.1016/j.nano.2015.07.016
7. Lim PN, Chang L, Thian ES. Development of nanosized silver-substituted apatite for biomedical applications: a review. Nanomed-Nanotechnol. 2015;11(6):1331–1344. doi:10.1016/j.nano.2015.03.016
8. Li J, Liu X, Qiao Y, Zhu H, Ding C. Antimicrobial activity and cytocompatibility of Ag plasma-modified hierarchical TiO2 film on titanium surface. Colloids Surf B Biointerfaces. 2014;113:134–145. doi:10.1016/j.colsurfb.2013.08.030
9. Devlin-Mullin A, Todd NM, Golrokhi Z, et al. Atomic layer deposition of a silver nanolayer on advanced titanium orthopedic implants inhibits bacterial colonization and supports vascularized de novo bone ingrowth. Adv Healthc Mater. 2017;6(11):1700033. doi:10.1002/adhm.201700033
10. AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–290. doi:10.1021/nn800596w
11. Hsieh JH, Chiu CH, Li C, Wu W, Chang SY. Development of anti-wear and anti-bacteria TaN-(Ag,Cu) thin films - a review. Surf Coat Tech. 2013;233:159–168. doi:10.1016/j.surfcoat.2013.05.013
12. Hsieh JH, Li C, Lin YC, Chiu CH, Hu CC, Chang YH. Antibacteria and anti-wear TaN-(Ag,Cu) nanocomposite thin films deposited on polyether ether ketone. Thin Solid Films. 2015;584:277–282. doi:10.1016/j.tsf.2015.02.063
13. Hsieh JH, Yeh TH, Li C, Chiu CH, Huang CT. Antibacterial properties of TaN-(Ag,Cu) nanocomposite thin films. Vacuum. 2013;87:160–163. doi:10.1016/j.vacuum.2012.02.016
14. Hsieh JH, Lai YH, Lin YC, et al. Structure analysis, mechanical property, and biocompatibility of TaOxNy thin films. Surf Coat Tech. 2016;303:54–60. doi:10.1016/j.surfcoat.2016.03.047
15. Chuang CK, Wong TH, Hwang SM, et al. Baculovirus transduction of mesenchymal stem cells: in vitro responses and in vivo immune responses after cell transplantation. Mol Ther. 2009;17(5):889–896. doi:10.1038/mt.2009.30
16. Hung FC, Chang YH, Sue LC, Chao CCK. Gas7 mediates the differentiation of human bone marrow-derived mesenchymal stem cells into functional osteoblasts by enhancing Runx2-dependent gene expression. J Orthop Res. 2011;29(10):1528–1535. doi:10.1002/jor.21425
17. Nyman JS, Munoz S, Jadhav S, et al. Quantitative measures of femoral fracture repair in rats derived by micro-computed tomography. J Biomech. 2009;42(7):891–897. doi:10.1016/j.jbiomech.2009.01.016
18. O’Neill KR, Stutz CM, Mignemi NA, et al. Micro-computed tomography assessment of the progression of fracture healing in mice. Bone. 2012;50(6):1357–1367. doi:10.1016/j.bone.2012.03.008
19. Bernthal NM, Stavrakis AI, Billi F, et al. A mouse model of post-arthroplasty staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010;5(9):e12580. doi:10.1371/journal.pone.0012580
20. Guan M, Chen YMF, Wei Y, et al. Long-lasting bactericidal activity through selective physical puncture and controlled ions release of polydopamine and silver nanoparticles-loaded TiO2 nanorods in vitro and in vivo. Int J Nanomed. 2019;14:2903–2914. doi:10.2147/IJN.S202625
21. Kose N, Otuzbir A, Peksen C, Kiremitci A, Dogan A. A silver ion-doped calcium phosphate-based ceramic nanopowder-coated prosthesis increased infection resistance. Clin Orthop Relat Res. 2013;471(8):2532–2539. doi:10.1007/s11999-013-2894-x
22. Zhang BGX, Myers DE, Wallace GG, Brandt M, Choong PFM. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int J Mol Sci. 2014;15(7):11878–11921. doi:10.3390/ijms150711878
23. Qing YA, Cheng L, Li RY, et al. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomed. 2018;13:3311–3327. doi:10.2147/IJN.S165125
24. Morgan EF, Mason ZD, Chien KB, et al. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44(2):335–344. doi:10.1016/j.bone.2008.10.039
25. Zeng X, Xiong S, Zhuo S, et al. Nanosilver/poly (dl-lactic-co-glycolic acid) on titanium implant surfaces for the enhancement of antibacterial properties and osteoinductivity. Int J Nanomedicine. 2019;14:1849–1863.
26. Subbiah R, Jeon SB, Park K, Ahn SJ, Yun K. Investigation of cellular responses upon interaction with silver nanoparticles. Int J Nanomedicine. 2015;10(Spec Iss):191–201.
27. Yonekura Y, Miyamoto H, Shimazaki T, et al. Osteoconductivity of thermal-sprayed silver-containing hydroxyapatite coating in the rat tibia. J Bone Joint Surg Br. 2011;93b(5):644–649. doi:10.1302/0301-620X.93B5.25518
28. Shandiz SAS, Montazeri A, Abdolhosseini M, et al. Functionalization of Ag nanoparticles by glutamic acid and conjugation of Ag@Glu by thiosemicarbazide enhances the apoptosis of human breast cancer MCF-7 cells. J Clust Sci. 2018;29(6):1107–1114. doi:10.1007/s10876-018-1424-0
29. Shokoofeh N, Moradi-Shoeili Z, Naeemi AS, Jalali A, Hedayati M, Salehzadeh A. Biosynthesis of Fe3O4@Ag nanocomposite and evaluation of its performance on expression of norA and norB efflux pump genes in ciprofloxacin-resistant staphylococcus aureus. Biol Trace Elem Res. 2019;191(2):522–530. doi:10.1007/s12011-019-1632-y
30. Huang HL, Chang YY, Chen HJ, Chou YK, Lai CH, Chen MYC. Antibacterial properties and cytocompatibility of tantalum oxide coatings with different silver content. J Vac Sci Technol A. 2014;32(2):02B117. doi:10.1116/1.4862543
31. Huang Y, Song GQ, Chang XT, et al. Nanostructured Ag+-substituted fluorhydroxyapatite-TiO2 coatings for enhanced bactericidal effects and osteoinductivity of Ti for biomedical applications. Int J Nanomed. 2018;13:2665–2684. doi:10.2147/IJN.S162558
32. Lomeli-Marroquin D, Cruz DM, Nieto-Arguello A, et al. Starch-mediated synthesis of mono- and bimetallic silver/gold nanoparticles as antimicrobial and anticancer agents. Int J Nanomed. 2019;14:2171. doi:10.2147/IJN.S192757
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.