Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
BCc1 Nanomedicine Therapeutic Effects in Streptozotocin and High-Fat Diet Induced Diabetic Kidney Disease
Authors Fakharzadeh S, Argani H, Dadashzadeh S , Kalanaky S , Mohammadi Torbati P, Nazaran MH , Basiri A
Received 2 December 2019
Accepted for publication 31 March 2020
Published 17 April 2020 Volume 2020:13 Pages 1179—1188
DOI https://doi.org/10.2147/DMSO.S240757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Konstantinos Tziomalos
Saideh Fakharzadeh,1,2 Hassan Argani,1 Simin Dadashzadeh,3 Somayeh Kalanaky,2 Peyman Mohammadi Torbati,4 Mohammad Hassan Nazaran,2 Abbas Basiri5
1Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran; 3Department of Pharmaceutics and Nanotechnology, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 4Department of Pathology, Labbafinejad Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran; 5Urology and Nephrology Research Center, Shahid Labbafinejad Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Correspondence: Mohammad Hassan Nazaran
Department of Research and Development, Sodour Ahrar Shargh Company, Tehran, Iran
Tel/ Fax +98 21 88992123
Email [email protected]
Abbas Basiri
Urology and Nephrology Research Center, Shahid Labbafinejad Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Tel/ Fax +98 21 22567222
Email [email protected]
Background: One common feature of chronic diseases, such as cancer, diabetes and chronic kidney disease (CKD), is the disruption of iron metabolism and increase in labile iron pool, which can result in excessive production of harmful oxidative stress. The proper management of iron metabolism in this situation can be a valuable tool to ameliorate pathological events.
Materials and Methods: In the previous studies, the anti-neoplastic effects of BCc1, a nanochelating-based nanomedicine with iron-chelating property, were demonstrated in cell culture, animal models and clinical trials. In the present study, the therapeutic effects of BCc1 in animal model of diabetic kidney disease (DKD), induced by streptozotocin injection (35 mg/kg) and high-fat diet consumption, were evaluated.
Results: The results showed that BCc1 significantly decreased HOMA-IR index, uric acid, blood urea nitrogen, malondialdehyde and 8-isoprostane. In addition, it reduced urinary albumin excretion rate and albumin-to-creatinine ratio in comparison to DKD control rats. This nanomedicine had no negative impact on liver iron content, hemoglobin level, red blood cell count, hematocrit and mean corpuscular volume, while it significantly decreased aspartate aminotransferase and alanine aminotransferase compared to DKD control group. Moreover, the histopathological assessment indicated that lesser glomerular basement membrane and wrinkling, mesangial matrix expansion and pathological changes in proximal cortical tubules were seen in the kidney samples of BCc1-treated rats.
Conclusion: In conclusion, BCc1 as an iron-chelating agent shows promising impacts in DKD animal model, which can ameliorate biochemical and pathological events of this disease.
Keywords: BCc1, diabetic kidney disease, chronic kidney disease, nanochelating technology, iron
Introduction
Chronic kidney disease (CKD) is a common disease defined as a long-term impairment of kidney function normally diagnosed in the presence of other comorbidities, especially diabetes, hypertension and cardiovascular disease.1–3 In fact, diabetes is the main cause of CKD in the world and diabetic kidney disease (DKD) continues to be a chronic and highly destructive complication of this disease.4
In recent decades, numerous studies have focused on the critical role of iron metabolism disruption (with or without iron overload) in diabetes, CKD, cancer and other chronic diseases initiation, progression and development.5–7 Although iron as a trace element has vital roles in the physiology of human beings, it is a redox-active element, so a vicious cycle of iron dis-homeostasis, inflammation and oxidative stress is formed mostly through free radicals induced by iron in chronic diseases,8–12 and therefore manipulation of iron homeostasis in chronic diseases, especially in DKD, is evaluated in dozens of studies using iron chelators to assess their impacts.13–17 In fact, iron chelation in these situations means iron re-distribution, not iron excretion.18
In the previous studies, anti-neoplastic effects of BCc1 nanostructure, synthetized based on nanochelating technology, were evaluated in animal models of breast cancer, proving increase in survival and reduction in tumor size growth.19 In addition, in a randomized, double-blind, placebo-controlled study, overall survival and quality of life in gastric cancer patients were significantly improved by BCc1.20
One important feature of BCc1 is its iron-chelating property.19 In light of this fact and based on the demonstrated role of iron metabolism changes in chronic diseases, especially in DKD, and the effects of iron chelators in chronic diseases, in the present study, the effects of BCc1 on pathological events in DKD using the experimental model of kidney disease induced by streptozotocin and a high-fat diet were assessed.
Materials and Methods
Animal Use
All studies were conducted as per national and international guidelines of Shahid Beheshti University of Medical Sciences for the care and use of laboratory animals approved by the ethics committee of Shahid Beheshti University of Medical Sciences.
Male Wistar rats (150–180 g), with 3 rats in every polycarbonate conventional cages, were used in the present study, which began 1 week after animals were adapted environmentally, and were kept under 12-hr dark/light cycles with ease of access to feed and water.
Protocol of Study
In the present study, diabetes was induced using a high-fat diet following intraperitoneal (i.p) injection of a low dose of streptozotocin (STZ) (35 mg/kg dissolved in 0.1 M sodium citrate buffer, pH 4.4).21 Three weeks later, rats with fasting blood glucose (FBS) levels >250 mg/dL were considered diabetic according to which grouping was performed (8 rats in each group): group H; healthy rats, group DKD control; diabetic kidney disease control rats, group LDB; low dose of BCc1 (0.4 µg/kg/day, i.p), group MDB; medium dose of BCc1 (4 µg/kg/day, i.p), group HDB; high dose of BCc1 (40 µg/kg/day, i.p). In week 10 (before using BCc1) and 18 (8 weeks after BCc1 injection) of the study, urine and blood samples were collected and then rats were euthanized by an overdose of isoflurane anesthesia, and finally, right kidney and liver of each rat were separated for further analyses. The timeline of the study is shown in Figure 1.
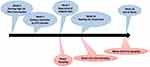 |
Figure 1 The timeline of study. |
Blood Sample Collection and Analysis
In week 10 and 18 of the study and after rats’ fasting for 12 hrs, blood samples were collected and the hematologic parameters were assayed using automated blood analyzer (Sysmex, Japan) to determine red blood cell (RBC) count, hemoglobin (Hb), hematocrit (Hct) and mean cell volume (MCV) levels. The plasma of blood samples was separated using refrigerated micro centrifuge (Cleaver Scientific, England). They were then used for biochemical assay of glucose, creatinine (Cr), blood urea nitrogen (BUN), uric acid, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) by commercial ELISA kits (Pars Azmoon, Tehran, Iran), and then microplate reader (Bioteck, USA). Insulin was assayed by ELISA kits (Zellbio, Ulm, Germany) and homeostasis model assessment of insulin resistance (HOMA-IR) index was computed based on fasting plasma insulin and glucose levels according to HOMA-IR=(fasting insulin (µg/L) × fasting blood glucose (mg/dL)/405)22,23 formula.
Urine Sample Collection and Analysis
In weeks 10 and 18 of the study (Figure 1) and before using BCc1, all rats were placed in individual metabolic cages (Tecniplast Company, Italy) for the collection of 24-hr urine. After the measurement of 24-hr urine volume, the samples were centrifuged using refrigerated micro centrifuge (Cleaver Scientific, England) at 4°C and 3000 rpm for 5 min. Then, they were kept in a −80°C freezer for evaluation of 24-hr urinary albumin excretion rate (UAER), calculated according to UAER= (urinary albumin (mg/mL) x 24-h urine volume (mL)) formula (commercial ELISA kit, Pars Azmoon, Tehran, Iran). Albumin-to-creatinine ratio (ACR) was calculated according to ACR= (urine albumin (mg/24 hours)/urine creatinine (mg/24 hours)) formula.
Assessment of Oxidative Stress
As malondialdehyde (MDA) and 8-isoprostane are two important markers of lipids oxidative damage,24,25 their concentration in urine was measured using ELISA kits (Zellbio, Ulm, Germany) and then microplate reader (BioTeck, USA), which were expressed relative to the level of urine creatinine.
Hepatic Iron Level Assay
As BCc1 is an iron-chelating agent, hepatic iron content of all rats were examined to assess the effect of BCc1 treatments on this iron storage parameter. The hepatic iron content was calculated using atomic absorption spectrometry (PerkinElmer, USA) with a deuterium background correction using acetylene-air flame atomization. Furthermore, an analytical line of 248.3 nm in a spectral interval of 0.2 nm was utilized to make the measurements. Also, the standard addition method was used to determine iron concentration. Sample digestion was accomplished in an MDS-2000 microwave sample-preparation system (CEM, Matthews, NC, USA) in Teflon cartridges by a mixture of nitric acid (5 mL) and H2O2 (2 mL) for 20 minutes at 120 psi pressure, and eventually, the final product was evaluated directly in Teflon cartridges.26
Histopathological Examination of the Kidney Tissue
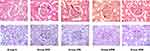
At the end of the study and after sacrificing the animals, the right kidneys were removed and fixed in 10% paraformaldehyde for Periodic-Acid Schiff (PAS) and Hematoxylin and Eosin (H and E) staining for further histology studies while they were magnified 400 times using bright field microscope (Olympus BX-41, USA). Glomerular basement membrane (GBM) thickening, GBM wrinkling, mesangial matrix expansion and hyper-cellularity and pathological changes in proximal cortical tubules were analyzed in the samples. The histopathological samples were observed blindly concerning the identity of the kidney samples and scored for severity of changes using the following scale: -, no damage; +, mild damage; ++, moderate damage; and +++, severe damage.27
Statistics
The results were expressed as means ± S.D. The Statistical differences between healthy and DKD rats and also between DKD and BCc1-treated rats were compared by Bonferroni test for post-hoc analysis after one-way analysis of variance (ANOVA) test. Statistical differences were considered significant if P-value were <0.05. The Statistical Package for Social Sciences (SPSS; 24, IBM, USA) software was used and a value of P < 0.05 was considered statistically significant.
Results
Effect of Nanomedicine on UAER
Albuminuria is a predictive feature for the progression of DKD,28 and besides, urinary ACR increase can be an indicator of renal dysfunction.29 In the present study, diabetic induction using a high-fat diet and STZ resulted in significant UAER and ACR increase in all DKD rats (P≤0.05). An 8-week treatment using BCc1 in all three doses decreased UAER and ACR significantly compared to DKD control group (P≤0.05), where MBD and HBD rats (medium and high dose of nanomedicine) had the lowest UAER and ACR level (Figure 2).
Effect of BCc1 on Plasma FBS, Cr, BUN, Uric Acid, AST, ALT, HOMA-IR and CBC
In clinical diabetic nephropathies, testing BUN and uric acid is common to confirm the kidney’s malfunction and to evaluate the associated pathology,30 and besides HOMA-IR index increase is a parameter showing insulin resistance.31 In addition, ALT and AST (aminotransferases) are routinely used to monitor and evaluate hepatic function and in CKD patients, changes in these enzymes is reported in several studies.32,33
Eight weeks after STZ injection in the current study, FBS, BUN and uric acid elevated dramatically (P≤0.05) in all DKD-induced groups (DKD, LDB, MDM and HDB), and no significant difference was seen between groups in Cr level during the study (Figure 3).
In week 18, significant increase in BUN, uric acid, HOMA-IR, AST and ALT was observed in DKD control rats compared to healthy ones (P≤0.05), while BCc1 treatment at high dose decreased uric acid, BUN, AST and ALT compared to DKD control rats (P≤0.05), reduced uric acid, ALT, AST and HOMA-IR levels at medium dose (P≤0.05), and had significant effects on uric acid and ALT at low dose. This nanomedicine had no significant effects on FBS level. Moreover, there was no significant difference in RBC, Hb, hematocrit and Hct levels between healthy and all DKD-induced groups (Figure 4), yet there was only a significant decrease in MCV in DKD control rats (P≤0.05).
BCc1 and Oxidative Stress-Related Parameters
Generally, human and animal studies have revealed that lipid oxidation plays a major role in anticipating renal diseases progression and reaction to treatments (Figure 5). MDA and isoprostanes34 are well-known markers of lipid oxidation in kidney diseases.
In the present study, urine 8-isoprostane and urine MDA increased in all DKD-induced rats compared to healthy ones (P≤0.05), and BCc1 had no effects on urine 8-isoprostane and MDA at low dose, but it had a significant effect on urine 8-isoprostane and MDA at medium dose (P≤0.05). The treated rats by a high dose of this nanomedicine had the least urine 8-isoprostane and MDA compared to DKD control rats (P≤0.05).
Effect of Nanomedicine on Liver Iron Content
As BCc1 is an iron-chelating agent, in the present study, the liver iron content in all groups was assayed using atomic absorption method to evaluate the effects of this nano chelator on liver iron as one of the most strategic body iron storages, the results of which indicated that BCc1 treatment had no negative effects on this parameter (Figure 6).
 |
Figure 6 Liver iron content in each group. |
BCc1 and Histopathological Study of Kidney Samples
In H&E and also PAS stained samples from DKD control rats, marked GBM thickening, GBM wrinkling, mesangial matrix expansion and hyper-cellularity and pathological changes in proximal cortical tubules were noticed. The scoring of observations showed that there was amelioration of pathological signs in tissue samples of BCc1-treated rats in all three doses in comparison to DKD controls, and HDB samples had minimal pathological changes (Figure 7, Table 1).
 |
Table 1 Scoring the Histopathological Observations |
Discussion
Iron is a vital micronutrient incorporated by heme proteins, iron sulfur clusters or other functional groups,35 and plays a vital role in cellular and organismal function.36 Since iron is a redox-active element, its metabolism is tightly controlled through binding proteins (ferritin, transferrin, ceruloplasmin, etc.) and different pathways.37 In other words, if the level of non-protein bound iron (labile iron pool) increases in cell and cell compartments, it results in production of high reactive and harmful oxygen species, which can damage cell biomolecules.38
In chronic diseases, such as cancer, diabetes and CKD, increase in labile iron pool ends in a vicious cycle of iron dis-homeostasis, inflammation and oxidative stress.39–41 The altered iron metabolism can be due to greater dietary iron intake from food42 or chronic iron administration,43–45 or it can be related to systemic and local dys-regulations of iron homeostasis, without having real iron overload.46 To break the harmful cycle, several studies have evaluated the effects of iron chelators in these situations.14–17
In the previous studies, anti-neoplastic effects of BCc1 nanomedicine with iron-chelating property were demonstrated in several experiments.19,20,47 In the present study, this nanomedicine was tested in animal model of DKD to evaluate its impacts on various biochemical and histopathological parameters of this disease.
The biochemical analysis of plasma showed that BCc1-treated animals at medium dose had significantly lower HOMA-IR index compared to DKD controls, but the low and high doses of BCc1 had no significant effects on this parameter. The different effects of BCc1 doses on this parameter could be due to the close relationship between the dose and therapeutic effects of drugs, especially nanomedicines, as this fact has been also reported in the previous studies such one study by Mao eta al, who showed that only one out of three doses of Nanoencapsulated Echinacea purpurea Ethanol Extract demonstrated anti-diabetic effects.48
The beneficial effect of iron manipulation on insulin resistance, using iron restriction or iron chelation, is confirmed in several studies. Hodaei et al50 in a randomized, double-blind clinical trial showed that daily administration of curcumin, considered as a natural iron chelator studied in cancer patients,49 has positive effects on reduction of FBS in patients with type 2 diabetes.50 In another study, Cooksey et al reported that dietary iron restriction or iron chelation exhibited significant increase in insulin sensitivity and beta-cell function in obese (ob/ob lep-/-) mice.51 In a study by Mihailidou et al, they showed that using an iron chelator, named ciclopirox, enhanced pancreatic islet health in vitro and in diabetic mice in vivo. In another study by Zou et al, it was reported that using deferiprone reduced insulin resistance and MDA level in high-carbohydrate-fat diet and streptozotocin model of DKD.52
In the present study, BCc1 decreased uric acid, BUN, UAER and ACR and ameliorated pathological signs in kidney samples compared to DKD controls. Consistence with our results, in one study by Zou et al, they showed that using deferiprone, an oral iron chelator, decreases ACR and tubulointerstitial fibrosis in animal model of DKD.53 Also Marks et al, reported that the mentioned iron chelator could delay the onset of albuminuria and reduced BUN concentrations.15
It should be noted that due to the role of iron in different vital processes, especially hematopoiesis, any interventions by chelation therapy must have no negative effects on iron-related physiological parameters, and due to the importance of this, in the present study, hepatic iron content and RBC count, Hb, Hct and MCV levels were assayed, the results of which showed no negative effects of using BCc1 on important iron-related physiological parameters. On the other hand, this nanomedicine significantly decreased urine MDA and 8-isoprostane concentration as the well-known markers of oxidative stress,54,55 and previous studies have already proved that the production of these markers is closely related to iron metabolism disruption.56,57 So since BCc1 nanomedicine has iron-chelating properties, it can reduce free radicals by iron manipulation and finally affect DKD pathological process through iron redistribution without any negative effects on functional iron-related parameters. Similarly, the previous study on another nanochelating-based structure (DIBc metal-organic framework) showed that this nanostructure could ameliorate diabetes-related biochemical parameters such as FBS, HOMA-IR and plasma MDA without any negative effects on iron-related physiological parameters.21
It is well known that ALT and AST activities are tightly related to normal liver function and on the other hand studies have revealed that iron disorder and increase in labile iron pool is one of the important causes of liver function damage and the activity of these aminotranspherases.7,58-60 In a study by An et al, it was reported that elevation of serum transaminase activities can be associated with iron metabolism changes and hyper-ferritinemia and in this situation hepcidine increase may reflect a protective response.61 In another study, Wang et al demonstrated that STZ, disrupts iron metabolism through significant reduction of liver hepcidin level and increase in intestinal iron absorption.62 In the present study, there was remarkable increase in plasma concentration of ALT and AST in DKD controls, whereas BCc1 could ameliorate AST and ALT activities, indicating that this nanomedicine protects liver function from STZ toxicity.
In conclusion, using BCc1 nanomedicine in an animal model of DKD can ameliorate HOMA-IR index, uric acid, BUN, ALT, AST and urine MDA, plus 8-isoprostane, ACR and UAER. It can also decrease DKD-related pathological changes without any negative effects on iron-related physiological parameters. Therefore, BCc1, as an iron-chelating agent, shows promising results in the DKD animal model as the leading cause of CKD.
Acknowledgment
The support for this work was provided by the Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences and Department of Research and Development at Sodour Ahrar Shargh Knowledge-based Company.
Disclosure
Mohammad Hassan Nazaran is the owner of Nanochelating Technology and Executive Manager and Chairman of Management Board of Sodour Ahrar Shargh Company, Tehran, Iran. He also reports a patent US8288587B2 with royalties paid. The authors report no other conflicts of interest in this work.
References
1. Fraser SD, Roderick PJ, Aitken G, et al. Chronic kidney disease, albuminuria and socioeconomic status in the Health Surveys for England 2009 and 2010. J Public Health (Bangkok). 2014;36(4):577–586. doi:10.1093/pubmed/fdt117
2. Fraser SD, Roderick PJ, May CR, et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2015;16(1):193. doi:10.1186/s12882-015-0189-z
3. Tonelli M, Wiebe N, Guthrie B, et al. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int. 2015;88(4):859–866. doi:10.1038/ki.2015.228
4. Pyram R, Kansara A, Banerji MA, Loney-Hutchinson L. Chronic kidney disease and diabetes. Maturitas. 2012;71(2):94–103. doi:10.1016/j.maturitas.2011.11.009
5. Kontoghiorghe CN, Kontoghiorghes GJ. New developments and controversies in iron metabolism and iron chelation therapy. World J Methodol. 2016;6(1):1–19. doi:10.5662/wjm.v6.i1.1
6. Panwar B, Gutierrez OM. Disorders of iron metabolism and anemia in chronic kidney disease. Semin Nephrol. 2016;36(4):252–261. doi:10.1016/j.semnephrol.2016.05.002
7. Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21(Suppl 1):S6–S20. doi:10.1111/hdi.12542
8. Osterholm EA, Georgieff MK. Chronic inflammation and iron metabolism. J Pediatr. 2015;166(6):1351–1357 e1351. doi:10.1016/j.jpeds.2015.01.017
9. Oberley TD. Oxidative damage and cancer. Am J Pathol. 2002;160(2):403–408. doi:10.1016/S0002-9440(10)64857-2
10. Wawer AA, Jennings A, Fairweather-Tait SJ. Iron status in the elderly: a review of recent evidence. Mech Ageing Dev. 2018;175:55–73. doi:10.1016/j.mad.2018.07.003
11. Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011;12(1):21–29. doi:10.3909/ricm0555
12. Miranda MA, Lawson HA. Ironing out the details: untangling dietary iron and genetic background in diabetes. Nutrients. 2018;10(10):1437. doi:10.3390/nu10101437
13. Lehmann C, Islam S, Jarosch S, et al. The utility of iron chelators in the management of inflammatory disorders. Mediators Inflamm. 2015;2015:516740. doi:10.1155/2015/516740
14. Shah SV, Rajapurkar MM. The role of labile iron in kidney disease and treatment with chelation. Hemoglobin. 2009;33(5):378–385. doi:10.3109/03630260903212233
15. Marks ES, Bonnemaison ML, Brusnahan SK, et al. Renal iron accumulation occurs in lupus nephritis and iron chelation delays the onset of albuminuria. Sci Rep. 2017;7(1):12821. doi:10.1038/s41598-017-13029-4
16. Morita T, Nakano D, Kitada K, et al. Chelation of dietary iron prevents iron accumulation and macrophage infiltration in the type I diabetic kidney. Eur J Pharmacol. 2015;756:85–91. doi:10.1016/j.ejphar.2015.03.053
17. Ikeda Y, Ozono I, Tajima S, et al. Iron chelation by deferoxamine prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. PLoS One. 2014;9(2):e89355. doi:10.1371/journal.pone.0089355
18. Kakhlon O, Breuer W, Munnich A, Cabantchik ZI. Iron redistribution as a therapeutic strategy for treating diseases of localized iron accumulation. Can J Physiol Pharmacol. 2010;88(3):187–196. doi:10.1139/Y09-128
19. Kalanaky S, Hafizi M, Fakharzadeh S, et al. BCc1, the novel antineoplastic nanocomplex, showed potent anticancer effects in vitro and in vivo. Drug Des Devel Ther. 2016;10:59–70. doi:10.2147/DDDT.S89694
20. Hafizi M, Kalanaky S, Moaiery H, et al. A randomized, double-blind, placebo-controlled investigation of BCc1 nanomedicine effect on survival and quality of life in metastatic and non-metastatic gastric cancer patients. J Nanobiotechnology. 2019;17(1):52. doi:10.1186/s12951-019-0484-0
21. Fakharzadeh S, Kalanaky S, Hafizi M, Nazaran MH, Zardooz H. DIBc, a nanochelating-based nano metal-organic framework, shows anti-diabetic effects in high-fat diet and streptozotocin-induced diabetic rats. Int J Nanomedicine. 2019;14:2145–2156. doi:10.2147/IJN.S196050
22. Antunes LC, Elkfury JL, Jornada MN, Foletto KC, Bertoluci MC. Validation of HOMA-IR in a model of insulin-resistance induced by a high-fat diet in Wistar rats. Arch Endocrinol Metabol. 2016;60(2):138–142. doi:10.1590/2359-3997000000169
23. Al-Trad B, Alkhateeb H, Alsmadi W, Al-Zoubi M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/streptozotocin-induced diabetic rat. Life Sci. 2019;216:183–188. doi:10.1016/j.lfs.2018.11.034
24. Krata N, Zagozdzon R, Foroncewicz B, Mucha K. Oxidative stress in kidney diseases: the cause or the consequence? Arch Immunol Ther Exp (Warsz). 2018;66(3):211–220. doi:10.1007/s00005-017-0496-0
25. Gyuraszova M, Gurecka R, Babickova J, Tothova L. Oxidative stress in the pathophysiology of kidney disease: implications for noninvasive monitoring and identification of biomarkers. Oxid Med Cell Longev. 2020;2020:5478708. doi:10.1155/2020/5478708
26. Fakharzadeh S, Sahraian MA, Hafizi M, et al. The therapeutic effects of MSc1 nanocomplex, synthesized by nanochelating technology, on experimental autoimmune encephalomyelitic C57/BL6 mice. Int J Nanomedicine. 2014;9:3841–3853. doi:10.2147/IJN.S64630
27. Dogukan A, Tuzcu M, Juturu V, et al. Effects of chromium histidinate on renal function, oxidative stress, and heat-shock proteins in fat-fed and streptozotocin-treated rats. J Ren Nutr. 2010;20(2):112–120. doi:10.1053/j.jrn.2009.04.009
28. Savage S, Estacio RO, Jeffers B, Schrier RW. Urinary albumin excretion as a predictor of diabetic retinopathy, neuropathy, and cardiovascular disease in NIDDM. Diabetes Care. 1996;19(11):1243–1248. doi:10.2337/diacare.19.11.1243
29. Uchida M, Sakaguchi Y, Miyamoto Y. A novel vitamin K1 2,3-epoxide reductase (VKOR) inhibitor, 3-acetyl-5-methyltetronic acid, reduces experimental glomerulonephritis. J Vet Med Sci. 2012;74(7):863–869. doi:10.1292/jvms.11-0530
30. Sasatomi Y, Kaneoka H, Abe Y, et al. Anemia and hypertension are risk factors for both renal prognosis and survival in patients with diabetes mellitus. Clin Exp Nephrol. 2009;13(5):473–479. doi:10.1007/s10157-009-0191-5
31. Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med. 2000;342(14):1040–1042. doi:10.1056/NEJM200004063421408
32. Renke M, Knap N, Tylicki L, et al. [Isoprostanes - important marker of the oxidative stress estimation in patients with chronic kidney disease]. Pol Merkur Lekarski. 2013;34(199):14–17. Polish.
33. Garrido P, Ribeiro S, Fernandes J, et al. Iron-hepcidin dysmetabolism, anemia and renal hypoxia, inflammation and fibrosis in the remnant kidney rat model. PLoS One. 2015;10(4):e0124048. doi:10.1371/journal.pone.0124048
34. Scholze A, Jankowski J, Pedraza-Chaverri J, Evenepoel P. Oxidative stress in chronic kidney disease. Oxid Med Cell Longev. 2016;2016:8375186. doi:10.1155/2016/8375186
35. Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61(6):933–952. doi:10.1136/gut.2010.214312
36. Martin W, Russell MJ. On the origins of cells: a hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos Trans R Soc London B Biol Sci. 2003;358(1429):
37. Papanikolaou G, Pantopoulos K. Iron metabolism and toxicity. Toxicol Appl Pharmacol. 2005;202(2):199–211. doi:10.1016/j.taap.2004.06.021
38. Imam MU, Zhang S, Ma J, Wang H, Wang F. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients. 2017;9(7):671. doi:10.3390/nu9070671
39. Gonzalez N, Prieto I, Del Puerto-Nevado L, et al. 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget. 2017;8(11):18456–18485. doi:10.18632/oncotarget.14472
40. Bystrom LM, Rivella S. Cancer cells with irons in the fire. Free Radic Biol Med. 2015;79:337–342. doi:10.1016/j.freeradbiomed.2014.04.035
41. Shah SV, Fonseca VA. Iron and diabetes revisited. Diabetes Care. 2011;34(7):1676–1677. doi:10.2337/dc11-0700
42. Leggett BA, Brown NN, Bryant SJ, Duplock L, Powell LW, Halliday JW. Factors affecting the concentrations of ferritin in serum in a healthy Australian population. Clin Chem. 1990;36(7):1350–1355. doi:10.1093/clinchem/36.7.1350
43. Zhuang T, Han H, Yang Z. Iron, oxidative stress and gestational diabetes. Nutrients. 2014;6(9):3968–3980. doi:10.3390/nu6093968
44. Day SM, Duquaine D, Mundada LV, et al. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003;107(20):2601–2606. doi:10.1161/01.CIR.0000066910.02844.D0
45. Lee DH, Folsom AR, Jacobs DR. Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: the Iowa Women’s Health Study. Diabetologia. 2004;47(2):185–194. doi:10.1007/s00125-003-1307-1
46. Gozzelino R, Arosio P. Iron homeostasis in health and disease. Int J Mol Sci. 2016;17(1):130. doi:10.3390/ijms17010130
47. Hafizi M, Soleimani M, Noorian S, et al. Effects of BCc1 nanoparticle and its mixture with doxorubicin on survival of murine 4T1 tumor model. Onco Targets Ther. 2019;12:4691–4701. doi:10.2147/OTT.S200446
48. Mao CF, Zhang XR, Johnson A, He JL, Kong ZL. Modulation of diabetes mellitus-induced male rat reproductive dysfunction with micro-nanoencapsulated echinacea purpurea ethanol extract. Biomed Res Int. 2018;2018:4237354. doi:10.1155/2018/4237354
49. Hatcher HC, Singh RN, Torti FM, Torti SV. Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Med Chem. 2009;1(9):1643–1670. doi:10.4155/fmc.09.121
50. Hodaei H, Adibian M, Nikpayam O, Hedayati M, Sohrab G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol Metab Syndr. 2019;11(1):41. doi:10.1186/s13098-019-0437-7
51. Cooksey RC, Jones D, Gabrielsen S, et al. Dietary iron restriction or iron chelation protects from diabetes and loss of beta-cell function in the obese (ob/ob lep-/-) mouse. Am J Physiol Endocrinol Metab. 2010;298(6):E1236–1243. doi:10.1152/ajpendo.00022.2010
52. Zou C, Liu X, Liu R, et al. Effect of the oral iron chelator deferiprone in diabetic nephropathy rats. J Diabetes. 2017;9(4):332–340. doi:10.1111/1753-0407.12420
53. Zou C, Xie R, Bao Y, et al. Iron chelator alleviates tubulointerstitial fibrosis in diabetic nephropathy rats by inhibiting the expression of tenascinC and other correlation factors. Endocrine. 2013;44(3):666–674. doi:10.1007/s12020-013-9907-0
54. Cui X, Gong J, Han H, et al. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J Thorac Dis. 2018;10(5):3088–3097. doi:10.21037/jtd.2018.05.92
55. Fritz KS, Petersen DR. An overview of the chemistry and biology of reactive aldehydes. Free Radic Biol Med. 2013;59:85–91. doi:10.1016/j.freeradbiomed.2012.06.025
56. Gao W, Li X, Gao Z, Li H. Iron increases diabetes-induced kidney injury and oxidative stress in rats. Biol Trace Elem Res. 2014;160(3):368–375. doi:10.1007/s12011-014-0021-9
57. Thethi T, Rajapurkar M, Walker P, et al. Urinary catalytic iron in patients with type 2 diabetes without microalbuminuria–a substudy of the ACCORD Trial. Clin Chem. 2011;57(2):341–344. doi:10.1373/clinchem.2010.155887
58. Sturm B, Goldenberg H, Scheiber-Mojdehkar B. Transient increase of the labile iron pool in HepG2 cells by intravenous iron preparations. Eur J Biochem. 2003;270(18):3731–3738. doi:10.1046/j.1432-1033.2003.03759.x
59. Powell EE, Ali A, Clouston AD, et al. Steatosis is a cofactor in liver injury in hemochromatosis. Gastroenterology. 2005;129(6):1937–1943. doi:10.1053/j.gastro.2005.09.015
60. Bernardo B. [Induced sterilization and ethics]. Servir. 1992;40(2):60–61. Portuguese.
61. An P, Wang H, Wu Q, et al. Elevated serum transaminase activities were associated with increased serum levels of iron regulatory hormone hepcidin and hyperferritinemia risk. Sci Rep. 2015;5(1):13106. doi:10.1038/srep13106
62. Wang H, Li H, Jiang X, Shi W, Shen Z, Li M. Hepcidin is directly regulated by insulin and plays an important role in iron overload in streptozotocin-induced diabetic rats. Diabetes. 2014;63(5):1506–1518. doi:10.2337/db13-1195
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.