Back to Journals » Journal of Pain Research » Volume 10
Bay11-7082 attenuates neuropathic pain via inhibition of nuclear factor-kappa B and nucleotide-binding domain-like receptor protein 3 inflammasome activation in dorsal root ganglions in a rat model of lumbar disc herniation
Authors Zhang AL, Wang K, Ding LH, Bao XN, Wang X, Qiu XB, Liu JB
Received 15 August 2016
Accepted for publication 2 December 2016
Published 13 February 2017 Volume 2017:10 Pages 375—382
DOI https://doi.org/10.2147/JPR.S119820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Enrica Santarcangelo
Ailiang Zhang, Kun Wang, Lianghua Ding, Xinnan Bao, Xuan Wang, Xubin Qiu, Jinbo Liu
Spine Surgery, Third Affiliated Hospital of Soochow University, Changzhou, People’s Republic of China
Abstract: Lumbar disc herniation (LDH) is an important cause of radiculopathy, but the underlying mechanisms are incompletely understood. Many studies suggested that local inflammation, rather than mechanical compression, results in radiculopathy induced by LDH. On the molecular and cellular level, nuclear factor-kappa B (NF-κB) and nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome have been implicated in the regulation of neuroinflammation formation and progression. In this study, the autologous nucleus pulposus (NP) was implanted in the left L5 dorsal root ganglion (DRG) to mimic LDH in rats. We investigated the expression of NF-κB and the components of NLRP3 inflammasome in the DRG neurons in rats. Western blotting and immunofluorescence for the related molecules, including NLRP3, apoptosis-associated speck-like protein containing caspase-1 activator domain (ASC), caspase-1, interleukin (IL)-1β, IL-18, IκBα, p-IκBα, p65, p-p65, and calcitonin gene-related peptide (CGRP) were examined. In the NP-treated group, the activations of NLRP3, ASC, caspase-1, IL-1β, IL-18, p-IκBα, and p-p65 in DRG neurons in rats were elevated at 1 day after surgery, and the peak occurred at 7 days. Treatment with Bay11-7082, an inhibitor of the actions of IKK-β, was able to inhibit expression and activation of the molecules (NLRP3, ASC, caspase-1, IL-1β, IL-18, p-IκBα, and p-p65) and relieve the pain in rats. Our study shows that NF-κB and NLRP3 inflammasome are involved in the maintenance of NP-induced pain, and that Bay11-7082 could alleviate mechanical allodynia and thermal hyperalgesia by inhibiting NF-κB and NLRP3 inflammasome activation.
Keywords: pain, NLRP3, NF-κB, dorsal root ganglion, nucleus pulposus
Introduction
Clinically, lumbar disc herniation (LDH) is a common disorder that can cause low back pain and severe radiculopathy, characterized by hyperalgesia, allodynia.1 Long-term chronic pain seriously compromises the quality of life of these patients. Radicular pain, one of the most common types of neuropathic pain, is mostly triggered by the mechanical compression and the local inflammation.2 Although the symptoms and physical sign are clear in clinic, the pathophysiology of neuropathic pain associated with LDH is incompletely clarified. The current therapeutic strategies are incapable of completely relieving the neuropathic pain.
Nuclear factor-kappa B (NF-κB) is a transcription factor that has a pivotal role in the onset of inflammation. In the quiescent state, NF-κB proteins exist in an inactive state as homo- or heterodimers bound to its inhibitor of NF-κB (IκB) molecules in the cytoplasm. The p65/p50 heterodimer appears to be the most abundant form of NF-κB in mammals. IκB is phosphorylated by the IκB kinase (IKK) complex, which is mainly composed of the subunits IKKα and IKKβ. Subsequently, NF-κB is activated and translocated to the nucleus, and then initiates the transcription of downstream target genes involving inflammation.3 Ma and Bisby reported that the expression of genes induced by NF-κB in the dorsal root ganglion (DRG) neurons may finally lead to events responsible for the pain behaviors after partial sciatic nerve injury.4
The nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome is a cytoplasmic multiprotein complex. It consists of the regulatory subunit NLRP3, the adaptor apoptosis-associated speck-like protein containing caspase-1 activator domain (ASC) and the effector subunit caspase-1. NLRP3 inflammasome can be activated by various pathogens such as bacteria, virus and fungi, and has the ability to sense the sterile tissue damage or metabolic stress. Under the hits of the aforementioned stimuli, caspase-1 is cleaved and subsequently switches pro-interleukin (IL)-1β and pro-IL-18 into their active forms.5 In addition, previous studies have showed that proinflammatory cytokine IL-1β and L-18 play an important role in the progression of pain hypersensitivity and the positive feedback between neurons and glial cells.6,7
Bay11-7082 is a selective inhibitor of IKK-β and it irreversibly inhibits IκBα phosphorylation with resulting in the downregulation of NF-κB activation. Previous study reported that Bay11-7082 was a potent inhibitor of the inflammasome independent of their inhibitory effect on the NF-κB pathway.8 In this study, we examined whether Bay11-7082 attenuates neuropathic pain via inhibiting the activations of NLRP3 inflammasome and NF-κB in the LDH-mimicry rat model.
Experimental procedures
Animals
Ninety adult Sprague Dawley rats (female, 200–250 g) were used in this study. The animals were maintained on a 12-h light/dark cycle with food and water provided ad libitum. Housing was kept at a constant room temperature and humidity level. All surgical procedures and experiments were approved by the Ethical Committee of Soochow University and were in accordance with the Guidelines for the Care and Treatment of Laboratory Animals of the US National Institutes of Health. Animal treatments and procedures were performed according to the Guidelines of the International Association for the Study of Pain. All efforts were made to minimize their pain, discomfort and suffering.
Surgery
The rats were anesthetized with sodium pentobarbital (40 mg/kg, intraperitoneal), and all surgical procedures were instrumented with sterile techniques. As described previously,9,10 rats were placed in a prone position and an incision was made at the spinal midline at levels L4–L6. The fascia was incised just to the left of spinous process of the fifth lumbar vertebrae. Multifidus muscles were gently moved laterally to expose the facet joint and interlaminar space. The left L5 DRG and spinal nerve were exposed by partial unilateral laminotomy and facetectomy without injury of dura mater and mechanical compression at the time of surgery. Nucleus pulposus (NP, ~3 mg) was harvested from between the second and third vertebrae of each tail, and the amount harvested was almost the same for different rats. Then the obtained NP was gently implanted next to the left L5 nerve root just proximal to the DRG without mechanical compression at the time of surgery. All surgical procedure for sham control was identical with NP-treated group, including harvesting of autologous NP from each tail, except the implantation of autologous NP. After the surgical procedures, the fascia and skin were closed.
Drug preparation
The dosage of Bay11-7082 (Sigma-Aldrich, St. Louis, MO, USA) was determined according to previous studies with minor modifications.11 Bay11-7082 was dissolved in 10% (v/v) dimethyl sulfoxide/phosphate-buffered saline. The treatment group received intraperitoneal injections thrice a week of Bay11-7082 (5 mg/kg) for 4 weeks, whereas the animals in the control groups received only the vehicle.
Assessment of mechanical allodynia and thermal hyperalgesia
The rats were tested for 4 days before surgery and on days 1, 3, 7, 14, and 28 after surgery. In a quiet room, animals were placed individually in acrylic cages with a mesh floor and allowed to acclimate for 30 min before the start of testing. Mechanical allodynia was defined by the incidence of foot withdrawal in response to non-noxious mechanical indentation of each hind paw using a probe with an ~0.5 mm2 polypropylene tip (Electronic von Frey Anesthesiometer, IITC Life Science, Woodland Hills, CA, USA). The mechanical withdrawal threshold in each animal are the means of four tests performed with intervals of at least 6 min.12
Thermal hyperalgesia was measured by foot withdrawal latency to heat stimulation according to the manufacturer’s protocol.13 Briefly, each rat was placed in a box containing a smooth glass floor. The temperature of the glass was maintained at 26°C±0.5°C. An analgesia meter (IITC Model 336 Analgesia Meter, Life Science) was used to provide a heat source. A heat source beneath a glass floor was focused on the plantar surface of the hind paw. The heat stimulus shut off automatically when the hind paw moved (or after 20 s to prevent tissue damage). Four measurements of withdrawal latency were taken for each hind paw in each test at 6-min intervals. The values are the mean of the four measurements of withdrawal latency.
Western blotting
To quantify temporal changes in protein levels, Western blot analysis was performed as previous reported.14 L4–L5 DRGs were removed quickly from deeply anesthetized rats and homogenized in an ice-cold lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mmol/L sodium orthovanadate, 1 mmol/L sodium fluoride, 1 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L phenylmethylsulfonyl fluoride, and 1% cocktail of protease inhibitors (Sigma-Aldrich). The lysates were then clarified by centrifugation at 12,000× g for 20 min at 4°C. Protein concentration was quantitated by the protein assay kit (Bio-Rad). Equal amount of samples was run on a 10% SDS-polyacrylamide gel electrophoresis and transferred to a polyphorylated difluoride membrane. The membranes were incubated with 5% nonfat dried milk for 60 min and then incubated with the primary antibodies at 4°C overnight. The membranes were incubated with an horseradish peroxidase-labeled secondary antibody and developed using the ECL detection kit (Millipore, Billerica, MA, USA). Protein expression was normalized against β-actin. Western blot bands were scanned and analyzed with the Quantity One image software (Bio-Rad).
Immunohistochemistry
Sacrificed rats were perfused transcardially with warm saline followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer, followed by 4% paraformaldehyde in 0.1 mol/L phosphate buffer. After the perfusion, the L5 DRG was dissected out from four rats in each group. Tissues were fixed in 4% paraformaldehyde overnight at 4°C and placed in 30% sucrose solution for 24 h at 4°C. Frozen sections of DRG (16 μm) were cut on a freezing microtome. For immunofluorescence staining, the sections were blocked with 5% donkey serum in 0.3% Triton X-100 for 2 h at room temperature and incubated in the first primary antibody at 4°C overnight. The dilution of antibodies were used as follows: anti-NLRP3 (1:100; Abcam), anti-caspase-1 p20 (1:50; Santa Cruz Biotechnology), and anti-calcitonin gene-related peptide (CGRP) (1:100; Abcam). After the primary antibody incubation, the sections were incubated with secondary antibodies according to manufacturer’s protocol for 2 h in the dark at room temperature. The images of the stained sections were captured by fluorescence microscope.
Statistical analysis
All experiments were done in triplicate, with independent treatments, each of which showed essentially the same results. The experimental data were analyzed using Student’s t-test by SPSS statistical software. All the results were presented as mean ± standard deviation (SD). For all analyses a two-sided p value of <0.05 was taken as statistically significant.
Results
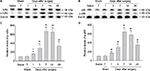
NF-κB activation in DRGs of NP-treated rats
NF-κB in the DRG neurons may finally lead to events responsible for the pain behaviors after partial sciatic nerve injury.4 Here, we want to investigate whether NF-κB signaling is activated in our NP-treated rat model. We found that the phosphorylation of IκBα was significantly elevated in DRGs of NP-treated rats compared to that of naive and sham rats (Figure 1). The phosphorylation of IκBα was upregulated with a peak 7 days after surgery in DRGs of NP-treated rat (Figure 1A, C). Also, the phosphorylation of p65 was significantly elevated in DRGs of NP-treated rats compared to that of naive and sham rats (Figure 1B, D). The phosphorylation of p65 was upregulated with a peak 7 days after surgery in DRGs of NP-treated rat (Figure 1B, D).
Activation of NLRP3 inflammasome in DRGs of NP-treated rats
NF-κB plays a crucial role in the regulated production of inflammatory cytokines and in the cytokine-induced expression of major proteins of inflammatory responses.15 In our NP-treated rat model, the expression level of NLRP3 was significantly elevated in DRGs of NP-treated rats compared to that of naive and sham rats (Figure 2A, B). NLRP3 was upregulated with a peak 7 days after surgery in DRGs of NP-treated rat (Figure 2A, B). In consistent with the results of Western blotting, immunofluorescent assay showed that NLRP3 also significantly elevated in DRGs of NP-treated rats 7 days after surgery compared to that of naive and sham rats (Figure 2C).
ASC, the component of NLRP3 inflammasome, was significantly upregulated in DRGs of NP-treated rats compared to sham rats (Figure 2D). Caspase-1 p20 expression, the indicative of caspase-1 activation, was also significantly increased in DRGs of NP-treated rats 7 days after surgery (Figure 2D, E). In consistent with the results of Western blotting, immunofluorescent assay showed that Caspase-1 p20 also significantly elevated in DRGs of NP-treated rats 7 days after surgery compared to that of naive and sham rats (Figure 2F).
Next, we found that the expression of IL-1β and IL-18, the downstream of NLRP3 inflammasome, were significantly elevated in DRGs of NP-treated rats compared to that of naive and sham rats (Figure 3). IL-1β and IL-18 were upregulated with a peak 7 days after surgery in DRGs of NP-treated rat (Figure 3).
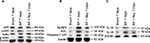
Bay11-7082 inhibited the activations of NF-κB and NLRP3 inflammasome in DRGs of NP-treated rats
Bay11-7082 was a potent inhibitor of the inflammasome independent of their inhibitory effect on the NF-κB pathway.8 We found that the phosphorylations of p65 and IκBα were both dramatically inhibited by Bay11-7082 treatment in DRGs of NP-treated rats compared to vehicle-treated group (Figure 4A). The formation of NLRP3 inflammasome was inhibited by Bay11-7082 treatment (Figure 4B). The expressions of NLRP3, ASC, and Caspase-1 p20 were significantly reduced after Bay11-7082 treatment compared to vehicle-treated rats (Figure 4B). The expression levels of IL-1β and IL-18 were significantly decreased in the Bay11-7082-treated rats (Figure 4C).
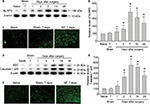
Suppression of CGRP expression in DRG neurons of NP-treated rats by Bay11-7082
The expression level of CGRP, which defined as a pain marker, was upregulated in DRG neurons in the rat lumbar degenerative intervertebral disc model.16 Immunofluorescence showed that CGRP was not detected in the DRG neurons of sham group (Figure 5). Cytoplasm staining of CGRP was obviously displayed in rat DRG neurons after autologous NP implantation group (Figure 5). Bay11-7082 treatment was able to inhibit the expression of CGRP in rat DRG neurons (Figure 5).
Behavioral test
Finally, we assessed the alleviating action of Bay11-7082 on NP-induced mechanical allodynia and thermal hyperalgesia. Rats in all groups showed stable conditions before surgery in response to mechanical or thermal stimulation. In the NP-treated group, the mechanical or thermal withdrawal thresholds were significantly decreased during the 28 days after surgery compared with the sham group (Figure 6). In the Bay11-7082-treated group, mechanical or thermal thresholds were significantly decreased during the 28 days after surgery in comparison with the NP-treated group (Figure 6).
Discussion
Regarding pain, some recent reports show the NF-κB pathway has a direct pain-related role. For instance, NF-κB activation was increased in rat lumbar DRG neurons following partial sciatic nerve injuries.4 Lentiviral-mediated targeted blockade of NF-κB activity in dorsal spinal cord glia attenuated sciatic nerve injury-induced neuropathic pain in the rats.17 Suzuki et al further reported that NF-κB was transduced into DRGs in vivo improved mechanical allodynia and thermal hyperalgesia in a rat LDH model.18 NF-κB plays a vital role in regulating the gene expression of proinflammatory cytokine IL-1β. Moreover, this positive feedback loop may amplify local inflammatory responses.19 Here, our data showed the upregulation of NF-κB in DRG neurons after the autologous implantation of NP and the significant inhibition of NF-κB expression under the Bay11-7082 treatment, accompanied with the reducing production of proinflammatory cytokine IL-1β. These data suggest that the effect of Bay11-7082 on NP-induced neuropathic pain was partly due to the inhibition of NF-κB activation.
The inflammasome plays vital roles in a variety of human autoimmune and inflammatory diseases, such as atherosclerosis, gout, myocardial infarction, and type II diabetes.20 The NLRP3 inflammasome is the most intensively studied complex of the inflammasomes and can be activated by various stimulating factors (pathogens, stress, or other danger-induced substances). A recent study has showed that NLRP3 inflammasome activation may be involved in the genesis of headaches by promoting IL-1β maturation in the trigeminal ganglia.21 In the present study, we found that the expression levels of IL-1β and IL-18 were significantly increased in DRG neurons of NP-implanted rats. The administration of Bay11-7082 significantly inhibited IL-1β and IL-18 expressions and had an effect on a pain-related behavior. This further provided another possible explanation for Bay11-7082 alleviating neuropathic pain by inhibiting NLRP3/caspase-1/IL-1β or IL-18 pathway. The implicate mitochondrion-associated signals mediated by Ca2+ signaling, generated by mitochondrial destabilization or dysfunction, in many settings of NLRP3 inflammasome activation.22 In particular, mtDNA released into the cytosol and externalized cardiolipin can interact with and activate the NLRP3 inflammasome, potentially serving as ligands of the complex.22
NP from animals and humans has been found antigenic, and antibodies to NP have been detected in serum from human patients and animal models.23 Inflammation mediators (phospholipase A2, prostaglandin E2, IL-1α, IL-1β, IL-6, tumor necrosis factor-α, and NO) have been identified in and around the intervertebral disk in numerous in vitro and in vivo studies.23 These mediators stimulate the release of metalloproteases, which play a pivotal role in disk degeneration.24
CGRP is a 37-amino acid neuropeptide and is primarily released from sensory nerves. The upregulation of CGRP expression in DRG neurons induces mechanical allodynia.25 In a prospective study of patients with LDH, preoperative levels of CGRP in plasma were significantly correlated with the degree of sciatica as determined by visual analog scale. Moreover, the expression level of CGRP significantly decreased after lumbar discectomy, which was in accordance with the relief of pain symptoms.26 In consistent with previous reports, our study suggests that autologous implantation of NP induced an upregulation of CGRP in DRG neurons and that the treatment of Bay11-7082 significantly inhibited expression of CGRP protein in the neurons.
In conclusion, our findings show that NF-κB and NLRP3 inflammasome are involved in the development and maintenance of NP-induced neuropathic pain. Bay11-7082 reduces NF-κB and NLRP3 inflammasome activation along with attenuation of the pain in rats. The therapy targeting NF-κB and NLRP3 inflammasome might be a potential strategy in the treatment of neuropathic pain.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20151177), Jiangsu Provincial Commission of Health and Family Planning (Z201614), the National Science Foundation of China (81471263), Changzhou High-Level Medical Talents Training Project (2016ZCLJ005), and the Science and Technology Bureau of Changzhou (number CJ20130029).
Disclosure
The authors report no conflicts of interest in this work.
References
Dower A, Chatterji R, Swart A, Winder MJ. Surgical management of recurrent lumbar disc herniation and the role of fusion. J Clin Neurosci. 2016;23:44–50. | ||
Elkan P, Sten-Linder M, Hedlund R, Willers U, Ponzer S, Gerdhem P. Markers of inflammation and fibrinolysis in relation to outcome after surgery for lumbar disc herniation. A prospective study on 177 patients. Eur Spine J. 2016;25(1):186–191. | ||
Seidel P, Sun Q, Costa L, Lardinois D, Tamm M, Roth M. The MNK-1/eIF4E pathway as a new therapeutic pathway to target inflammation and remodelling in asthma. Cell Signal. 2016;28(10):1555–1562. | ||
Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain Res. 1998;797(2):243–254. | ||
Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. | ||
del Rey A, Yau HJ, Randolf A, et al. Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus. Pain. 2011;152(12):2827–2835. | ||
Hasegawa S, Kohro Y, Shiratori M, et al. Role of PAF receptor in proinflammatory cytokine expression in the dorsal root ganglion and tactile allodynia in a rodent model of neuropathic pain. PLoS One. 2010;5(5):e10467. | ||
Juliana C, Fernandes-Alnemri T, Wu J, et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. 2010;285(13):9792–9802. | ||
Park HW, Ahn SH, Kim SJ, et al. Changes in spinal cord expression of fractalkine and its receptor in a rat model of disc herniation by autologous nucleus pulposus. Spine (Phila Pa 1976). 2011;36(12):E753–E760. | ||
You CC, Zhu K, Liu XQ, et al. Tumor necrosis factor-alpha-dependent infiltration of macrophages into the dorsal root ganglion in a rat disc herniation model. Spine. 2013;38(23):2003–2007. | ||
Zhao J, Zhang H, Huang Y, et al. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-kappaB activation. Int Immunopharmacol. 2013;17(1):116–122. | ||
Yamamoto H, Shimoshige Y, Yamaji T, Murai N, Aoki T, Matsuoka N. Pharmacological characterization of standard analgesics on mechanical allodynia in streptozotocin-induced diabetic rats. Neuropharmacology. 2009;57(4):403–408. | ||
Song XJ, Vizcarra C, Xu DS, Rupert RL, Wong ZN. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol. 2003;89(4):2185–2193. | ||
Liu J, Zhang Y, Xu R, et al. PI3K/Akt-dependent phosphorylation of GSK3beta and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell Signal. 2013;25(2):447–456. | ||
Lin TH, Tamaki Y, Pajarinen J, et al. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Biomater. 2014;10(1):1–10. | ||
Orita S, Miyagi M, Kobori S, et al. IkappaB kinase beta inhibitor downregulates pain-related neuropeptide production in the sensory neurons innervating injured lumbar intervertebral discs in the dorsal root ganglia of rats. Spine J. 2013;13(3):284–288. | ||
Meunier A, Latremoliere A, Dominguez E, et al. Lentiviral-mediated targeted NF-kappaB blockade in dorsal spinal cord glia attenuates sciatic nerve injury-induced neuropathic pain in the rat. Mol Ther. 2007;15(4):687–697. | ||
Suzuki M, Inoue G, Gemba T, et al. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009;18(7):1001–1007. | ||
Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. | ||
Benetti E, Chiazza F, Patel NSA, Collino M. The NLRP3 inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediators Inflamm. 2013;2013:678627. | ||
Chen L, Li X, Huang L, Wu Q, Chen L, Wan Q. Chemical stimulation of the intracranial dura activates NALP3 inflammasome in trigeminal ganglia neurons. Brain Res. 2014;1566:1–11. | ||
Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35(6):253–261. | ||
Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related sciatica. I. – Evidence supporting a chemical component. Joint Bone Spine. 2006;73(2):151–158. | ||
Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine (Phila Pa 1976). 1998;23(14):1612–1626. | ||
Miki K, Fukuoka T, Tokunaga A, Noguchi K. Calcitonin gene-related peptide increase in the rat spinal dorsal horn and dorsal column nucleus following peripheral nerve injury: up-regulation in a subpopulation of primary afferent sensory neurons. Neuroscience. 1998;82(4):1243–1252. | ||
Takeuchi H, Kawaguchi S, Ohwada O, et al. Plasma neuropeptides in patients undergoing lumbar discectomy. Spine. 2007;32(2):E79–E84. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.