Back to Journals » Cancer Management and Research » Volume 13
Baseline Serum Cholesterol Levels Predict the Response of Patients with Advanced Non-Small Cell Lung Cancer to Immune Checkpoint Inhibitor-Based Treatment
Authors Tong III J , Mao Y, Yang Z, Xu Q, Zheng Z, Zhang H, Wang J, Zhang S, Rong W, Zheng III L
Received 30 January 2021
Accepted for publication 4 May 2021
Published 18 May 2021 Volume 2021:13 Pages 4041—4053
DOI https://doi.org/10.2147/CMAR.S304022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Seema Singh
Jingtao Tong III,1,* Yifei Mao,2,* Ziru Yang,1 Quan Xu,1 Zhen Zheng,1 Hui Zhang,1 Jingjing Wang,1 Sandian Zhang,1 Weibo Rong,3 Lu Zheng III1
1Department of Radiation Oncology, Ningbo Medical Treatment Center Li Huili Hospital, Ningbo, 315040, People’s Republic of China; 2Department of Emergency Medicine, Ninghai First Hospital, Ningbo, 315600, People’s Republic of China; 3Department of Pharmacy, Ningbo Medical Treatment Center Li Huili Hospital, Ningbo, 315040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Zheng
Department of Radiation Oncology, Ningbo Medical Treatment Center Li Huili Hospital, No. 57, Xing Ning Road, Yinzhou District, Ningbo, 315040, People’s Republic of China
Tel +86 13486688768
Fax +86 574 83872180
Email [email protected]
Purpose: Although predictive markers of immune checkpoint inhibitor (ICI)-based treatments have been extensively studied, with the exception of programmed death ligand 1 (PD-L1), most are not widely used in the clinic due to poor effects or defective practicability. The aim of this study was to identify those patients with high baseline serum cholesterol who benefit from ICI-based treatments.
Patients and Methods: Patients with advanced non-small cell lung cancer (NSCLC) treated at Ningbo Medical Center, Li Huili Hospital between August 2017 and December 2019 were enrolled in this retrospective study. The Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) were used to evaluate the efficacy of the ICI-based treatment. Progression-free survival (PFS) and overall survival (OS) were calculated using the Kaplan–Meier survival curves and compared using the log rank test. Univariate and multivariate analyses were conducted using the logistic regression analysis and Cox proportional hazards model. A receiver operating characteristic curve was created, and the area under the curve (AUC) was calculated to compare the predictive value of baseline serum cholesterol with PD-L1 expression for patient response to ICI-based treatment.
Results: In our cohort of 169 NSCLC patients, the objective response rate (ORR) and disease control rate (DCR) of the treatment were significantly higher in patients with hypercholesterolemia (> 5.18 mmol/L) than in those with hypocholesterolemia (ORR: 33.67% vs 14.08%, P=0.004; DCR: 68.37% vs 42.25%, P=0.001). The median PFS was 7.9 months in the hypercholesterolemia group, significantly longer than in the hypocholesterolemia group (4.4 months, 95% CI: 4.620– 7.380, P< 0.001). The median OS in the two groups were 11 months and 8 months, with 95% CIs of 8.980– 10.420 (P< 0.001). The AUC for the baseline level of cholesterol was 0.706 (P< 0.001), while it was 0.643 (P=0.001) for PD-L1 expression.
Conclusion: The baseline serum cholesterol level is predictive of a clinical benefit for advanced NSCLC patients who undergo ICI-based treatment, and hence it is a promising prognostic indicator for ICI-based treatment of NSCLC.
Keywords: non-small cell lung cancer, NSCLC, serum cholesterol, immune checkpoint inhibitors, ICIs, prognostic marker
Corrigendum for this paper has been published
Introduction
Lung cancer is associated with high morbidity and mortality throughout the world, with 2,093,876 new cases and 1,761,007 new deaths in 2018.1 Non-small cell lung cancer (NSCLC) is the dominant subtype. After chemotherapy and targeted therapy, immune-checkpoint inhibitors (ICIs) have offered a new approach to advanced NSCLC.2 ICIs target the programmed cell death protein 1 (PD-1)/programmed death ligand-1 (PD-L1) axis, and include PD-1 inhibitors like nivolumab and pembrolizumab, and PD-L1 inhibitors like atezolizumab and durvalumab. ICI-based treatment has been standard for NSCLC patients in recent years.3,4 Still, the number of patients who benefit from such treatment is small, and predictive biomarkers are urgently needed. Tumor mutation burden, tumor-infiltrating lymphocytes, changes in peripheral blood CD8+ T cells, and immune cell repertoires are potential biomarkers that have been identified in clinical practice.5–7
Metabolic diseases have recently attracted a lot of attention in patients with metastatic cancer. For example, obesity, body mass index (BMI), diabetes, and dyslipidemia were reported to be risk or prognostic factors.8,9 Recent studies have also demonstrated that such metabolic conditions are associated with NSCLC patient response to ICI treatment.10,11 High serum cholesterol, known as hyperlipidemia, is a type of dyslipidemia. In preclinical studies, high serum cholesterol interacted with immune cells to enhance their anti-tumor properties.12,13 Retrospective studies revealed that high serum cholesterol offers positive prognostic value in some types of cancers, including NSCLC, treated with ICIs,14 and statins have been observed to stimulate immune responses and synergize with anti-PD-1 antibodies in NSCLC.15
This study compared clinical outcomes with different levels of serum cholesterol and identified high baseline serum cholesterol levels as being associated with the benefits of ICI-based treatments for NSCLC patients.
Patients and Methods
Patient Eligibility
This study enrolled patients with advanced NSCLC treated at Ningbo Medical Treatment Center, Li Huili Hospital from August 2017 to December 2019. Enrolled patients satisfied the following criteria: (1) diagnosed with histologically or cytologically confirmed NSCLC without mutation of EGFR/ALK/ROS1, (2) presented with measurable lesions, and (3) treated with ICI-based therapy. Treatment was stopped when there were serious infusion-related adverse events, disease progression, or patients refused to continue treatment. Patients’ clinicopathological features were examined. The end of the follow-up period was December 31st 2019.
This study was conducted in accordance with the Declaration of Helsinki. And it was approved by the Ethics Committee of Ningbo Medical Treatment Center, Li Huili Hospital. Written informed consent from patients was waived because of the observational nature.
Definitions of Variables
Patient response to ICI-based treatment was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1). Disease control rate (DCR) was defined as complete or partial response plus stable disease. Progression-free survival (PFS) was defined as the time from the start of ICI-based treatment to confirmed disease progression or death, whichever occurred first. Overall survival (OS) was measured from the date of the first ICI-based treatment to death or the last day of follow-up. If patients were still alive without disease progression before the deadline for analysis or last follow-up date, the data were censored.
PD-L1 expression was detected by the 22C3 antibody, and categorized using TPS (tumor proportion score) cutoffs of 1% and 50%: negative (<1%), low (1–49%), and high (≥50%).16,17 There were two types of treatment: ICI-based treatment alone, classified as monotherapy, with nivolumab (3mg/kg q2w), pembrolizumab (200mg q3w), camrelizumab (200mg q3w), and sintilimab (200mg q3w). Or ICIs plus chemotherapy, combined with pemetrexed/paclitaxel plus platinum (cisplatin/carboplatin) based on PD-L1 expression using standard doses and schedules.
We used a value of 5.18 mmol/L total cholesterol (TC) to divide patients into hypercholesterolemia and hypocholesterolemia groups according to American Heart Association Guidelines,18 with cutoff values of 1.76 mmol/L for triglyceridemia (TG), 3.37 mmol/L for low-density lipoprotein (LDL) cholesterol, and 1.04 mmol/L for high-density lipoprotein (HDL) cholesterol. Fasting serum levels of total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol were measured before ICI-based treatment. BMI was calculated at the time of treatment initiation, with 25kg/m2 serving as the cut-off value. If a patient had a history of statin use, then statin therapy was recorded as “yes”.
Statistical Analysis
SPSS version 22.0 was used for statistical analyses. Pearson’s χ2 and independent t-tests were used to make comparisons using patients’ clinicopathological data. Kaplan-Meier was used to estimate PFS and OS, and a Log rank test was used to compare survival rates. Univariate and multivariate analyses for objective response was performed by logistic regression analysis. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and corresponding 95% confidence interval (CI). Receiver-operating characteristic (ROC) curves were used to estimate the sensitivity and specificity of biomarkers by calculating the area under the curve (AUC). Statistical significance was indicated by a two-sided P value <0.05.
Results
Patients Characteristics
A total of 193 patients with advanced NSCLC who received ICI-based treatment at Ningbo Medical Treatment Center, Li Huili Hospital from August 1, 2017 to December 31, 2019 were screened, and 169 patients (87.6%) were enrolled. At the time of data collection, 72 patients had experienced progression (42.60%) and 98 patients (57.99%) were had died. The baseline characteristics of the patients with hypocholesterolemia or hypocholesterolemia and efficacy outcomes of ICI-based treatment, are summarized in Table 1. Patients with hypercholesterolemia were more likely in stage IV (P=0.043) or with BMI≥25kg/m2 (P=0.013), and had a better response to ICI-based treatment according to the higher rate of ORR (P=0.004) and DCR (P=0.001).
 |
Table 1 Baseline Characteristics of Patients (n=169) |
Patients were divided into two groups based on PD-L1 expression. In the Unknown/no/low-PD-L1 group (n=121), patients with hypercholesterolemia had higher baseline TG (P=0.041) or with BMI≥25kg/m2 (P=0.027) and a better response to ICI-based treatment as judged by their higher rate of PR (P=0.001) and the lower rate of PD (P=0.001, Table 2). Although in the high-PD-L1 group, patients with hypercholesterolemia had lower ECOG (Eastern Cooperative Oncology Group) scores (P=0.050), there was no significant difference in their response to ICI-based treatment compared with patients with hypocholesterolemia (Table 3).
 |
Table 2 Baseline Characteristics of Patients in Unknown/No/Low PD-L1 Group (n=121) |
 |
Table 3 Baseline Characteristics of Patients in High PD-L1 Group (n=48) |
Objective Response to ICI-Based Treatment
Patients with hypercholesterolemia had a higher objective response rate (ORR) (33.67%) and disease control rate (DCR) (68.37%) than those with hypocholesterolemia (ORR: 14.08%, P=0.004; DCR: 42.25%, P=0.001 Figure 1A). And in the unknown/no/low PD-L1 group, the patients with hypercholesterolemia also responded more favorably to ICI-based treatment. The ORR of patients with hypercholesterolemia was 30.56%, while it was 6.12% in patients with hypocholesterolemia (P<0.001). The DCR was 62.50% vs 32.65% (P=0.002) for the same two groups, respectively (Figure 1B). In the high-PD-L1 group, the response was similar (ORR: 42.31% vs 31.82%, P=0.454; DCR: 84.62% vs 63.64, P=0.094; Figure 1C).
Survival Analysis
The median PFS times of patients with hypercholesterolemia and hypocholesterolemia were 7.9 months vs 4.4 months, respectively (95% CI: 4.620–7.380, P<0.001, Figure 2A). And the median OS times in the two groups were 11 months and 8 months, respectively (95% CIs of 8.980–10.420, P<0.001, Figure 2B). In the unknown/no/low PD-L1 group, the median PFS of the patients with hypercholesterolemia was 7.5 months, which was higher than that of patients with hypocholesterolemia (3.5 months, 95% CI: 4.477–5.923, P<0.001, Figure 2C). Also, the median OS in the two groups was 10 months and 7.2 months, respectively (95% CIs of 7.751–9.249, P<0.001, Figure 2D). The mean PFS in the high-PD-L1 group with hypercholesterolemia was higher than that with hypocholesterolemia (8.9 months vs 4.9 months, 95% CI: 4.620–7.380, P<0.001; Figure 2E). Also, the median OS in the high-PD-L1 group with hypercholesterolemia was higher than that with hypocholesterolemia (13.9 months vs 9.9 months, 95% CI: 9.911–12.089, P<0.001, Figure 2F).
Based on univariate analysis for PFS among the entire cohort, patients over 60 years old or at a later stage had decreased PFS (P=0.000, HR =6.226; P=0.018, HR =2.998) while high PD-L1 expression and baseline hypercholesterolemia were associated with increased PFS (P=0.003, HR =0.394; P=0.000, HR =0.268). Furthermore, BMI≥25kg/m2 and statin therapy resulted in better PFS compared with BMI<25kg/m2 and non-statin therapy (P=0.000, HR =0.704; P=0.002, HR =0.370). Based on multivariate analysis, age, statin therapy, baseline hypercholesterolemia, and PD-L1 expression were associated with PFS (Table 4).
 |
Table 4 Univariate and Multivariate Analyses of Clinical Parameters of PFS in Overall Patients |
Based on univariate analysis for OS among the entire cohort, patients with later stage or squamous carcinoma had decreased OS (P=0.005, HR =1.660; P=0.050, HR =0.650), while TC≥5.18mmol/L and TG≥1.76mmol/L were associated with increased OS (P=0.000, HR =0.295; P=0.038, HR =0.599). Furthermore, BMI≥25kg/m2 and ICIs combined with chemotherapy resulted in better OS compared with BMI<25kg/m2 and monotherapy (P=0.019, HR =0.368; P=0.050, HR =1.478). Based on multivariate analysis, baseline hypercholesterolemia and PD-L1 expression were associated with OS (Table 5).
 |
Table 5 Univariate and Multivariate Analyses of Clinical Parameters of OS in Overall Patients |
Based on univariate analysis for objective response among the entire cohort, patients over 60 years old or at a later stage had decreased objective response (P=0.000, HR =1.121; P=0.000, HR =0.158), while high PD-L1 expression and statin therapy were associated with better objective response (P=0.025, HR =2.304; P=0.000, HR =4.894;). Furthermore, TC≥5.18mmol/L, TG≥1.76mmol/L, HDL cholesterol≥1.04 and BMI≥25kg/m2 resulted in more objective response compared with TC<5.18mmol/L, TG<1.76mmol/L, HDL cholesterol<1.04 and BMI<25kg/m2 (P=0.005, HR =3.097; P=0.032, HR =2.279; P=0.006, HR =2.928; P=0.000, HR =9.792). Based on multivariate analysis, age, BMI≥25kg/m2, stage, statin therapy, baseline hypercholesterolemia and HDL cholesterol were associated with objective response (Table 6).
 |
Table 6 Univariate and Multivariate Analyses of Clinical Parameters of Objective Response in Overall Patients |
Predictive Value of Baseline Cholesterol Level
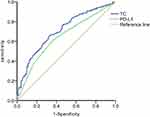
In the ROC curve for disease control in the total population, the AUC for baseline cholesterol level was 0.706 (p<0.001) based on a 5.265mmol/L cut-off, While the AUC was 0.643 (p=0.001) for PD-L1 expression (Figure 3), thus indicating lower sensitivity and specificity in the total population.
Discussion
Cholesterol has been reported to substantially contribute to progression of several types of cancer.19–21 ICIs have frequently been used to treat NSCLC in recent years. In this study, we found that baseline hypercholesterolemia was associated with a positive response to ICI-based treatment and with longer PFS and OS in advanced NSCLC patients. The level of serum cholesterol is therefore a potential marker for prediction of efficacy and survival of patients with advanced NSCLC undergoing ICI-based therapy.
Cholesterol plays an important role in the metabolism and growth of every type of mammalian cell, stabilizes cell membranes, and is a precursor of vitamins and hormones.22 Recently, cholesterol has gained a lot of attention for its association with cancer. Earlier research indicated that cholesterol has a significant role in initiating and promoting some types of cancer.23–25 Meanwhile, the relationship between cholesterol and immune cells has also frequently been studied. On the one hand, some research has shown that in T cells, the biosynthesis of cholesterol is highly upregulated by liver X receptor (LXR) inactivation, which activates T cells.26,27 This was related to cholesterol maintaining the rigidity of immune cell membranes and transmitting cellular signals by receptor co-localization, thus demonstrating the positive role of cholesterol in the immune response.28 On the other hand, other studies showed that for tumor-infiltrating T cells, accumulation of cholesterol induced the expression of immune checkpoints PD-1 and 2B4, leading to T cell exhaustion.29,30 Reducing cholesterol may enhance T cell-based immunotherapy.31 However, we suspected increasing expression of PD-1 on the surface of tumor-infiltrating T cells may in some cases increase PD-L1 binding and the response to ICI-based treatment. In addition to T cells, it was also observed that cholesterol in NK cells was associated with activation of immune signaling.32 Cholesterol influx into DC cells has been reported to enhance antigen presentation.33
In our study, we found the level of serum cholesterol to be significantly associated with better responses to ICI-based treatment. Our results are consistent with two retrospective studies. In one study, 55 patients with metastatic NSCLC were enrolled and received ICI monotherapy.21 In another study, 131 patients with advanced NSCLC were enrolled and received monotherapy or a combination of two ICIs.14 Neither study analyzed the ORR or DCR of the ICI-based treatment, and the baseline cholesterol level was obtained a few months before treatment. In our study, more patients were included, and ICIs combined with chemotherapy were used for the majority of patients, and we assessed the serum cholesterol level immediately before the ICI-based treatment. Furthermore, we found that ORR and DCR were higher in hypercholesterolemia patients than in hypocholesterolemia patients. The result indicated that along with PD-L1, serum cholesterol was an independent prognostic factor.
We also divided the patients into two groups by PD-L1 expression and found that ORR and DCR of the hypercholesterolemia patients were markedly different from those with hypocholesterolemia in the unknown/no/low PD-L1 group, which was not observed in the high PD-L1 group. Thus, baseline cholesterol may become a biomarker for response to ICI-based treatment, especially in unknown/no/low PD-L1 populations that previously lacked a valid marker. Furthermore, we calculate the sensitivity and specificity of PD-L1 and serum cholesterol for predicting the effectiveness of ICI-based treatment in patients with advanced NSCLC, and found that serum cholesterol was a slightly better predictor than PD-L1. We also observed that statin use led to longer PFS and better ORR in the cohort, consistent with earlier research.15
However, our study has some limitations. First, the retrospective design and small sample size may not get a definitive conclusion, and second, we did not analyze outcomes using different ICI drugs. Therefore, larger prospective studies will be required to validate the present results, and studies using other ICI drugs will be needed to test whether our results represent a generalized response.
Conclusion
In conclusion, the present study indicated that baseline serum cholesterol level was associated with clinical benefit for advanced NSCLC patients who undergo ICI-based treatment. And it could be a promising prognostic predictor for ICI-based treatment in NSCLC.
Funding
This study was supported by the Natural Science Foundation of Ningbo (grant 2019A610226).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2020;1–24.
2. Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist. 2017;22(1):81–88. doi:10.1634/theoncologist.2016-0189
3. Gandhi L, Rodríguez D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
4. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi:10.1056/NEJMoa1716948
5. Mitsuhashi A, Okuma Y. Perspective on immune oncology with liquid biopsy, peripheral blood mononuclear cells, and microbiome with non-invasive biomarkers in cancer patients. Clin Transl Oncol. 2018;20:966–974. doi:10.1007/s12094-017-1827-7
6. Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi:10.1038/nrc.2016.36
7. Kim KH, Cho J, Ku BM, et al. The first-week proliferative response of peripheral blood PD-1+CD8+T cells predicts the response to anti-PD-1 therapy in solid tumors. Clin Cancer Res. 2019;25:2144–2154. doi:10.1158/1078-0432.CCR-18-1449
8. Taghizadeh N, Boezen HM, Schouten JP, et al. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10(4):e0125261. doi:10.1371/journal.pone.0125261eCollection2015
9. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi:10.1155/2014/943162
10. Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57–67. doi:10.1186/s40425-019-0527-y
11. Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell-lung-carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29:1853–1860. doi:10.1093/annonc/mdy218
12. Ito A, Hong C, Oka K, et al. Cholesterol accumulation in CD11c + immune cells is a causal and targetable factor in autoimmune disease. Immunity. 2016;45(6):1311–1326. doi:10.1016/j.immuni.2016.11.008
13. Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. doi:10.1038/nature17412
14. Perrone F, Minari R, Bersanelli M, et al. The prognostic role of high blood cholesterol in advanced cancer patients treated with immune checkpoint inhibitors. J Immunother. 2020;43(6):196–203. doi:10.1097/CJI.0000000000000321
15. Omori M, Okuma Y, Hakozaki T, et al. Statins improve survival in patients previously treated with nivolumab for advanced non-small cell lung cancer: an observational study. Mol Clin Oncol. 2019;10(1):137–143. doi:10.3892/mco.2018.1765
16. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomized, open-label, controlled, Phase 3 trial. Lancet. 2019;393:1819–1830. doi:10.1016/S0140-6736(18)32409-7
17. Reck M, Rodríguezabreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi:10.1056/NEJMoa1606774
18. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol. 2019;73:3168–3209.
19. Liu Z, Liu X, Liu S, et al. Cholesterol promotes the migration and invasion of renal carcinoma cells by regulating the KLF5/miR-27a/FBXW7 pathway. Biochem Biophys Res Commun. 2018;502:69–75. doi:10.1016/j.bbrc.2018.05.122
20. Wang Y, Liu C, Hu L. Cholesterol regulates cell proliferation and apoptosis of colorectal cancer by modulating miR-33a-PIM3 pathway. Biochem Biophys Res Commun. 2019;511:685–692. doi:10.1016/j.bbrc.2019.02.123
21. Galli G, Corsetto P, Ferrara R, et al. Impact of cholesterolemia and body mass index on outcome of metastatic non-small cell lung cancer treated with immunotherapy. J Clin Oncol. 2019;37(suppl):e20691. doi:10.1200/JCO.2019.37.15_suppl.e20691
22. Ding X, Zhang W, Li S, Yang H. The role of cholesterol metabolism in cancer. Am J Cancer Res. 2019;9:219–227.
23. Finlayschultz J, Sartorius CA. Steroid hormones, steroid receptors, and breast cancer stem cells. J Mammary Gland Biol Neoplasia. 2015;20:39–50. doi:10.1007/s10911-015-9340-5
24. Degirolamo C, Modica S, Palasciano G, Moschetta A. Bile acids and colon cancer: solving the puzzle with nuclear receptors. Trends Mol Med. 2011;17:564–572. doi:10.1016/j.molmed.2011.05.010
25. Heir T, Falk RS, Robsahm TE, et al. Cholesterol and prostate cancer risk: a long-term prospective cohort study. BMC Cancer. 2016;16(1):643. doi:10.1186/s12885-016-2691-5
26. Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi:10.1038/nri1710
27. Bensinger SJ, Bradley MN, Joseph SB, et al. LXR signalling couples’ sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi:10.1016/j.cell.2008.04.052
28. Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J.Immunol. 2011;187:1529–1535. doi:10.4049/jimmunol.1100253
29. Ma X, Bi E, Huang C, et al. Cholesterol negatively regulates IL-9-producing CD8+T cell differentiation and antitumor activity. J Exp Med. 2018;215:1555–1569. doi:10.1084/jem.20171576
30. Xingzhe M, Enguang B, Yong L, et al. Cholesterol induces CD8+T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156. doi:10.1016/j.cmet.2019.04.002
31. Xingzhe M, Qing Y. Cholesterol induces T cell exhaustion. Aging. 2019;11(18):7334–7335. doi:10.18632/aging.102305
32. Qin WH, Yang ZS, Li M, et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158(6):1713–1727. doi:10.1053/j.gastro.2020.01.028
33. Villablanca EJ, Raccosta L, Zhou D, et al. Tumor-mediated liver X receptor-alpha activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat Med. 2010;16(1):98–105. doi:10.1038/nm.2074
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.