Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Barrier Effect of a New Topical Agent on Damaged Esophageal Mucosa: Experimental Study on an ex vivo Swine Model
Authors Salaroli R , Ventrella D , Bernardini C , Elmi A , Zannoni A , Bacci ML, Forni M , Calanni F, Ferrieri A, Baldi F
Received 2 July 2020
Accepted for publication 7 October 2020
Published 13 November 2020 Volume 2020:13 Pages 569—576
DOI https://doi.org/10.2147/CEG.S269568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Roberta Salaroli,1 Domenico Ventrella,1 Chiara Bernardini,1 Alberto Elmi,1 Augusta Zannoni,1,2 Maria Laura Bacci,1,2 Monica Forni,1,2 Fiorella Calanni,3 Antonella Ferrieri,4 Fabio Baldi5
1Department of Veterinary Medical Science, University of Bologna, Ozzano Emilia, Bologna, Italy; 2Health Sciences and Technologies Interdepartmental Center for Industrial Research, University of Bologna, Bologna, Italy; 3Pre-Clinical Research Department, Alfasigma, Bologna, Italy; 4Division of Clinical Research, Department of Research and Development, Alfasigma, Bologna, Italy; 5Center for the Study of Diseases of the Esophagus, University of Bologna and Gruppo Villa Maria Care & Research, Ravenna, Italy
Correspondence: Monica Forni
Department of Veterinary Medical Science, University of Bologna, Via Tolara, 50, Ozzano Emilia 40064, Bologna, Italy
Tel +39 051 20 9 7913
Email [email protected]
Purpose: AL2106 is a new medical device based on a mixture of chondroitin sulphate in a xyloglucan and glycerol solution made to maximize its bioadhesive capability to the esophageal mucosa. The aim of the present study was twofold to evaluate the AL2106 protective effect on the esophageal mucosa when exposed to an acidic solution mimicking gastric reflux and to assess the resilience of this effect to saline washing.
Materials and Methods: A porcine ex vivo model was used and the effects of the new medical device were compared to a sodium alginate suspension (SAS) already present on the market which was assumed as reference. Mucosal damage was induced in 19 porcine esophagi by perfusion with an acidic solution added with pepsin, and Evans blue dye (EBD) tissue uptake was used as an indicator of mucosal permeability. The EBD penetration, expressed as EBD μg/g of dry tissue, was assessed in specimens of untreated damaged mucosa and in specimens treated with AL2106 or SAS. The same evaluation was carried out after washing with normal saline.
Results: Both topical agents tested significantly reduced the EBD uptake by more than 60% (AL2106 8.4± 4.5, SAS 3.6± 2.7 vs control 23.2± 13.1, p< 0.01). The saline washing did not cause any significant reduction in the protective effect of AL2106 (8.6± 5.9), while it significantly reduced that of SAS (5.9± 4.3, p< 0.05).
Conclusion: The new AL2106 medical device showed a good barrier effect against a reflux-like damaging solution and preserved this effect after the mucosal washing test, thus suggesting its possible relevance for the treatment of gastroesophageal reflux disease.
Keywords: bioadhesion, Evans blue dye, EBD, animal model, esophagus, gastroesophageal reflux disease, GERD
Introduction
Gastroesophageal reflux disease (GERD) is a condition affecting 10–20% of the Western population. It is now well established that the mechanisms underlying symptoms in GERD patients are not only related to esophageal acid exposure, but also to enhanced esophageal sensitivity due to increased mucosal permeability, thus explaining the partial failure of anti-secretory therapy in these patients.1–4
The esophageal mucosa consists of a stratified squamous epithelium which acts as a tight defensive barrier against the injurious components of the gastroesophageal refluxate, and its impairment has been demonstrated not only in patients with mucosal erosions (esophagitis) but also in those with an apparently normal mucosa (NERD), who represent the most common GERD phenotype.5–7
In recent years, several experimental studies aimed at demonstrating the possibility of strengthening the mucosal barrier by means of topical products containing combinations of hyaluronic acid, chondroitin sulfate, alginates and antacids have been carried out on both animal models and humans.7–11
In a previous study, the Authors demonstrated how perfusion with a hyaluronic acid- and chondroitin sulfate-based product (Esoxx®) was able to reduce the permeability of the acid/peptic-damaged esophageal mucosa in a porcine ex vivo model in which the epithelial permeability was assessed using Evans blue dye (EBD) visualization on microscope sections.7
AL2106 is a new formulation for medical device, potentially useful for the topical treatment of GERD symptoms, based on a new mixture of chondroitin sulphate (CS), xyloglucan and glycerol aimed at increasing its bioadherence to the esophageal mucosa.
The aim of the present study was to evaluate, using a porcine ex vivo established model, the potential barrier protective effect on the esophageal mucosa of this new original chemical formulation and its bioadhesive capability by assessing its resilience to washing events.
Materials and Methods
Organ Collection and Preparation
The esophagi were collected from commercially available European breed pigs, weighing approximately 120 kg, at a local slaughterhouse immediately upon slaughter, and transferred within 2 hours in cooled saline to the physiology labs of the Department of Veterinary Medical Sciences of the University of Bologna. The organs were obtained at the slaughterhouse from carcasses earmarked for human consumption, thus replacing the slaughter of animals for tissue sampling in accordance with the 3Rs (Reduction, Replacement and Refinement). Therefore, it ethical approval was not needed as no animal had been sacrificed for experimental purposes.
Only organs without visible lesions were used for the experimental purposes (n=19). Upon arrival, each esophagus was thoroughly rinsed with tap water (inside and outside), and the cranial and caudal sphincters were ablated. The adventitial and the muscular layers were removed, maintaining only the submucosal and mucosal layers; the organs were then pinned upright (cranial end at the top and caudal end at the bottom) on an polystyrene support and placed in a thermostatic hood (Climatic Hood 810; ASAL s.r.l, Cernusco sul Naviglio, Italy) set at 37°C with an inclination of approximately 45 degrees as previously described7 (Figure 1).
Once positioned, the cranial opening of the esophagus was secured by means of a surgical knot to an extension tube connected to a 50 mL syringe attached to a perfusion pump. At the beginning of the experiment in order to obtain regular and uniform perfusion, the caudal end was closed by means of a surgical knot, and the esophagus was filled with 40 mL of the damaging solution for 5 minutes. At this point, the caudal surgical knot was removed and perfusion with the damaging solution started. The perfusion rate was set to 60 mL/h and lasted 60 minutes. Due to an inclination of approximately 45 degrees, the damaging solution was also slowly cleared from the aperistaltic esophagi.
Damaging Solution
Mucosal damage was achieved with an acidic-peptic solution. In particular, the damaging solution was prepared by adding porcine pepsin (2000 U/mL, Sigma-Aldrich, St. Louis, MO, USA) to normal saline acidified to pH 2 with HCl 0.1 N and warmed to 37°C. In the Authors’ previous study, it was found that this solution was able to induce microscopic mucosal damage on all the esophagi perfused.7
Design of the Study
At the end of the perfusion with the damaging solution, the esophagi were longitudinally sectioned, and the exposed inner mucosa was washed for 5 minutes in normal saline. At this point, six tissue fragments (~1cm x 1cm) were obtained from the middle portion of each organ and were used for the EBD quantitative analysis. The quantitative data were corroborated by a qualitative evaluation of tissue fragments using a microscope to indicate the depth of the EB penetration. The esophageal fragments underwent different treatments according to the strategy schematized in Figure 2. Briefly:
- One untreated fragment was used as a control for the epifluorescence evaluation.
- One fragment was exposed to the EBD solution (10 mg/mL in normal saline solution; Sigma-Aldrich, St. Louis, MO, USA) for 10 minutes.
- Two fragments were treated with each of the two products tested for 10 minutes, and were then exposed to the EBD solution for 10 minutes.
The products were each in stick packs of 10 mL:
- AL2106: a new mixture of chondroitin sulphate in a bioadhesive solution of xyloglucan and glycerol polymer.
- SAS: suspension of sodium alginate and potassium bicarbonate.
At the end of the above-mentioned procedures, all the fragments were washed twice in normal saline solution; the first wash was very rapid consisting of two dips in 100 mL of saline solution while the second one was 5 minutes long in 50 mL of saline. The fragments were then trimmed and divided into three approximately equal samples: one earmarked for epifluorescence evaluation and two as technical duplicates for EBD quantification.
Mucosal Permeability Assessment
The presence of the EBD inside the tissue was used as an indicator of increased permeability caused by peptic/acid perfusion since intact mucosa does not absorb the dye. To evaluate the quantity of EBD penetrated, a method of EBD extraction and spectrophotometric quantification was used.
In addition, a qualitative evaluation with an epifluorescence microscope was carried out.
Extraction and Quantification of the EBD
All the samples were dried at 37°C in a thermostatic hood for 30 minutes and were then immediately weighed, placed in a 15 mL conic tube with 3 mL of formamide (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 48 hours at 50°C. The quantity of EBD extracted from the tissue was evaluated using spectrophotometric measurements (Gene Quant 1300; GE Healthcare, UK) at the absorption maximum for Evans Blue (620 nm). Micrograms of EBD per g of tissue were quantified using a standard curve (0.025–25μg/mL EBD in formamide) and each of the sample weights. The data of the two technical replicates were averaged.
Evaluation of EBD Penetration Using Epifluorescence Microscopy
To evaluate the EBD penetration using epifluorescence microscopy, the esophagus fragments were fixed in 4% buffered formic aldehyde (pH 7.4) overnight at 4°C. The next day, after a short wash in phosphate buffered saline (PBS), the fragments were transferred into a 25% sucrose PBS solution at + 4°C to give protection against subsequent cryopreservation. Upon complete precipitation, the samples were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, USA) and stored at −80°C. Ten μm thick esophageal mucosal/submucosal serial sections were cut at a Leica CM1950 cryostat (Leica, Wetzlar, Germany) and were placed on microscope slides. The samples were observed using an Eclipse E600 epifluorescence microscope (Nikon Europe BV, Badhoevedorp, The Netherlands) equipped with a Nikon DXM 1200 digital camera.
Statistical Analysis
All the quantitative EBD tissue uptake data were expressed as mean ± standard deviation (SD); single comparisons were carried out using the paired and unpaired Student’s t-test (parametric data) where appropriate. Significance was set at a value of p < 0.05.
Results
Assessment of Mucosal Permeability
In the present study, 19 esophagi were damaged using an acidic/peptic solution, and 6 mucosal fragments were collected from each of them. According to the design of the study (Figure 2), of the 114 samples, 95 were stained with the EBD solution, and the presence of the dye inside the fragment was used as a measure of the mucosal permeability. The EBD absorption values were expressed as EBD µg/g of tissue.
The first analysis was carried out by comparing the EBD absorbed values in the two sample subgroups. As expected, the absorption values of the mucosal samples treated were significantly lower than those of the damaged but untreated mucosal fragments (Figure 3). A statistically significant reduction (p<0.01) in EBD penetration was observed after treatment with both AL2106 (8.4±4.5 µg/g) and SAS (3.6±2.7 µg/g) when compared with the control fragments (23.2±13.1 µg/g), confirming their protective properties. Assessment of the barrier effect after washing with saline showed that the effect persisted without variation for AL2016 (8.6±5.9 µg/g) while it showed a significant barrier effect reduction for SAS (5.9±4.3 µg/g).
Since the absorbance values observed in the untreated samples were rather variable (range 7.6–52.7 µg/g), potentially due to the variability depending on different animals and “on field” sampling conditions, the difference between each treatment and the corresponding control value obtained from the same esophagus was calculated, assuming that the fragments obtained from the same esophagus had the same behavior. Figure 4 shows the difference observed after the treatments with respect to their control values; higher values indicated a greater reduction in EBD absorption due to an improvement in the mucosal barrier. The results in the unwashed samples showed a reduction of 14.8±14.9 µg/g for AL2106 and of 19.6±12.3 µg/g for SAS. As observed for the absolute values, the saline washing did not modify the effect of AL2106 (14.6±14.3 µg/g) but it reduced that of SAS. (17.3±12.4 µg/g, p<0.05).
Evaluation of EBD Penetration in Epifluorescence Microscopy
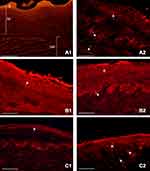
To evaluate the depth of mucosal penetration of the dye, mucosal samples were collected from all esophagi as described above and as schematized in Figure 2. The representative images obtained are shown in Figure 5.
Analysis of these samples suggested that the damaged esophageal mucosa displayed a very high orange background as shown in the control samples (perfused with damaging solution, but not treated with any topical agents, and not stained with EBD) (Figure 5A1).
In the samples not treated with topical agents but stained with EBD, an EBD-related signal present in all layers of the multilayered squamous epithelium was observed; the dye penetrated, affecting the lamina propria, passing through the muscularis mucosae and reaching the submucosa (Figure 5A2).
In the fragments treated with AL2106, the background signal was very high, but the EBD-related signal showed that the penetration did not affect all the layers of the epithelium (Figure 5B1). After washing in normal saline, only a minimal quantity of EBD penetrated the esophageal mucosa and submucosa (Figure 5B2).
Conversely, in the fragments treated with SAS, the presence of dye was noted in only a few layers of the epithelium (Figure 5C1). Washing in normal saline partially restored the EBD ability to penetrate the esophageal mucosa and submucosa (Figure 5C2). It could also be noted that, in the sections in which the epithelium still had keratin, it appeared intensely fluorescent.
Discussion
The aim of the present study was to evaluate the potential barrier effect and the bioadhesive capability of the new medical device called AL2106 on the esophageal mucosa. To achieve these goals, an ex vivo porcine model was used in which the damage was induced by the perfusion of an acidic/peptic solution, and the mucosal permeability was assessed by the absorption of a vital dye.7 The porcine esophagus is considered a proper model for ex vivo studies due to the great similarities between human and porcine gastrointestinal tracts.7,12 In addition, EBD is known to bind quantitatively to albumin in vivo and in vitro; therefore, it was used as an indicator of esophageal mucosal permeability.13–15 A previous experiment with the same model showed that EBD penetration was due to the increased mucosal permeability caused by exposure to the acidic/peptic solution since the intact mucosa did not absorb the dye.7 After perfusion with the damaging solution, all the untreated mucosal samples absorbed the EBD while the absorption of EBD was reduced when the esophagi were pre-treated with protective topical agents. Unlike the Authors’ previous study, a method of extraction and quantification of the dye was used to obtain a more objective measurement of EBD absorption instead of visual analysis of the staining.
In this study, the effect of AL2106, a new medical device with high bioadherence which contains a quantity of 0.5 to 5% chondroitin sulphate, a quantity of 0.05 to 5% xyloglucan and a quantity of 10 to 70% glycerol was investigated. Chondroitin sulphate is a natural glycosaminoglycan present in all connective tissue extracellular matrices, especially in the cartilage, skin, blood vessels, ligaments and tendons, where it carries out a number of biological functions (cell proliferation, differentiation, migration, tissue morphogenesis, organogenesis, infection and wound repair).16,17 Xyloglucan is a matrix polysaccharide which is present in the cell walls of all land plants. It is a natural biodegradable high-molecular weight branched polysaccharide (hemicellulose) derived from the tamarind seed. The configuration of this polysaccharide gives the product a “mucin-like” molecular structure, thus conferring optimal mucoadhesive properties. Xyloglucan possesses high swelling capacity, which is of great importance in initiating the bioadhesion process.18 Xyloglucan was used to increase the AL2106 adhesive capacity for its rheological profile; hypothetically, during swallowing, in which the stress on the formulation is high, the viscosity is low and AL2106 behaves like a liquid. On the contrary, when AL2106 adheres to the mucosa, the stress is null and it behaves like a solid, increasing molecular interaction and thus the viscosity.19
The reference compound was SAS, present on the market since 1987 and one of the most used medical devices for the treatment of GERD.8 It is composed of alginic acid, a linear polysaccharidic polymer distributed widely in the cell walls of brown algae; it is hydrophilic and forms a viscous gum when hydrated. Alginic acid derivatives, or alginates, act by creating a mechanical barrier which displaces the postprandial acid pocket. Due to its specific composition, it has been used to prevent the occurrence of reflux and to neutralize the acidity of the refluxate. Moreover, recent studies, both in vitro and in vivo, have shown its capacity of adhering to the esophageal mucosa and of preventing the mucosal impairment induced by the reflux components.6,20,21 In the present study, it was found that AL2106 was able to significantly reduce the amount of EBD absorbed by the damaged mucosa, an effect also shown by the reference device, and that this occurred even if the dye solution was applied after washing the mucosal fragments with saline.
To reduce the variability among the 19 esophagi examined and to better compare the two treatments, the difference between the dye absorbed after treatment and that of the corresponding untreated fragments was calculated. The entity of the variation induced by the treatments, either in unwashed or in washed fragments, was rather variable but the majority of the fragments showed a reduction in dye absorption ≥ 50%.
The persistence of a barrier effect after saline washing was particularly interesting since it could have represented a property displayed during more physiological conditions (ie during swallowing and salivation), therefore reflecting a critical role for the bioadhesive capability of the product.
Microscopic evaluation revealed that a damaged esophageal mucosa displayed great autofluorescence; however, it was possible to detect an EBD-related signal. In the samples not treated with any topical agents and stained with EBD, the dye penetrated the mucosa, affecting the lamina propria, passing through the muscularis mucosae and reaching the submucosa. In the samples treated with both AL2106 and the reference topical agent, the EBD signal was restricted to only some of the more superficial epithelial layers. A very high background signal was noted in the fragments treated with AL2106, probably due to some intrinsic properties of the specific formulation. Washing in saline did not seem to alter EBD distribution markedly, even if sporadic EBD-related signals were present in the submucosa and an additional increase in the background was observed. Even if the microscopic observation seemed to support the above-mentioned quantitative data, it is important to note that this type of analysis is widely influenced by both the operator and the sample tract analyzed.
Conclusion
In conclusion, in our experiments AL2106 showed a barrier protective effect on the esophageal mucosa similar to that of the topical agent used as reference and maintained its effect even after mucosal washing, thus suggesting superior resilience. Additional experimental and clinical studies may support its use as a valuable medical device for the treatment of GERD.
Acknowledgments
The Authors would like to thank Danilo Matteuzzi for his technical support in the organ sampling and CLAI slaughterhouse (Faenza, Ravenna, Italy) for organ supply. This study was supported by the University of Bologna (Programma di Ricerca Fondamentale Orientata, RFO331 MIUR 2016). Alfasigma Spa supplied the compounds tested and provided financial support for this study.
Disclosure
Alfasigma Spa was not involved in the study design, or in the collection, analysis and interpretation of the data. The views expressed in this article are the personal views of the authors and were not influenced by Alfasigma in any way. Alfasigma has agreed with the decision to submit this article for publication. Fiorella Calanni and Antonella Ferrieri are employees of Alfasigma. Maria Laura Bacci reports grants from Alfasigma during the conduct of the study. Fabio Baldi reports personal fees from Alfasigma during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
1. Klenzak S, Danelisen I, Brannan GD, Holland MA, van Tilburg MA. Management of gastroesophageal reflux disease: patient and physician communication challenges and shared decision making. World J Clin Cases. 2018;6(15):892–900. doi:10.12998/wjcc.v6.i15.892
2. Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61(9):1340–1354.
3. Baldi F. PPI-refractory GERD: an intriguing, probably overestimated, phenomenon. Curr Gastroenterol Rep. 2015;17(7):25. doi:10.1007/s11894-015-0451-3
4. Partial symptom‐response to proton pump inhibitors in patients with non‐erosive reflux disease or reflux oesophagitis – a post hoc analysis of 5796 patients - Bytzer - 2012 - Alimentary Pharmacology & Therapeutics - Wiley Online Library [Internet]. [cited April 23, 2020]. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/apt.12007.
5. Topical protection of human esophageal mucosal integrity. American journal of physiology-gastrointestinal and liver physiology [Internet]. [cited April 23, 2020]. Available from: https://journals.physiology.org/doi/full/10.1152/ajpgi.00424.2014.
6. Woodland P, Sifrim D. Esophageal mucosal integrity in nonerosive reflux disease. J Clin Gastroenterol. 2014;48(1):6–12.
7. Di Simone MP, Baldi F, Vasina V, et al. Barrier effect of Esoxx® on esophageal mucosal damage: experimental study on ex-vivo swine model. Clin Exp Gastroenterol. 2012; 5:103–107. doi: 10.2147/CEG.S31404
8. Leiman DA, Riff BP, Morgan S, et al. Alginate therapy is effective treatment for GERD symptoms: a systematic review and meta-analysis. Dis Esophagus. 2017;30(5):1–9.
9. Palmieri B, Merighi A, Corbascio D, Rottigni V, Fistetto G, Esposito A. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux.:7.
10. Batchelor HK, Banning D, Dettmar PW, Hampson FC, Jolliffe IG, Craig DQM. An in vitro mucosal model for prediction of the bioadhesion of alginate solutions to the oesophagus. Int J Pharm. 2002;238(1):123–132.
11. (14) (PDF) Oesophageal bioadhesion of sodium alginate suspensions: 2. Suspension behaviour on oesophageal mucosa [Internet]. ResearchGate. [cited May 22, 2020]. Available from: https://www.researchgate.net/publication/8103539_Oesophageal_bioadhesion_of_sodium_alginate_suspensions_2_Suspension_behaviour_on_oesophageal_mucosa.
12. Honkanen O, Pia L, Janne M, Sari E, Raimo T, Martti M. Bioavailability and in vitro oesophageal sticking tendency of hydroxypropyl methylcellulose capsule formulations and corresponding gelatine capsule formulations. Eur J Pharm Sci. 2002;15(5):479–488.
13. Saria A, Lundberg JM. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983;8:41–49. doi:10.1016/0165-0270(83)90050-X
14. Lundberg JM, Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983;302(5905):251–253.
15. Zhiyuan Q, Qingyong L, Shengming H, Hui M. Protective effect of rhEPO on tight junctions of cerebral microvascular endothelial cells early following traumatic brain injury in rats. Brain Inj. 2016;30(4):462–467. doi:10.3109/02699052.2015.1080386
16. Souich PD, García AG, Vergés J, Montell E. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J Cell Mol Med. 2009;13(8a):1451–1463.
17. Lauder RM. Chondroitin sulphate: a complex molecule with potential impacts on a wide range of biological systems. Complement Ther Med. 2009;17:56–62. doi:10.1016/j.ctim.2008.08.004
18. Singh R, Malviya R, Sharma PK. Extraction and characterization of tamarind seed polysaccharide as a pharmaceutical excipient. Pharmacogn J. 2011;3(20):17–19.
19. Lapasin R. Rheology of Industrial Polysaccharides: Theory and Applications. Springer Science & Business Media; 2012:633.
20. Zentilin P, Dulbecco P, Savarino E, et al. An evaluation of the antireflux properties of sodium alginate by means of combined multichannel intraluminal impedance and pH-metry. Aliment Pharmacol Ther. 2005;21(1):29–34.
21. Woodland P, Lee C, Duraysami Y, Farré R, Dettmar P, Sifrim D. Assessment and protection of esophageal mucosal integrity in patients with heartburn without Esophagitis. Am J Gastroenterol. 2013;108(4):535–543.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.