Back to Journals » OncoTargets and Therapy » Volume 12
Baicalin Inhibits Cell Viability, Migration and Invasion in Breast Cancer by Regulating miR-338-3p and MORC4
Authors Duan X, Guo G, Pei X, Wang X, Li L, Xiong Y, Qiu X
Received 25 May 2019
Accepted for publication 2 December 2019
Published 17 December 2019 Volume 2019:12 Pages 11183—11193
DOI https://doi.org/10.2147/OTT.S217101
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nicola Silvestris
Xin Duan, Guangcheng Guo, Xinhong Pei, Xinxing Wang, Lin Li, Youyi Xiong, Xinguang Qiu
Department of Breast Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, People’s Republic of China
Correspondence: Xinguang Qiu
Department of Breast Surgery, The First Affiliated Hospital of Zhengzhou University, No. 1 East Jianshe Road, Zhengzhou, Henan 450000, People’s Republic of China
Tel +86 371-67967247
Email [email protected]
Background: Baicalin is a natural compound from the roots of Scutellaria lateriflora Georgi, which plays anti-cancer role in multiple cancers. However, the exact role and potential underlying mechanism of baicalin in breast cancer (BC) remain poorly understood.
Methods: Thirty BC patients were recruited in this study. MCF-10A, MCF-7 and MDA-MB-231 cells were used to investigate the anti-cancer role of baicalin in vitro. Cell viability, migration, invasion and apoptosis were measured by MTT, trans-well and flow cytometry, respectively. The expression levels of microRNA-338-3p (miR-338-3p) and microrchidia family CW-type zinc-finger 4 (MORC4) were measured by quantitative real-time polymerase chain reaction or Western blot. The interaction between miR-338-3p and MORC4 was explored by luciferase reporter assay and RNA immunoprecipitation.
Results: We found that Baicalin treatment inhibited cell viability, migration and invasion but promoted apoptosis of BC cells. The expression of miR-338-3p was decreased in BC tissues and cells and miR-338-3p overexpression suppressed cell viability, migration and invasion but induced apoptosis. MiR-338-3p expression was reversed by baicalin exposure and inhibition of miR-338-3p attenuated the role of baicalin in viability, apoptosis, migration and invasion. MORC4 mRNA level was increased in BC tissues and cells, which was decreased by baicalin exposure. MORC4 was a target of miR-338-3p and its overexpression alleviated the effect of miR-338-3p on cell viability, apoptosis, migration and invasion.
Conclusion: In conclusion, baicalin suppressed cell viability, migration and invasion but promoted apoptosis in BC cells by regulating miR-338-3p and MORC4, indicating the promising pharmacological value of baicalin in BC treatment.
Keywords: breast cancer, baicalin, miR-338-3p, MORC4
Introduction
Breast cancer (BC) is the most common tumor malignancy with high incidence in women around the world because of the lifestyle changes, such as obesity, alcohol consumption and tobacco use.1–3 In recent years, many prognostic and predictive biomarkers (such as estrogen and progesterone receptors, human epidermal growth factor receptor 2 and some microRNA (miRNAs)) have been investigated for the treatment of BC patients with the improvement of understanding pathogenesis.4 The exploration of methods for screening and prevention contributes to improving the outcomes of BC patients.5 Multiple strategies including surgical resection, radiotherapy, endocrine therapy, chemotherapy and immunotherapy have been applied in the clinical treatment of BC.6,7 However, the effective strategies are limited. Hence, there is a need for exploring novel avenues for the therapeutics of BC.
The Chinese herbal medicines have been regarded as promising adjuvant therapy for patients with BC.8 Baicalin is a benzylisoquinoline alkaloid from Chinese herbal medicine Scutellaria lateriflora Georgi and its structure is exhibited as previous study,9 which has been demonstrated to play anti-proliferation, anti-inflammatory and anti-metastatic roles in human cancers or diseases.4, More importantly, previous study suggests that baicalin could inhibit BC development in vitro and in vivo.15 However, the regulatory mechanism that underlies the anti-cancer role of baicalin in BC remains largely unclear.
MiRNAs are a class of noncoding RNA molecules with 18–25 nucleotides in length, which have been reported to act as important biomarkers for diagnosis, prognosis and therapy of BC at early stage.16 Increasing evidences suggest that miR-338-3p could function as a tumor suppressor by inhibiting proliferation, metastasis and promoting apoptosis in gastric cancer, osteosarcoma and prostate cancer.17–19 In BC, a former work describes that down-regulation of miR-338-3p promotes cell growth, migration and invasion in vitro and contributes to lung metastasis in vivo.20 Microrchidia family CW-type zinc-finger (MORC) family, including MORC1, MORC2, MORC3 and MORC4, plays pivotal roles in human cancer development.21 MORC4 mRNA, located at chromosome Xq22.3, is widely expressed in tissues, which is increased in diffuse large B-cell lymphoma.22 Furthermore, emerging effort reveals that MORC4 mRNA acts as a novel oncogene and reduction of MORC4 protein induces apoptosis production in BC cells.23 Bioinformatics analysis searches the binding sites between miR-338-3p and MORC4. Hence, we assumed that miR-338-3p/MORC4 might be associated with the anti-cancer role of baicalin in BC. In the present study, we investigated the pharmacological effect of baicalin on cell viability, apoptosis, migration and invasion in BC cells. Moreover, we explored whether miR-338-3p/MORC4 axis was involved in the mechanism addressed by baicalin.
Materials and Methods
Patients and Clinical Samples
Thirty tumor tissues and matched para-tumor normal samples were harvested from patients who were diagnosed with BC at the First Affiliated Hospital of Zhengzhou University. The written informed consent has been provided and this research was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The tissues were stored at −80°C and used for RNA extraction.
Cell Culture and Treatment
Normal human breast epithelial cell line MCF-10A and BC cell lines (MCF-7 and MDA-MB-231) were purchased from Cell Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All cells were cultured in an incubator with 5% CO2 atmosphere at 37°C. The culture medium was premixed RPMI-1640 medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum (Gibco) and exchanged every 3 days. To investigate the effect of baicalin on BC progression, MCF-7 and MDA-MB-231 cells were exposed to the indicated concentration of baicalin (Sigma, St Louis, MO, USA) for the indicated time.
For cell transfection, pcDNA targeting the MORC4 overexpression vector (MORC4), pcDNA, miR-338-3p mimic (miR-338-3p), miRNA negative control (miR-NC), miR-338-3p inhibitor (anti-miR-338-3p) and inhibitor negative control (anti-miR-NC) were synthesized by Genepharma (Shanghai, China). Cell transfection was performed in MCF-7 and MDA-MB-231 cells using Lipofectamine 3000 transfection reagent (Invitrogen, Carlsbad, CA, USA) when cells reached 70% confluence in 6-well plates. After 24 hrs of the transfection, cells were harvested for baicalin treatment or following analyses.
Cell Viability
Cell viability was measured using MTT Kit (Beyotime, Shanghai, China). MCF-10A, MCF-7 and MDA-MB-231 cells were seeded into 96-well plates at a density of 10, 000 cells per well overnight and then exposed to different concentrations (0, 25, 50, 100, 200 μM) of baicalin for 24 hrs or treated with 200 μM of baicalin for 0, 12, 24 or 48 hrs. Every sample was prepared in quadruplicate. At end point, the medium was replaced with fresh medium containing 0.5 mg/mL MTT solution and incubated for 4 hrs at 37°C. Subsequently, 100 μL formazan dissolving solution in the kit was added into each well. The absorbance at 570 nm was determined using a microplate reader (Bio-Rad, Hercules, CA, USA). Cell viability was normalized to non-treated control group.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was isolated from tissues or cells with or without treatment of baicalin using Trizol reagent (Thermo Fisher, Wilmington, DE, USA). The RNA was used for reverse transcribe using Taqman™ miRNA or mRNA Reverse Transcription Kit (Thermo Fisher) and qRT-PCR was performed using SYBR and special primers. The samples were prepared in duplicate. The primers synthesized by Sangon Biotech (Shanghai, China) were listed as follows: MORC4 (Forward, 5ʹ-TGGATTGAGCACCAGACTGT-3ʹ; Reverse, 5ʹ-AACTGGCCTCTTTCTCCACA-3ʹ); β-actin (Forward, 5ʹ-ATGGGTCAGAAGGATTCCTATGTG-3ʹ; Reverse, 5ʹ-CTTCATGAGGTAGTCAGTCAGGTC-3ʹ); miR-338-3p (Forward, 5ʹ-TGCGGTCCAGCATCAGTGAT-3ʹ; Reverse, 5ʹ-CCAGTGCAGGGTCCGAGGT-3ʹ); U6 (Forward, 5ʹ-CTCGCTTCGGCAGCACA-3ʹ; Reverse, 5ʹ-AACGCTTCACGAATTTGCGT-3ʹ). β-Actin or U6 was used as endogenous controls. The expression levels of miR-338-3p and MORC4 were analyzed via 2−ΔΔCt method.24
Trans-Well Assay
For the migration and invasion assays, 24-well trans-well chambers (Corning, Corning, NY, USA) were used. Invasion assay featured a member pre-coated with Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA). After transfection, cells were digested and seeded into upper chambers (1×104 cells for migration assay and 5×104 cells for invasion assay). After incubation with or without 200 μM of baicalin for 12 hrs at 37°C, cells passed the membrane to the lower surface were stained with 0.1% crystal violet (Sigma). The stained cells were counted under an inverted microscope (Olympus, Tokyo, Japan) with three random fields.
Cell Apoptosis
Cell apoptosis was measured by flow cytometry. In brief, transfected cells were exposed to different concentrations (0, 25, 50, 100, 200 μM) of baicalin for 24 hrs or treated with 200 μM of baicalin for 0, 12, 24 or 48 hrs, followed by collection for apoptosis analysis using Annexin V-FITC/PI apoptosis detection kit (Vazyme, Nanjing, China) according to the manufacturer’s instructions. The apoptotic rate was analyzed with a flow cytometer (BD Biosciences, San Jose, CA, USA).
Luciferase Reporter Assay and RNA Immunoprecipitation (RIP)
The putative binding sites of miR-338-3p and MORC4 were predicted using TargetScan Release 7.2. The 3ʹ-untranslated region (UTR) sequences of MORC4 containing the wild-type (UGCUGGA) or mutant (GUUCUAG) binding sites of miR-338-3p were inserted into the downstream of luciferase reporter gene in pmirGLO vector (Promega, Madison, WI, USA) to form the luciferase reporter constructs targeting MORC4 (MORC4-WT or MORC4-MUT). For luciferase reporter assay, MCF-7 cells were transfected with miR-338-3p or miR-NC and wild-type or mutant luciferase reporter constructs using Lipofectamine 3000 transfection reagent. Luciferase activity was detected at 48 hrs after post-transfection using a luciferase reporter assay kit (Promega).
RIP assay was performed to verify the interaction between miR-338-3p and MORC4 through Ago2 immunoprecipitation. For RIP assay, a Magna RNA immunoprecipitation kit (Millipore, Billerica, MA, USA) was used. Briefly, 1 × 107 MCF-7 cells transfected with miR-338-3p or miR-NC were lysed in RIP buffer and incubated with magnetic beads pre-coated with Ago2 or IgG antibody for 6 hrs at 4°C. The RNA-induced silencing complex was incubated with Proteinase K (Sigma) and then the mRNA level of MORC4 in the complex was detected by qRT-PCR.
Western Blot
After the transfection, MCF-7 and MDA-MB-231 cells were harvested and lysed for total protein extraction. The equal amounts (20 μg) of proteins were subjected to SDS-PAGE and transferred to nitrocellulose membranes (Millipore). Each sample was prepared in triplicate. The membranes were blocked with 5% skimmed milk, probed with antibodies against MORC4 (ab121099, Abcam, Cambridge, MA, USA) or β-actin (ab8227, Abcam) at 4°C, and interacted for 1 hr with horseradish peroxidase-labeled secondary antibody (ab6721, Abcam), followed by incubation of BeyoECL Plus (Beyotime). Protein blot was visualized in the dark using a film (Carestream Health, Rochester, NY, USA) and analyzed using Quantity One software (Bio-Rad). β-Actin was used as an endogenous control.
Statistical Analysis
GraphPad Prism 7 software (GraphPad Inc., La Jolla, CA, USA) was used for the statistical tests. The data were expressed as the mean ± standard deviation (S.D.) and compared by Student’s t-test or ANOVA followed by Dunnett’s test. All experiments were repeated three times. P<0.05 indicated a significant difference.
Results
Baicalin Inhibits BC Cell Viability, Migration and Invasion but Promotes Apoptosis
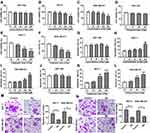
To investigate the potential role of baicalin in BC progression, MCF-10A, MCF-7 and MDA-MB-231 cells were exposed to different concentrations (0, 25, 50, 100 or 200 Μm) of baicalin for 24 hrs. The results of MTT assay displayed that 200 μM of baicalin treatment significantly decreased cell viability of MCF-7 and MDA-MB-231 cells, while it showed little cytotoxicity to MCF-10A cells (Figure 1A–C). Moreover, MCF-10A, MCF-7 and MDA-MB-231 cells were treated with 200 μM of baicalin for 12, 24 or 48 hrs. As demonstrated in Figure 1D–F, cell viability of MCF-7 and MDA-MB-231 was obviously inhibited at 24 and 48 hrs after the treatment of baicalin, whereas MCF-10A cell viability was not affected by the treatment of baicalin at 12, 24 or 48 hrs. Meanwhile, stimulation of baicalin-induced apoptosis of MCF-7 and MDA-MB-231 cells in concentration- and time-dependent manners, while it did not trigger the apoptosis of MCF-10A cells (Figure 1G–L). In addition, the effect of baicalin on cell migration and invasion was investigated in MCF-7 and MDA-MB-231 cells after the treatment of 200 μM of baicalin for 12 hrs. The results showed that the treatment of baicalin significantly repressed the migration and invasion of MCF-7 and MDA-MB-231 cells (Figure 1M and N). These findings suggested that baicalin could suppress viability, migration and invasion of BC cells.
miR-338-3p Suppresses BC Cell Viability, Migration and Invasion but Induces Apoptosis
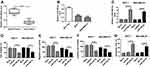
qRT-PCR was performed to measure the expression of miR-338-3p in BC tissues and cell lines. Compared with that in normal samples, the expression of miR-338-3p was aberrantly reduced in BC tissues (n=30) (Figure 2A). Moreover, miR-338-3p level was lower in MCF-7 and MDA-MB-231 cells than that in MCF-10A cells (Figure 2B). To explore the role of miR-338-3p in BC development, MCF-7 and MDA-MB-231 cells were transfected with miR-338-3p mimic or miR-NC. As shown in Figure 2C, the abundance of miR-338-3p was effectively elevated in MCF-7 and MDA-MB-231 cells after the transfection of miR-338-3p mimic compared with that in miR-NC or mock group. Moreover, overexpression of miR-338-3p significantly suppressed cell viability, migration and invasion but increased cell apoptosis in MCF-7 and MDA-MB-231 cells (Figure 2D–G). These data indicated that miR-338-3p could repress suppress viability, migration and invasion of BC cells.
Baicalin Suppresses Viability, Migration and Invasion but Promotes Apoptosis of BC Cells by Up-Regulating miR-338-3p
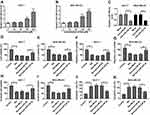
Treatment of baicalin progressively increased the expression level of miR-338-3p in MCF-7 and MDA-MB-231 cells (Figure 3A and B). These findings indicated that miR-338-3p might be involved in baicalin-mediated BC progression. To explore whether the anti-cancer role of baicalin in BC was associated with miR-338-3p, MCF-7 and MDA-MB-231 cells were transfected with anti-miR-338-3p or anti-miR-NC and then treated with 200 μM of baicalin for 24 hrs. The transfection efficacy was validated with the result that miR-338-3p abundance was efficacy reduced by transfection of anti-miR-338-3p compared with that in anti-miR-NC and mock group (Figure 3C). Moreover, knockdown of miR-338-3p attenuated baicalin-induced viability suppression in MCF-7 and MDA-MB-231 cells (Figure 3D and E). Additionally, the data of trans-well assay showed that down-regulation of miR-338-3p significantly weakened baicalin-mediated inhibition of cell migration and invasion in MCF-7 and MDA-MB-231 cells (Figure 3F–I). Furthermore, analysis of flow cytometry revealed that knockdown of miR-338-3p protected against baicalin-induced cell apoptosis in MCF-7 and MDA-MB-231 cells (Figure 3J and K). These results showed that baicalin might inhibit viability, migration and invasion by regulating miR-338-3p in BC cells.
MORC4 Is a Target of miR-338-3p in BC Cells
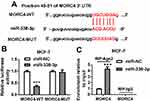
To further explore the mechanism, the potential target of miR-338-3p was searched by TargetScan Release 7.2, which showed the potential binding sites of miR-338-3p and MORC4, suggesting that MORC4 might be a target of miR-338-3p (Figure 4A). To validate this prediction, the wild-type and mutant luciferase reporter vector MORC4-WT and MORC4-MUT were constructed and luciferase reporter assay was performed in MCF-7 cells co-transfected with these constructs and miR-338-3p mimic or miR-NC. As shown in Figure 4B, overexpression of miR-338-3p significantly decreased the luciferase activity in MCF-7 cells with transfection of MORC4-WT, while it failed to show efficacy in MORC4-MUT group. Moreover, miR-338-3p overexpression resulted in higher enrichment level of MORC4 mRNA in MCF-7 cells after Ago2 RIP assay (Figure 4C). These findings uncovered that MORC4 could act as a target of miR-338-3p in BC cells.
Baicalin Represses MORC4 Expression by miR-338-3p in BC Cells
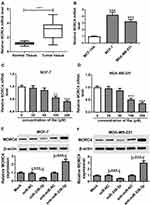
Subsequently, the expression level of MORC4 mRNA was detected in BC tissues and cells. The qRT-PCR assay demonstrated that the abundance of MORC4 mRNA was obviously elevated in BC tissues (n=30) and cells in comparison to that in normal samples or MCF-10A cells, respectively (Figure 5A and B). However, exposure of baicalin progressively decreased the expression level of MORC4 mRNA in MCF-7 and MDA-MB-231 cells (Figure 5C and D). In addition, the effect of miR-338-3p on MORC4 protein level was investigated in MCF-7 and MDA-MB-231 cells. The results of Western blot described, as displayed in Figure 5E and F, that the abundance of MORC4 protein was notably decreased in the two cell lines by miR-338-3p overexpression, but was increased by knockdown of miR-338-3p. These results uncovered that baicalin could decrease MORC4 expression by up-regulating miR-338-3p in BC cells.
Up-Regulation of miR-338-3p Decreases Viability, Migration and Invasion but Promotes Apoptosis by Targeting MORC4 in BC Cells
To explore whether MORC4 is required for miR-338-3p-mediated BC progression, MCF-7 and MDA-MB-231 cells were transfected with miR-NC, miR-338-3p, miR-338-3p and pcDNA or MORC4. As displayed in Figure 6A and B, the protein level of MORC4 was significantly decreased by miR-338-3p overexpression in MCF-7 and MDA-MB-231 cells, which was restored by introduction of MORC4. Furthermore, restoration of MORC4 conspicuously attenuated miR-338-3p-mediated viability inhibition in MCF-7 and MDA-MB-231 cells (Figure 6C and D). In addition, introduction of MORC4 evidently abated miR-338-3p-induced suppression of migration and invasion in MCF-7 and MDA-MB-231 cells (Figure 6E–H, Supplementary Figure 1A and B). Besides, up-regulation of MORC4 weakened miR-338-3p-mediated promotion of apoptosis in MCF-7 and MDA-MB-231 cells (Figure 6I and J). These data revealed that miR-338-3p inhibited viability, migration and invasion but promoted apoptosis by targeting MORC4 in BC cells.
Discussion
In the current study, we demonstrated the therapeutic effect of baicalin on BC progression. Specially, baicalin treatment induced the up-regulation of miR-338-3p and down-regulation of MORC4. Exposure of baicalin inhibited cell viability by promoting apoptosis and decreased cell migration and invasion in BC cells. Moreover, we found that the mechanism underlying the anti-cancer role of baicalin in BC development might be associated with miR-338-3p and MORC4. This research elucidates a novel molecular mechanism of baicalin in the development and therapeutics of BC.
Proliferation and metastasis are two main challenges in cancer development and therapeutics. Baicalin is a natural medicine having capability of cancer therapy by decreasing inflammation, proliferation and metastasis. Gao et al showed that baicalin inhibited BC cell proliferation through G1 cell cycle arrest and apoptosis production by blocking the nuclear factor-ĸB (NF-ĸB) pathway.15 Wang et al also demonstrated the anti-proliferation of baicalin by G1 arrest and apoptosis in BC cells.25 In the present study, our data displayed that baicalin decreases cell viability by promoting apoptosis in BC cells, while whether cell cycle process is affected needs more investigation in further study. Migration and invasion are two key forms of metastasis. Moreover, previous works provided that baicalin inhibited migration and invasion of BC cells by suppressing epithelial-to-mesenchymal transition or mitogen-activated protein kinase (MAPK) activity.26,27 Consistently with these findings, we also proved the anti-metastasis role of baicalin in BC cells by trans-well assay. However, the potential pathway induced migration and invasion is absent in the current paper, which should be explored in future. This study focused on exploring the underlying molecular mechanism addressed by baicalin/miRNA/mRNA.
Previous studies demonstrated that baicalin could play the anti-cancer role by targeting miRNAs. For example, baicalin could alleviate the inflammatory response in chondrocytes by regulating miR-126.12 Moreover, Wu et al reported that baicalin displays an anti-cancer role in hepatic stellate cells by regulating miR-3595.13 In our study, we found that miR-338-3p expression was decreased in BC tissues and cells, suggesting that miR-338-3p might be a tumor suppressor in BC. In addition, gain-of-function experiments exhibited that overexpression of miR-338-3p led to inhibition of viability, migration and invasion and increase of apoptosis, indicating the suppressive role of miR-338-3p in BC progression, which is also in agreement with previous studies.20,28 Besides, Kodahl et al reported that miR-338-3p expression was lower in serum of post-operative BC patients than that in pre-operative samples.29 And, Sueta et al suggested that high expression of miR-338-3p in serum was associated with the recurrence of BC patients.30 These two reports summarized that miR-338-3p in serum might be used to monitor the prognosis of patients, and it was not controversial to our study because of the varying microenvironment between tumor tissues and serum. Furthermore, here we found that miR-338-3p expression was up-regulated by baicalin treatment and knockdown of miR-338-3p weakened the effect of baicalin on BC development, indicating that baicalin could play the anti-cancer role in BC by increasing miR-338-3p.
One of the main mechanisms that allow miRNAs participating in cancer progression is to regulate target genes expression. Former works have indicated multiple targets of miR-338-3p, such as protein-tyrosine phosphatase 1B (PTP1B), runt-related transcription factor 2 (RUNX2), cyclin-dependent kinase 4 (CDK4) and Ras-Related Protein 23 (RAB23).17–19 To further elucidate the mechanism of baicalin, this research validated that MORC4 could act as a target of miR-338-3p, revealed by luciferase reporter and RIP assays. The emerging evidence indicated MORC4 as an oncogene in BC, uncovered by which its knockdown suppressed cell growth by promoting apoptosis.23 Consistently with our study, we also found that MORC4 expression was enhanced in BC tissues and cells and its restoration counteracted the therapeutic effect of miR-338-3p. Moreover, treatment of baicalin could decrease the MORC4 level. These data reflected that miR-338-3p/MORC4 was associated with the impact of baicalin in BC. Previous studies showed that BC is a stem cell disease and phosphatidylinositol 3 kinase (PI3K)–protein kinase B (AKT)–mammalian targets of rapamycin (mTOR) signaling is one important pathway for BC development.31,32 Hence, the effect of baicalin on stem cell function and potential signaling pathway should be explored in future. Moreover, the poor bioavailability limited the application of baicalin in clinical. Thus, the drug delivery system, such as gold nanoparticles,33 that might improve the bioavailability of baicalin should be investigated in further study. Meanwhile, Yang et al indicated that miR-193b-3p could take part in BC development by serving as a gene regulator of MORC4.23 However, whether this miRNA was associated with baicalin-mediated mechanism is unclear. Here, we found that miR-193b-3p abundance in MCF-7 and MDA-MB-231 cells was up-regulated (Supplementary Figure 2A and B). Hence, we hypothesized that miR-193b-3p might be also responsible for baicalin-mediated regulation in BC development, which should be confirmed in the future.
In conclusion, our findings demonstrated the therapeutic effect of baicalin on BC progression, revealed by inhibition of viability, migration and invasion and increase of apoptosis, possibly by up-regulating miR-338-3p and down-regulating MORC4. This study first provided that miR-338-3p and MORC4 were involved in the baicalin-mediated mechanism, indicating the clinical value of baicalin on BC treatment.
Disclosure
There are no conflicts of interest to declare.
References
1. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi:10.1016/S0140-6736(16)31891-8
2. Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Semin Cancer Biol. 2004;14(6):473–486. doi:10.1016/j.semcancer.2004.06.010
3. Teegarden D, Romieu I, Lelièvre SA. Redefining the impact of nutrition on breast cancer incidence: is epigenetics involved? Nutr Res Rev. 2012;25(1):68–95. doi:10.1017/S0954422411000199
4. Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol. 2018;52(Pt 1):56–73. doi:10.1016/j.semcancer.2017.08.010
5. Nattinger AB, Mitchell JL. Breast cancer screening and prevention. Ann Intern Med. 2016;164(11):ITC81–ITC96. doi:10.7326/AITC201606070
6. Harbeck N, Penault-Llorca F, Cortes J, ` Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. doi:10.1038/s41572-019-0111-2
7. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi:10.1001/jama.2018.19323
8. Zhu L, Li L, Li Y, Wang J, Wang Q. Chinese herbal medicine as an adjunctive therapy for breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:9469276. doi:10.1155/2016/9469276
9. Huang T, Liu Y, Zhang C. Pharmacokinetics and bioavailability enhancement of baicalin: a review. Eur J Drug Metab Pharmacokinet. 2019;44(2):159–168. doi:10.1007/s13318-018-0509-3
10. Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49(4):285–292. doi:10.1002/pros.10024
11. Wang J, Masika J, Zhou J, et al. Traditional Chinese medicine baicalin suppresses mESCs proliferation through inhibition of miR-294 expression. Cell Physiol Biochem. 2015;35(5):1868–1876. doi:10.1159/000373997
12. Yang X, Zhang Q, Gao Z, Yu C, Zhang L. Baicalin alleviates IL-1beta-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed Pharmacother. 2018;99:184–190. doi:10.1016/j.biopha.2018.01.041
13. Wu X, Zhi F, Lun W, Deng Q, Zhang W. Baicalin inhibits PDGF-BB-induced hepatic stellate cell proliferation, apoptosis, invasion, migration and activation via the miR-3595/ACSL4 axis. Int J Mol Med. 2018;41(4):1992–2002. doi:10.3892/ijmm.2018.3427
14. Tao Y, Zhan S, Wang Y, et al. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci Rep. 2018;8(1):14477. doi:10.1038/s41598-018-32734-2
15. Gao Y, Liu H, Wang H, et al. Baicalin inhibits breast cancer development via inhibiting IkB kinase activation in vitro and in vivo. Int J Oncol. 2018;53(6):2727–2736. doi:10.3892/ijo.2018.4594
16. Nassar FJ, Nasr R, Talhouk R. MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther. 2017;172:34–49. doi:10.1016/j.pharmthera.2016.11.012
17. Sun F, Yu M, Yu J, et al. miR-338-3p functions as a tumor suppressor in gastric cancer by targeting PTP1B. Cell Death Dis. 2018;9(5):522. doi:10.1038/s41419-018-0611-0
18. Jia F, Zhang Z, Zhang X. MicroRNA-338-3p inhibits tumor growth and metastasis in osteosarcoma cells by targeting RUNX2/CDK4 and inhibition of MAPK pathway. J Cell Biochem. 2019;120(4):6420–6430. doi:10.1002/jcb.27929
19. Wang Y, Qin H. miR-338-3p targets RAB23 and suppresses tumorigenicity of prostate cancer cells. Am J Cancer Res. 2018;8(12):2564–2574.
20. Liang Y, Xu X, Wang T, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8(7):e2928. doi:10.1038/cddis.2017.325
21. Hong G, Qiu H, Wang C, et al. The emerging role of morc family proteins in cancer development and bone homeostasis. J Cell Physiol. 2017;232(5):928–934. doi:10.1002/jcp.25665
22. Liggins AP, Cooper CD, Lawrie CH, et al. MORC4, a novel member of the MORC family, is highly expressed in a subset of diffuse large B-cell lymphomas. Br J Haematol. 2007;138(4):479–486. doi:10.1111/j.1365-2141.2007.06680.x
23. Yang Z, Zhuang Q, Hu G, Geng S. MORC4 is a novel breast cancer oncogene regulated by miR-193b-3p. J Cell Biochem. 2019;120(3):4634–4643. doi:10.1002/jcb.27751
24. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
25. Wang N, Tang LJ, Zhu GQ, et al. Apoptosis induced by baicalin involving up-regulation of P53 and bax in MCF-7 cells. J Asian Nat Prod Res. 2008;10(11–12):1129–1135. doi:10.1080/10286020802410664
26. Zhou T, Zhang A, Kuang G, et al. Baicalin inhibits the metastasis of highly aggressive breast cancer cells by reversing epithelial-to-mesenchymal transition by targeting beta-catenin signaling. Oncol Rep. 2017;38(6):3599–3607. doi:10.3892/or.2017.6011
27. Wang XF, Zhou QM, Du J, Zhang H, Lu YY, Su SB. Baicalin suppresses migration, invasion and metastasis of breast cancer via p38MAPK signaling pathway. Anticancer Agents Med Chem. 2013;13(6):923–931. doi:10.2174/18715206113139990143
28. Vivacqua A, Sebastiani A, Miglietta AM, et al. miR-338-3p is regulated by estrogens through gper in breast cancer cells and cancer-associated fibroblasts (cafs). Cells. 2018;7(11):E203. doi:10.3390/cells7110203
29. Kodahl AR, Zeuthen P, Binder H, Knoop AS, Ditzel HJ. Alterations in circulating miRNA levels following early-stage estrogen receptor-positive breast cancer resection in post-menopausal women. PLoS ONE. 2014;9(7):e101950. doi:10.1371/journal.pone.0101950
30. Sueta A, Yamamoto Y, Tomiguchi M, Takeshita T, Yamamoto-Ibusuki M, Iwase H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget. 2017;8(41):69934–69944. doi:10.18632/oncotarget.19482
31. Dittmer J. Breast cancer stem cells: features, key drivers and treatment options. Semin Cancer Biol. 2018;53:59–74. doi:10.1016/j.semcancer.2018.07.007
32. Dey N, De P, Leyland-Jones B. PI3K-AKT-mTOR inhibitors in breast cancers: from tumor cell signaling to clinical trials. Pharmacol Ther. 2017;175:91–106. doi:10.1016/j.pharmthera.2017.02.037
33. Lee D, Ko WK, Hwang DS, et al. Use of baicalin-conjugated gold nanoparticles for apoptotic induction of breast cancer cells. Nanoscale Res Lett. 2016;11(1):381. doi:10.1186/s11671-016-1586-3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.