Back to Journals » Drug Design, Development and Therapy » Volume 16
Baicalin Alleviates Contrast-Induced Acute Kidney Injury Through ROS/NLRP3/Caspase-1/GSDMD Pathway-Mediated Proptosis in vitro
Authors Li Y, Wang J, Huang D, Yu C
Received 22 June 2022
Accepted for publication 16 September 2022
Published 28 September 2022 Volume 2022:16 Pages 3353—3364
DOI https://doi.org/10.2147/DDDT.S379629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Yanyan Li,1,2 Junda Wang,2 Dan Huang,2 Chao Yu1
1College of Pharmacy, Chongqing Medical University, Chongqing, People’s Republic of China; 2Chongqing Traditional Chinese Medicine Hospital, Chongqing, People’s Republic of China
Correspondence: Chao Yu, College of Pharmacy, Chongqing Medical University, Yixueyuan Road, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86 23-68485589, Fax +86 23-68486294, Email [email protected]
Purpose: To investigate the effect of baicalin on the reactive oxygen species (ROS)/ NOD-like receptor protein 3 (NLRP3)/Caspase-1/gasdermin-D (GSDMD) inflammasome pathway and its related mechanism in regulating pyroptosis of human renal tubular epithelial cells (HK-2) induced by contrast media.
Methods: Iohexol was used to act on HK-2 cells to establish a renal tubular cell pyroptosis model; and the signal pathway genes were silenced, cytokines were detected by enzyme-linked immunosorbent assay (ELISA), and cell viability, gene expression, and protein expression were evaluated by double fluorescence staining and flow cytometry. To assess the cytotoxicity caused by the contrast agent; cells were pretreated with different concentrations of baicalin; and then the cells were exposed to iohexol again, and the relevant indicators were tested again.
Results: After HK-2 cells were exposed to iohexol, the NLRP3 inflammasome pathway markers NLRP3, interleukin (IL)-1β, and IL-18 mRNA levels as well as the protein expression levels of NLRP3, ASC, Caspase-1, and GSDMD were up-regulated. In addition, the effect also significantly increased the IL-18, IL-1β, lactate dehydrogenase (LDH), superoxide dismutase (SOD), malondialdehyde (MDA) release, and cellular ROS levels. The results of Annexin V-FITC/PI flow cytometry showed that the level of apoptosis was increased. However, after the intervention of baicalin, the changes in the above indexes caused by iohexol stimulation of HK-2 cells were inhibited.
Conclusion: Exposure to iohexol can induce pyroptosis of HK-2 cells through the ROS/NLRP3/Caspase-1/GSDMD signaling pathway. Baicalin ameliorated iohexol-induced pyroptosis in HK-2 cells by regulating the NLRP3 inflammasome pathway.
Keywords: pyroptosis, baicalin, iohexol, ROS/NLRP3/Caspase-1/GSDMD pathway, kidney injury
Introduction
The kidney plays an important role in maintaining the osmotic pressure balance in the body and promoting the metabolism of substances in the body. It is the main metabolic organ of the body, and the renal tubular epithelial cells are the main working cells.1 But the kidneys are susceptible to many pathogenic injuries, including metabolic disturbances, drug retention, contrast agents, and more. Among them is an increase in acute kidney injury (AKI) caused by nephrotoxic drugs and contrast media, which often leads to subsequent chronic kidney disease (CKD). On the other hand, accumulating evidence also suggests that CKD is also a predisposing factor for new-onset AKI.2 CKD affects 26–30 million adults in the United States and remains a major public health problem. The US Centers for Disease Control and Prevention project that 47% of 30-year-olds will develop CKD during their lifetime. Eleven percent of individuals with stage 3 CKD will eventually progress to end-stage renal disease (ESRD), requiring dialysis or kidney transplantation. CKD is also one of the strongest risk factors for cardiovascular disease. The costs to care for CKD (US$49 billion) are more than twice as large as ESRD costs ($23 billion) reported in Explor Med.3
In radiology procedures, contrast media (CI) are used for interventional therapy in order to determine various diseases. Most of the commonly used contrast agents are drugs containing different iodine concentrations, that is, iodine contrast agents. Because iodinated contrast agents have the advantages of low contrast density, low toxicity and good tolerance. Therefore, at present, iodine contrast agent is the most widely used X-ray contrast agent. Among them, iohexol has the highest safety and is currently the first-line representative drug of non-ionic monomeric iodine contrast agents. However, when chemical contrast agents are injected or administered into human tissues or organs, they can cause cellular damage, especially acute kidney injury (AKI).4 Among them, the iodinated contrast agent (iohexol) is the most widely used, so AKI caused by iohexol is currently the most common causative factor. Some injured patients may experience adverse reactions after intravascular injection of iodinated contrast agents, such as AKI, a phenomenon known as contrast agent nephropathy (CI-AKI).5 Early studies suggested that CI-AKI induced cytotoxicity, leading to cellular dysfunction and apoptosis, was mainly achieved by disrupting the function of renal tubular cells.6 Numerous studies have shown that the occurrence of CI-AKI is accompanied by an increase in the level of oxidative stress, leading to the accumulation of excessive reactive oxygen species (ROS), which in turn promotes cellular inflammation and pyroptosis.7 Recent studies have found that the inflammatory response of the kidney is also involved in CI-AKI.8
NOD-like receptor protein 3 (NLRP3) inflammasome is involved in the immune regulation system of the body and is an important component of the immune system, and it plays an important role in the body’s response to inflammation and immune responses.9 The inflammasome is formed, after NLRP3 is stimulated and activated, and the inflammatory factors interleukin (IL)-1β and IL-18 are activated and they participate in the process of inflammatory response.10 Emerging studies have shown that cellular inflammation, apoptosis, and tissue damage triggered by the NLRP3 inflammasome are found during AKI development.11 However, the mechanism of action of NLRP3 inflammasome in renal injury in CI-AKI remains unclear.
Programmed death of inflammatory cells comprises the traditional form of apoptosis, while pyroptosis is a newly discovered type of programmed cell death. The occurrence of pyroptosis is usually accompanied by the activation of the NLRP3 inflammasome and the release of pro-inflammatory cytokines.12
An important active ingredient in traditional Chinese medicine Scutellaria baicalensis Georgi, baicalin contains a variety of flavonoids, and it is used as a medicine in heat-clearing and detoxifying, hypoglycemic and lipid-lowering, liver protection, and anti-inflammatory treatment.13 In recent years, a number of studies have demonstrated that baicalin has an intervening effect on cell inflammatory damage. For example, Fu et al found that baicalin can improve the level of inflammatory injury in mice by regulating the inflammatory model pathway in the lipopolysaccharide (LPS)-induced inflammatory system in mice.14 Zhao et al found that baicalin ameliorated disease by inhibiting the NLRP3 inflammasome in mice.15 In addition, studies that baicalin can modulate the pyroptotic program have also been published. Fu et al verified that Scutellaria baicalensis isolated from Scutellaria baicalensis can reduce the occurrence of pyroptosis in Mtb-infected macrophages in vitro.16 Shi et al found that baicalin attenuated liver injury in a cell model of nonalcoholic steatohepatitis by inhibiting inflammasome-dependent GSDMD-mediated pyroptosis.17 Numerous research results show that baicalin has a significant effect on the regulation of inflammation and pyroptosis. In addition, whether baicalin plays a role in the CI-AKI model has not been confirmed.
Our previous study found that contrast agents induced renal tubular epithelial cell death, mainly manifested as apoptosis. However, the mechanism by which CI induce pyroptosis in renal tubular epithelial cells remains unclear. At present, the role of pyroptosis mediated by the ROS/NLRP3/Caspase-1/GSDMD pathway in the model of CI-AKI is less studied. Therefore, the purpose of this experiment was to reveal whether contrast agents can induce pyroptosis through the ROS/NLRP3/Caspase-1/GSDMD pathway, and to study the role of baicalin in inhibiting the occurrence of this pathway.
Materials and Methods
Cell Culture, Treatment, and Viability Assays
Renal tubular epithelial cells (HK-2) (SCSP-511, National Collection of Authenticated Cell Cultures, Shanghai, China) were cultured in DMEM-F12 (LA1320, Solarbio Life Sciences, Beijing, China) medium containing 5% fetal bovine serum (13011–8611, Sijiqing, Zhejiang, China) and Penicillin-streptomycin double antibody (P1400, Solarbio Life Sciences, Beijing, China), and they were cultured at 37°C, 5% CO2. After passage, cells were evenly seeded, and serum-free medium was added to synchronize for 24h when they grew to 80% confluence. Iohexol (15045921, GE Pharmaceuticals, Shanghai, China) was used as a source of contrast agent, and 20 mg/mL of mannitol (SM8120, Solarbio Life Sciences, Beijing, China) was used as a control. HK-2 cells were treated with a series of “I” concentrations (20, 30, and 40 mgI/mL) of iohexol, 20 mg/mL of mannitol, and baicalin (3021022, Solarbio Life Sciences, Beijing, China) (2.5, 5.0, 7.5, 10, 12.5, 17.5, and 20 mg/mL). After treatment for 24, 48, and 72 h, the cell viability was calculated using the CCK-8 (CA1210, Solarbio Life Sciences, Beijing, China) method.
Cell Transfection
The well-grown cells were seeded in 6-well plates at 5×105 CFU/mL, and 3 duplicate wells were set up. When the cell confluence reached 70%, the transfection experiment was carried out. The plasmid and liposome complexes (si-NLRP3, si-Caspase-1, and si-NC) were prepared according to the operation instructions of the Lipofectamine 3000 kit (L3000001, ThermoFisher Scientific) (siRNA sequences are provided in Table 1). After 6 h of transfection, the serum-free DMEM-F12 medium was changed to continue cultivation for 24 h.
 |
Table 1 siRNA Sequences |
RT-qPCR Method to Detect Target Gene Expression
Total RNA from HK-2 cells was extracted with a total RNA extraction kit (R1200, Solarbio Life Sciences, Beijing, China), cDNA was synthesized by reverse transcription (11137ES60, Yeasen, Shanghai, China), and amplification was performed by RT-qPCR cycling. The relative expression of target gene mRNA was expressed as the ratio of target gene to internal reference gene 2ΔΔCT.
Flow Annexin V-FITC/PI Double Staining Method to Detect Cell Apoptosis
After the cells were treated, 1 mL of cells (5×105 CFU/mL) were taken for centrifugation at 300 xg and 4°C, and the supernatant was discarded. The cells were rinsed with pre-cooled PBS and resuspended in 500 μL of mixed Binding Buffer. The cells were mixed with 5 uL Annexin V-FITC and 5 uL Propidium Iodide (CA1020, Solarbio Life Sciences, Beijing, China), and they were treated at room temperature for 15 min in the dark. Detection was performed on a flow cytometer (AccuriC6, BD Biosciences, San Jose, CA, USA). A total of 1~5×105 cells were collected for each experimental sample.
Detection of the Cytokine Content by Enzyme-Linked Immunosorbent Assay (ELISA)
After the experiment, the cells and cell culture medium were collected and centrifuged at 300 xg and 4°C, and the supernatant was discarded. Cytokine (IL-1β and IL-18) levels were detected using standard commercial ELISA kits (ml028595 and ml027422, mlbio, Shanghai, China). On the ELISA detector, after zeroing with the blank control well, the absorbance was detected at 450 nm, and the standard curve was drawn.
The Biochemical Reagent Method to Detection the Content of Cellular Oxidative Factors
After the experiment, the cells and cell culture medium were collected and centrifuged at 300 xg and 4°C, and the supernatant was discarded. The content of cellular oxidative factors (superoxide dismutase (SOD), malondialdehyde (MDA), and lactate dehydrogenase (LDH)) was detected using standard commercial biochemical detection kits (A001-3, A003-2, A020-2, Nanjing Jiancheng Biotechnology, JiangSu, China). The absorbance was detected on an enzyme label detector, and the protein content was calculated according to the kit instructions.
Detect the Content of Cellular Oxidative Factors ROS
After baicalin treatment HK-2 cells for 4 h, ROS inhibitor was added to intervene HK-2 cells, and then stimulated with 20 mg I/mL iohexol and 20 mg/mL mannitol, and the total intracellular ROS expression was detected by fluorescence method (50101ES01, Yeasen, Shanghai, China). The green fluorescence intensity is proportional to the reactive oxygen species level. Inverted fluorescence microscopy was used to observe intracellular ROS accumulation. Image J software calculated the mean fluorescence intensity (MGV, Mean gray value) for semi-quantitative analysis of ROS expression.
Western Blotting to Detect Target Protein Expression
After the experiment, the cells were collected, digested with 0.25% trypsin, collected into EP tubes, and protease inhibitors were added. The whole protein extraction kit (BC3710, Solarbio Life Sciences, Beijing, China) obtained the cell protein solution, and the BCA method (PC0020, Solarbio Life Sciences, Beijing, China) was used to detect the protein concentration. SDS-PAGE electrophoresis was performed, the protein was transferred to PVDF membrane and it was blocked with 5% nonfat milk powder for 1 h at room temperature, GAPDH (anti-GAPDH (MA1-16757, Invitrogen) was used as the normalizing antibody, and the corresponding primary antibodies (anti-NLRP3 (MA5-23919, Invitrogen), anti-ASC (PA5-83948, Invitrogen), anti-Caspase-1 (2225S, CST), and anti-GSDMD (39754S, CST)) were added and incubated at 4°C overnight. Horseradish peroxidase-labeled secondary antibody was used, and ECL color was developed, protein ladders are used to mark target protein positions (26617, thermo scientific). The gray value of the target band was analyzed with a gel image processing system (Image J software).
Statistical Methods
Statistical processing of data was performed using Prism Graphpad 7.0 statistical software. Data are presented as m±s, and comparisons between multiple samples were performed using Duncan’s multiplerange tests.
Results
The Effect of Baicalin on HK-2 Cell Viability
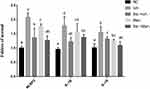
After HK-2 cell stimulation with iohexol, the cell viability decreased with an increase in the iohexol concentration and time. HK-2 cells exhibited similar cell viability after exposure to 20 mgI/mL iohexol and 20 mg/mL mannitol for 24 hours, and keep a high cell viability (Figure 1A). After HK-2 cells were exposed to different concentrations of baicalin, excessive concentrations could have certain effects on the cell viability (Figure 1B). According to the change in cell viability, 10.0 mg/L were selected as the intervention concentration of baicalin.
The Effect of Baicalin on Cell Viability
After HK-2 cells were stimulated with iohexol, the permeability of the cell membrane changed, obvious cell death occurred by flow cytometry, And the degree of death was concentration-dependent (Figure 2A). After sinking the expression of NLRP3 and Caspase-1, we found that the ratio of apoptosis in the NLRP3-siRNA and Caspase-1-siRNA Iohexol group was reduced compared with the control group (NC-siRNA). Baicalin inhibited iohexol-induced cell damage in both the control and gene silencing groups (Figure 2B).
The Effect of Baicalin on the Inflammasome
After HK-2 cells were exposed to iohexol, the expression levels of NLRP3 in the inflammasome were up-regulated, and the expression levels of inflammatory cytokines IL-1β and IL-18 were also up-regulated (Figure 3). Meanwhile, after HK-2 cells were treated with 10 mg/Lbaicalin, the expression levels of iohexol, NLRP3 were down-regulated when exposed to the same conditions, while the expression of inflammatory cytokines IL-1β, IL-18 was down-regulated.
The Effect of Baicalin on the Expression of Inflammatory Factors
After iohexol stimulated the HK-2 cells, the expression levels of IL-1β and IL-18 were increased (Figure 4). Baicalin had an intervention effect on iohexol to stimulate the expression of IL-1β and IL-18 in HK-2 cells, and the expression of IL-1β and IL-18 were reduced.
The Effect of Baicalin on the Expression of Oxidative Factors
After iohexol stimulated the HK-2 cells, the level of cellular oxidative stress was increased, and the detection levels of SOD, MDA, and LDH were increased (Table 2). Baicalin had an intervening effect on the expression of SOD, MDA, and LDH in HK-2 cells stimulated by iohexol.
 |
Table 2 Effects of Baicalin on the Expression of Oxidative Factors SOD, MDA and LDH |
The Effect of Baicalin on the Expression of ROS
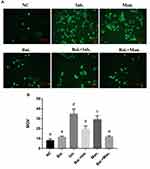
After iohexol stimulated the HK-2 cells, due to the level of cellular oxidative stress was increased, the release of ROS was increased, fluorescence detection results showed an increase in green fluorescence intensity (Figure 5). After the intervention of baicalin and stimulation with the same concentration of iohexol, the results of fluorescence staining showed that the expression of ROS was down-regulated.
The Effect of Baicalin on Protein Expression
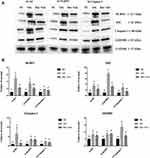
The results of Western Blot showed that the expression levels of NLRP3, ASC, IL-1β, and GSDMD were up-regulated after HK-2 cells were exposed to iohexol (Figure 6). The expression in the IM group was significantly higher than that in the BK group. After baicalin intervention, the expression levels of NLRP3, ASC, IL-1β, and GSDMD were significantly down-regulated.
Discussion
Kidney disease is a growing global public health problem with high morbidity and mortality. Kidney disease is often accompanied by cellular inflammation and oxidative stress. A large number of studies have shown that traditional Chinese medicine and its active ingredients can effectively promote the remission of kidney disease.18,19 Kidney is an important target organ for iohexol-induced cytotoxicity. Iohexol-induced AKI involves multiple pathophysiological mechanisms, including renal inflammation, pyroptosis, oxidative stress, proximal renal tubular injury, and vascular injury. Among them, inflammation involving multiple cytokines and chemokines, including NLRP3, IL-1β and IL-18, plays a crucial role.20 Pyroptosis is the latest direction in cytotoxic death research, a Caspase-1-dependent programmed cell death.21 The phenomenon of pyroptosis has been widely investigated because studies have found that the occurrence of pyroptosis is usually closely related to the cellular inflammatory process.22 In this study, based on the human renal tubular epithelial cell model, we investigated the pyroptosis mediated by iohexol based on the ROS/NLRP3/Caspase-1/GSDMD pathway, and we assessed the mechanism of baicalin in reducing iohexol-induced CI-AKI. Our results suggest that iohexol acts through the ROS/NLRP3/Caspase-1/GSDMD pathway in renal tubular epithelial cell pyroptosis, and that baicalin may attenuate iohexol-induced pyroptosis by inhibiting NLRP3/Caspase-1-dependent apoptosis.
Pyroptosis is the morphological change in cells after being stimulated by external factors, which is mainly manifested by continuous expansion of cells and secretion of vesicles. Finally, the cell membrane is ruptured, resulting in the release of cell contents and a severe inflammatory response.23 Number of studies have found that pyroptosis was closely related to cardiovascular diseases, tumors, kidney diseases, and other diseases.24 Accumulating evidence suggests that pyroptosis occurs as a state of massive body cell inflammatory death resulting from the activation of Caspase-1 upon NLRP3 inflammasome stimulation.25 Activation of the nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome is a major pathogenic mechanism of AKI.26 The NLRP3 inflammasome is one of the main programs of cellular inflammatory response, mainly comprising three domains, NLRP3, ASC, and pro-Caspase-1.27 Natural products ameliorated kidney injury via regulating NLRP3 that has been widely reported.28 It has been reported that Chinese traditional Chinese medicine can inhibit the activation of NF-κB/NLRP3 pathway by regulating exosome secretion, thereby alleviating renal dysfunction.29 The therapeutic effect of phloretin on HUA-induced renal injury in mice has been demonstrated due to its antioxidant and anti-inflammatory properties.30 Total astragalus flavonoids can ameliorate podocyte pyroptosis and injury in nephropathy under high glucose conditions by modulating NLRP3 inflammasome activation and PTEN/PI3K/Akt signaling.31 In addition, GSDMD is also a key molecule in the pyroptotic signaling pathway.32 It has been reported that the expression of pyroptosis executive factor (GSDMD) and NLRP3 inflammasome pathway molecules (NLRP3, caspase-1 and IL-1β) located in the renal tubules of DKD rats is down-regulated under treatment by alleviating renal lesions.33 It is known that pyroptosis dependent on Caspase-1 activation is related to the activation of the inflammasome. In this process, the interaction of NLRP3 and ASC protein will cause the aggregation of Pro-Caspase-1, resulting in Caspase-1 activation. Activation of Caspase-1 also leads to the cleavage of GSDMD, which after binding to phospholipid proteins on the cell membrane, forms a hole. Pro-inflammatory cytokines, such as I L-1β and IL-18, are released into the extracellular space to induce pyroptosis. Therefore, Caspase-1 plays a major role in the activation of the NLRP3 inflammasome, and NLRP3, ASC, and GSDMD play an important role in the onset of pyroptosis. In our study, we found that changes in NLRP3, ASC, and Caspase-1 gene and protein, increased IL-1β and IL-18 release, and inflammatory cell death program occurred in HK-2 cells after exposure to iohexol. After baicalin intervention, the corresponding data changed, and the inflammatory death of cells was reduced.
Oxidative stress and inflammation are important and key mediators in the development and progression of acute kidney disease (AKI) and its complications (CKD). Studies have shown that antioxidant and anti-inflammatory can prevent renal fibrosis and play an important role in the fight against AKI and CKD.34 It has been demonstrated that it has a significant preventive effect on renal insufficiency and fibrosis by inhibiting cellular inflammation and oxidative stress.35 A large number of research results have found that the inflammatory response is usually accompanied by an increase in the level of oxidative stress in the body, and excessive accumulation of ROS is one of the reasons for this phenomenon.36 The literature describes the overproduction of reactive oxygen species (ROS) and the increased release of LDH and MDA in diabetic nephropathy (DN), and antioxidant therapy is considered a promising strategy for the treatment of DN.37 We know that the activation of the NLRP3 inflammasome is accompanied by a large number of cellular inflammatory processes. In the process of oxidative stress in the body, excessive release of ROS plays an active role. The effect of ROS on cell pyroptosis mainly occurs on inflammatory stimuli, and the accumulated ROS can activate the NLRP3/Caspase-1 pathway, which leads to cell pyroptosis.38 It is well known that excessive ROS accumulation is a key factor in inducing oxidative stress. An imbalance of the oxidative stress level in cells will lead to abnormal expression of intracellular oxidative factors, such as the release of oxidative markers SOD and MDA, which further accelerates the cell pyroptosis process. In this study, it was found that under the stimulation with iohexol, the levels of cellular inflammation and oxidation were increased, ROS accumulated in HK-2 cells, and the levels of SOD and MDA were also increased. As a natural common and important antioxidant, baicalin is used as an important active oxygen free radical scavenger because it contains flavonoid polyphenol antioxidant substances. Therefore, in this study, baicalin was used as a ROS scavenger; another aspect to verify the relationship between ROS and iohexol-induced pyroptosis. Our results showed that baicalin treatment significantly reduced the accumulation of ROS compared with that in the iohexol group. In the intervention of inflammatory stimuli, baicalin significantly regulated the key indicators in the ROS/NLRP3/Caspase-1/GSDMD pathway, the mRNA expression levels of NLRP3, ASC, Caspase-1, IL-1β, and IL-18 as well as the levels of NLRP3, ASC, and Caspase-1. The protein level of GSDMD was found to decrease after the intervention. In addition, at the intervention level of oxidative stress, baicalin also significantly reduced the release of IL-1β, IL-18, SOD, and MDA induced by iohexol. Therefore, we speculate that the NLRP3 inflammasome can be activated by excessive ROS induced by iohexol, which in turn leads to pyroptosis of the ROS/NLRP3/Caspase-1/GSDMD pathway in renal tubular epithelial cells, and that baicalin can intervene to alleviate this symptom.
In addition, the content of LDH in the kidney is extremely high. Hence, after kidney disease, it will be accompanied by an increase in LDH; thus, the increase in LDH is generally related to kidney disease. It is known that in the process of pyroptosis, the increased release of LDH is also one of the main features to judge the occurrence of pyroptosis.39 Studies at the cellular level have shown that pyroptosis was induced upon activation of the NLRP3 inflammasome, in addition to changes at the gene and protein levels associated with NLRP3, ASC, Caspase-1, IL-1β, IL-8, and GSDMD.40 In addition, another study found that pyroptosis was also accompanied by LDH release.41 Similar changes were also found in our experimental results. After HK-2 cells were stimulated with iodine, the amount of LDH in the cell supernatant was increased; while in the experimental group after baicalin intervention, the LDH amount was down-regulated.
Conclusion
The present study investigated the role of the ROS/NLRP3/Caspase-1/GSDMD pathway in CI-AKI in vitro. In the HK-2 cell model, the NLRP3 inflammasome and ASC played critical roles in the ROS/NLRP3/Caspase-1/GSDMD pathway. Pyroptosis induction in HK-2 cells by iodinated contrast agents also occurred through this pathway. Iohexol could stimulate the expression of the NLRP3 inflammasome, thereby stimulating the release of inflammatory factors Caspase-1, IL-1β, and IL-18, and mediating the occurrence of signaling pathways. In addition, the release of ROS led to an increase in the level of cellular oxidative stress, and the release of SOD and MDA was increased. After the intervention of baicalin, the levels of inflammation and oxidative stress in HK-2 cells were reduced at the detection level of genes and cytokines. Collectively, we demonstrated that the ROS/NLRP3/Caspase-1/GSDMD pathway mediated CI-AKI via the pyroptotic pathway, and the intervention of baicalin could alleviate the associated inflammation and oxidation levels.
Acknowledgments
Natural Science Foundation of Chongqing in 2020 (cstc2020jcyj- msxmX0187), China; Chongqing Science and health joint traditional Chinese medicine science and technology project in 2020 (2020ZY3539), China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mihevc M, Petreski T, Maver U, et al. Renal proximal tubular epithelial cells: review of isolation, characterization, and culturing techniques. Mol Biol Rep. 2020;47(12):9865–9882. doi:10.1007/s11033-020-05977-4
2. Chen JH, Chiang CK. Uremic toxins and protein-bound therapeutics in AKI and CKD: up-to-date evidence. Toxins. 2021;14(1):8. doi:10.3390/toxins14010008
3. Humphreys BD. Mechanisms of Renal Fibrosis. Annu Rev Physiol. 2018;80:309–326. doi:10.1146/annurev-physiol-022516-034227
4. Vijayan A. Tackling AKI: prevention, timing of dialysis and follow-up. Nat Rev Nephrol. 2021;17(2):87–88. doi:10.1038/s41581-020-00390-3
5. Chandiramani R, Cao D, Nicolas J, et al. Contrast-induced acute kidney injury. Cardiovasc Interv Ther. 2020;35(3):209–217. doi:10.1007/s12928-020-00660-8
6. Singh K, Bhargava V, Brar JE, et al. Contrast induced acute kidney injury (CI- AKI) - myths and realities. J Assoc Physicians India. 2021;69(11):11–12.
7. Lei R, Zhao F, Tang CY, et al. Mitophagy plays a protective role in iodinated contrast-induced acute renal tubular epithelial cells injury. Cell Physiol Biochem. 2018;46(3):975–985. doi:10.1159/000488827
8. Jiang H, Li D, Xu T, et al. Systemic immune-inflammation index predicts contrast-induced acute kidney injury in patients undergoing coronary angiography: a cross-sectional study. Front Med. 2022;9:841601. doi:10.3389/fmed.2022.841601
9. Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803. doi:10.3389/fimmu.2020.591803
10. Zhao C, Zhao W. NLRP3 Inflammasome-A key player in antiviral responses. Front Immunol. 2020;11:211. doi:10.3389/fimmu.2020.00211
11. Zhu X, Li S, Lin Q, et al. αKlotho protein has therapeutic activity in contrast-induced acute kidney injury by limiting NLRP3 inflammasome-mediated pyroptosis and promoting autophagy. Pharmacol Res. 2021;167:105531. doi:10.1016/j.phrs.2021.105531
12. Zhang X, Zhang Y, Li R, et al. Salidroside ameliorates Parkinson’s disease by inhibiting NLRP3-dependent pyroptosis. Aging. 2020;12(10):9405–9426. doi:10.18632/aging.103215
13. Singh S, Meena A, Luqman S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol Res. 2021;164:105387. doi:10.1016/j.phrs.2020.105387
14. Fu YJ, Xu B, Huang SW, et al. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol Sin. 2021;42(1):88–96. doi:10.1038/s41401-020-0411-9
15. Li CG, Yan L, Mai FY, et al. Baicalin inhibits NOD-like receptor family, pyrin containing domain 3 inflammasome activation in murine macrophages by augmenting protein Kinase A signaling. Front Immunol. 2017;8:1409. doi:10.3389/fimmu.2017.01409
16. Fu Y, Shen J, Li Y, et al. Inhibition of the PERK/TXNIP/NLRP3 axis by baicalin reduces NLRP3 inflammasome-mediated pyroptosis in macrophages infected with Mycobacterium tuberculosis. Mediators Inflamm. 2021;2021:1805147. doi:10.1155/2021/1805147
17. Shi H, Zhang Y, Xing J, et al. Baicalin attenuates hepatic injury in non-alcoholic steatohepatitis cell model by suppressing inflammasome-dependent GSDMD-mediated cell pyroptosis. Int Immunopharmacol. 2020;81:106195. doi:10.1016/j.intimp.2020.106195
18. Zhou F, Zou X, Zhang J, Wang Z, Yang Y, Wang D. Jian-Pi-Yi-Shen formula ameliorates oxidative stress, inflammation, and apoptosis by activating the Nrf2 signaling in 5/6 nephrectomized rats. Front Pharmacol. 2021;12:630210. doi:10.3389/fphar.2021.630210
19. Wang X, Liu J, Tian R, et al. Sanqi oral solution mitigates proteinuria in rat passive heymann nephritis and blocks podocyte apoptosis via Nrf2/HO-1 pathway. Front Pharmacol. 2021;12:727874. doi:10.3389/fphar.2021.727874
20. Wen L, Tao SH, Guo F, et al. Selective EZH2 inhibitor zld1039 alleviates inflammation in cisplatin-induced acute kidney injury partially by enhancing RKIP and suppressing NF-κB p65 pathway. Acta Pharmacol Sin. 2022;43(8):2067–2080. doi:10.1038/s41401-021-00837-8
21. Yan H, Luo B, Wu X, et al. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer. Int J Biol Sci. 2021;17(10):2606–2621. doi:10.7150/ijbs.60292
22. Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81(22):4579–4590. doi:10.1016/j.molcel.2021.09.003
23. Jia C, Chen H, Zhang J, et al. Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol. 2019;67:311–318. doi:10.1016/j.intimp.2018.12.028
24. Yu P, Zhang X, Liu N, et al. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128. doi:10.1038/s41392-021-00507-5
25. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
26. Li H, Lu R, Pang Y, et al. Zhen-Wu-Tang protects IgA nephropathy in rats by regulating exosomes to inhibit NF-κB/NLRP3 pathway. Front Pharmacol. 2020;11:1080. doi:10.3389/fphar.2020.01080
27. Yu ZW, Zhang J, Li X, et al. A new research hot spot: the role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020;240:117138. doi:10.1016/j.lfs.2019.117138
28. Yang T, Feng X, Zhao Y, et al. Dexmedetomidine enhances autophagy via α2-AR/AMPK/mTOR pathway to inhibit the activation of NLRP3 inflammasome and subsequently alleviates lipopolysaccharide-induced acute kidney injury. Front Pharmacol. 2020;11:790. doi:10.3389/fphar.2020.00790
29. Li G, Guan C, Xu L, et al. Scutellarin ameliorates renal injury via increasing CCN1 expression and suppressing NLRP3 inflammasome activation in hyperuricemic mice. Front Pharmacol. 2020;11:584942. doi:10.3389/fphar.2020.584942
30. Cui D, Liu S, Tang M, et al. Phloretin ameliorates hyperuricemia-induced chronic renal dysfunction through inhibiting NLRP3 inflammasome and uric acid reabsorption. Phytomedicine. 2020;66:153111. doi:10.1016/j.phymed.2019.153111
31. Liu BH, Tu Y, Ni GX, et al. Total flavones of abelmoschus Manihot ameliorates podocyte pyroptosis and injury in high glucose conditions by targeting METTL3-dependent m6A modification-mediated NLRP3-inflammasome activation and PTEN/PI3K/Akt signaling. Front Pharmacol. 2021;12:667644. doi:10.3389/fphar.2021.667644
32. Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369(6511):1633–1637. doi:10.1126/science.abb9818
33. Li N, Zhao T, Cao Y, et al. Tangshen formula attenuates diabetic kidney injury by imparting anti-pyroptotic effects via the TXNIP-NLRP3-GSDMD axis. Front Pharmacol. 2021;11:623489. doi:10.3389/fphar.2020.623489
34. Luo LP, Suo P, Ren LL, et al. Shenkang injection and its three anthraquinones ameliorates renal fibrosis by simultaneous targeting IƙB/NF-ƙB and Keap1/Nrf2 signaling pathways. Front Pharmacol. 2021;12:800522. doi:10.3389/fphar.2021.800522
35. Gao P, Du X, Liu L, et al. Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front Pharmacol. 2021;11:610102. doi:10.3389/fphar.2020.610102
36. Sho T, Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol Appl Biochem. 2019;66(1):4–13. doi:10.1002/bab.1700
37. Du L, Wang L, Wang B, et al. A novel compound AB38b attenuates oxidative stress and ECM protein accumulation in kidneys of diabetic mice through modulation of Keap1/Nrf2 signaling. Acta Pharmacol Sin. 2020;41(3):358–372. doi:10.1038/s41401-019-0297-6
38. Daenen K, Andries A, Mekahli D, et al. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019;34(6):975–991. doi:10.1007/s00467-018-4005-4
39. Xiao C, Zhao H, Zhu H, et al. Tisp40 induces tubular epithelial cell GSDMD-mediated pyroptosis in renal ischemia-reperfusion injury via NF-κB signaling. Front Physiol. 2020;11:906. doi:10.3389/fphys.2020.00906
40. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550–559. doi:10.1038/s41590-021-00886-5
41. Zou Y, Luo X, Feng Y, et al. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chem Biol Interact. 2021;345:109573. doi:10.1016/j.cbi.2021.109573
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.