Back to Journals » OncoTargets and Therapy » Volume 14
Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/mTOR Pathway by the Regulation of circHIAT1/miR-19a-3p Axis
Authors Hu J, Wang R, Liu Y, Zhou J, Shen K, Dai Y
Received 18 September 2020
Accepted for publication 9 December 2020
Published 9 February 2021 Volume 2021:14 Pages 905—916
DOI https://doi.org/10.2147/OTT.S282790
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Jiaojiao Hu,1,* Runkun Wang,2,* Yi Liu,3 Jianbo Zhou,4 Ka Shen,4 Yun Dai1
1Department of Oncology, Suizhou Hospital, Hubei University of Medicine, Suizhou, People’s Republic of China; 2Department of Oncology, The First People’s Hospital of Guangshui, Guangshui, People’s Republic of China; 3Department of Traditional Chinese Medicine, Suizhou Hospital, Hubei University of Medicine, Suizhou, People’s Republic of China; 4Department of General Surgery, Suizhou Hospital, Hubei University of Medicine, Suizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Dai
Department of Oncology, Suizhou Hospital, Hubei University of Medicine, No. 60 Longmen Street, Suizhou, 441300, People’s Republic of China
Tel +86-13908666102
Email [email protected]
Background: Baicalein has a significant anti-cancerous function in the treatment of cervical cancer (CC). Its functional mechanism regarding circular RNA (circRNA) hippocampus abundant transcript 1 (circHIAT1) and microRNA-19a-3p (miR-19a-3p) was explored in this research.
Methods: CC cell viability and colony formation were determined using Cell Counting Kit-8 (CCK-8) and colony formation assay. Cell cycle progression and apoptosis were analyzed via flow cytometry. Protein markers of cell cycle, apoptosis and protein kinase B/mammalian target of rapamycin (AKT/mTOR) pathway were detected by Western blot. CircHIAT1 and miR-19a-3p levels were assayed through the quantitative real-time polymerase chain reaction (qRT-PCR). The interaction between circHIAT1 and miR-19a-3p was validated by dual-luciferase reporter and RNA pull-down assays. In vivo experiment was performed by xenograft model.
Results: CC cell growth and cell cycle progression were repressed while apoptosis was enhanced by baicalein. MiR-19a-3p was downregulated in baicalein-treated CC cells and miR-19a-3p overexpression lightened the baicalein-induced CC progression inhibition. Moreover, circHIAT1 was found to be a sponge of miR-19a-3p in CC cells. Baicalein-induced cell growth inhibition, cell cycle arrest and apoptosis promotion were neutralized by knockdown of circHIAT1 via targeting miR-19a-3p. Baicalein acted on the circHIAT1/miR-19a-3p to inactivate AKT/mTOR pathway. Baicalein also reduced CC tumor growth in vivo via regulating the levels of circHIAT1 and miR-19a-3p.
Conclusion: These findings demonstrated that the inhibitory function of baicalein in CC progression was dependent on the repression of AKT/mTOR pathway by upregulating circHIAT1 to sponge miR-19a-3p, showing a specific mechanism for baicalein in CC.
Keywords: baicalein, cervical cancer, circHIAT1, miR-19a-3p, AKT/mTOR
Introduction
Cervical cancer (CC) is one of the most common malignancies in women and high-risk subtypes of human papilloma virus (HPV) is the leading cause.1 Conservative, fertility-preserving surgical operation remains the primary therapeutic option for early-stage CC patients, with the concurrent chemoradiotherapy and brachytherapy.2 However, the overall prognosis for patients with metastatic and recurrent CC is still poor.3 To explore more therapeutic regimens is necessary for the management of CC.
Baicalein is a flavonoid coming from the root of Scutellaria baicalensis that is a traditional Chinese herb.4 Baicalein has important pharmacological activities, such as anti-tumorous, anti-inflammatory, antiviral and antioxidative effects.5 Issued reports have found the inhibitory effects of baicalein on various human cancers, including non-small cell lung cancer,6 hepatocellular carcinoma,7 breast cancer,8 thyroid cancer,9 and so on. Regarding the functional mechanisms of anticancer baicalein, it is mainly because of its inhibition on cell cycle progression by regulating cyclins, promotion of apoptosis by activating caspase-3/9, and the activity repression of mitogen-activated protein kinase (MAPK), protein kinase B (AKT) or mammalian target of rapamycin (mTOR).5 In the CC research, baicalein has been shown to suppress cell proliferation by Notch 1/Hes signaling pathway10 or Wnt signaling pathway,11 and induce cell apoptosis by affecting the activity of nuclear factor (NF)‑κB.12 The association of baicalein and AKT/mTOR pathway in CC has not been researched.
Increasing studies have indicated that the baicalein regulated the expression of microRNAs (miRNAs) in cancers. For example, baicalein retarded the progression of osteosarcoma via upregulating miR-183,13 bladder cancer by downregulating miR-106,14 and hepatocellular carcinoma through miR-3127 inhibition.15 MicroRNA-19a (miR-19a) served as a pro-cancer factor in CC.16,17 However, the relation between baicalein and miR-19a-3p is unreported. MiRNAs can be used as the targets of circular RNAs (circRNAs) that are covalently closed loops, and circRNAs function as regulatory molecules by sponging miRNAs in cancer progression.18 CircRNA hippocampus Abundant Transcript 1 (circHIAT1) restrained hepatocellular carcinoma cell growth by acting as a miR-3171 sponge19 and hampered gastric cancer progression via absorbing miR-21.20 This study will also address whether circHIAT1 can be a sponge for miR-19a-3p in CC.
Herein, the function of baicalein in CC cells and the potential regulation of baicalein on miR-19a-3p, circHIAT1 and AKT/mTOR pathway are investigated. The target relation between circHIAT1 and miR-19a-3p is confirmed.
Materials and Methods
Human Samples
Thirty-six CC patients have signed the written informed consent forms before surgical resection at Suizhou Hospital, Hubei University of Medicine. Thirty-six paired CC and non-tumorous tissue samples were collected into the sterile centrifugal tubes (Axygen, Santa Clara, CA, USA) during the surgery, then saved at −80°C instantly. All operations and use of these human samples were in accordance with the Declaration of Helsinki involving in the human subjects, based on getting authorization from the Ethics Committee of Suizhou Hospital, Hubei University of Medicine.
Cell Lines and Baicalein Stimulation
Human CC cell lines (HeLa and SiHa) and normal cervical epithelial cell line End1/E6E7 were bought from American Type Culture Collection (ATCC, Manassas, VA, USA). Cell culture was performed in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA) added with 10% (v/v) fetal bovine serum (FBS; Gibco) and 1% (v/v) penicillin & streptomycin solution (Gibco) in a 37°C incubator containing 5% CO2. Baicalein (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethylsulfoxide (DMSO; Invitrogen, Carlsbad, CA, USA) to reach the concentrations of 20 μg/mL and 40 μg/mL. HeLa and SiHa cells were treated with baicalein at 37°C for 24 h, and the DMSO treatment group (Baicalein 0 μg/mL) was used as the negative control. The baicalein concentration was used in a reasonable range referring to the previous studies.21,22
Cell Transfection
MiRNA mimics of miR-19a-3p (miR-19a-3p) and negative control (miR-NC), miRNA inhibitors of miR-19a-3p (anti-miR-19a-3p) and negative control (anti-miR-NC) were synthesized by GenePharma (Shanghai, China). Short hairpin RNA (shRNA) vector targeting circHIAT1 (sh-circHIAT1) and its negative control (sh-NC), pCE-RB-Mam-circHIAT1 overexpression vector (circHIAT1) and the control vector pCE-RB-Mam (vector) were purchased from RIBOBIO (Guangzhou, China). HeLa and SiHa cells were cultivated for 60% monolayer confluence, then cell transfection was conducted by Lipofectamine™ 3000 (Invitrogen).
Cell Counting Kit-8 (CCK-8) Assay
Viable cells were detected using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto, Japan). Then, 2 × 103 HeLa and SiHa cells were seeded into the 96-well plates overnight at 37°C. After baicalein treatment (24 h) and transfection (48 h), 10 μL CCK-8 solution was pipetted into each well of the 96-well plates to incubate for 3 h. Cell viability (%) was obtained after the absorbance determination by the microplate reader (Bio-Rad, Hercules, CA, USA).
Colony Formation Assay
Colony formation ability was assessed using colony formation assay. 2 × 102 treated or transfected HeLa and SiHa cells were harvested, then transplanted into the 6-well plates for cell growth at 37°C. After 2 weeks, methanol (Sigma-Aldrich) was added into the wells for fixing these colonies and crystal violet (Sigma-Aldrich) was used to stain colonies. Then, the stained colonies were manually counted.
Flow Cytometry
Flow cytometry was applied for analyzing cell cycle and apoptosis. For cell cycle, HeLa and SiHa cells were stained by propidium iodide (PI) according to the Cell Cycle Assay Kit - PI/RNase Staining (Dojindo Molecular Technologies), followed by the detection pf cell proportions at G0/G1, S and G2/M phases on the flow cytometer (BD Biosciences, San Diego, CA, USA). For cell apoptosis, HeLa and SiHa cells were stained by Annexin V-fluorescein isothiocyanate (FITC) and PI through Annexin V-FITC Apoptosis Detection Kit (Dojindo Molecular Technologies); then, the apoptotic cells (%) were discerned under the flow cytometer.
Western Blot
After cell harvest and tissue collection, total proteins were extracted via radioimmunoprecipitation assay (RIPA; Thermo Fisher Scientific, Waltham, MA, USA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to separate proteins from the loading products to the gels; then, proteins on the gels were transferred to the polyvinylidene difluoride (PVDF) membranes (Thermo Fisher Scientific). PVDF membranes were blocked in 5% skim milk (Invitrogen), followed by the incubation of primary antibodies against cyclin D1 (#2922, 1:1000), p21 (#2947, 1:1000), p27 (#3686, 1:1000), B-cell lymphoma-2 (Bcl-2; #4223, 1:1000), Bcl-2-associated X (Bax; #5023, 1:1000), phospho-AKT (p-AKT; #4060, 1:2000), AKT (#4691, 1:1000), phospho-mTOR (p-mTOR; #5536, 1:1000), mTOR (#2983, 1:1000) or internal control glyceraldehyde-phosphate dehydrogenase (GAPDH; #8884, 1:1000) at 4°C overnight. Enhanced chemiluminescence (ECL; Bio-Rad) was exploited for protein presentation after the membranes were incubated with a second antibody (#7074, 1:3000) for 1 h at room temperature. The protein bands were captured and analyzed by Image Lab software (Bio-Rad). The antibodies used in this study were purchased from Cell Signaling Technology (CST, Boston, MA, USA).
The Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The expression levels of circHIAT1 and miR-19a-3p were examined by qRT-PCR. Briefly, total RNA was isolated from tissues and cells using TRI Reagent (Sigma-Aldrich) and reversely transcribed to obtain the complementary DNA (cDNA) by ReadyScript cDNA Synthesis Mix (Sigma-Aldrich), followed by performing the qRT-PCR through SYBR® Green Quantitative RT-qPCR Kit (Sigma-Aldrich) and data analysis via the 2−∆∆Ct method. The nucleotide sequences of primers were shown as follows: forward (F), 5ʹ-GCAGTCCTTGGCATTCTTTC-3ʹ and reverse (R), 5ʹ-CAAGGTGGGTGCTGTCAATA-3ʹ for circHIAT1; F, 5ʹ-GGGGGGGTGTGCAAATCT-3ʹ and R, 5ʹ-GTGCGTGTCGTGGAGTCG-3ʹ for miR-19a-3p; 5ʹ-AAGGTGAAGGTCGGAGTCA-3ʹ and R, 5ʹ-GGAAGATGGTGATGGGATTT-3ʹ for GAPDH; 5ʹ-AGAGCCTGTGGTGTCCG-3ʹ and R, 5ʹ-CATCTTCAAAGCACTTCCCT-3ʹ for U6. CircHIAT1 and miR-19a-3p levels were respectively normalized by GAPDH and U6.
Dual-Luciferase Reporter Assay
CircBank was used for predicting the binding between circHIAT1 and miR-19a-3p. The fragment of circHIAT1 that contained the binding sites of miR-19a-3p was amplified and inserted into the pmirGLO (Promega, Madison, WI, USA). The formed wild-type (WT) luciferase plasmid was named as circHIAT1-WT. After the binding sites of miR-19a-3p in circHIAT1 sequence were mutated, the constructed mutant-type (MUT) plasmid was named as circHIAT1-MUT. CircHIAT1-WT or circHIAT1-MUT and miR-19a-3p or miR-NC were co-transfected into HeLa and SiHa cells for 48 h, then the luciferase activity was determined applying with the Dual-Luciferase Reporter Assay System (Promega).
RNA Pull-Down Assay
Biotin-coupled miRNA mimics: Biotin-miR-19a-3p-WT, Biotin-miR-19a-3p-MUT and Biotin-miR-NC were bought from RIBOBIO. At 48 h after transfection of each mimic, HeLa and SiHa cell lysates were harvested and incubated in streptavidin magnetic beads (Thermo Fisher Scientific) at 4°C overnight. After total RNA isolation from the magnetic beads, relative circHIAT1 expression was measured via qRT-PCR assay.
Animal Assay
This animal assay was ratified by the Animal Ethical Committee of Suizhou Hospital, Hubei University of Medicine. Female BALB/c nude mice (5-week-old, n=18) were purchased from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China) and divided into three groups with six mice/group. Transfected (sh-NC, sh-circHIAT1) HeLa cells (3 × 106) were subcutaneously injected into the flank of mice, then the mice were intraperitoneally treated with 100 μL DMSO (0.25%) in sh-NC group or 10 mg/kg baicalein in sh-NC and sh-circHIAT1 groups daily. Tumor volume was measured every 5 days using the equation: length × width2 × 0.5. Tumor was dissected from mice at the 30th day post-injection, and total RNA or protein was extracted for associated expression analysis. The care and euthanasia for mice followed the Management and Use Guidelines of Laboratory Animals issued by the National Institutes of Health (NIH).
Statistical Analysis
Each experiment was independently performed for three times and data were shown as the mean ± standard deviation (SD). The linear association between circHIAT1 and miR-19a-3p was analyzed using Spearman correlation coefficient. Data analysis was administrated by SPSS 24.0 and statistical difference was compared via Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s test. P<0.05 was expressed as a significant difference.
Results
Baicalein Caused CC Cell Growth Suppression, Cell Cycle Arrest and Cell Apoptosis Promotion
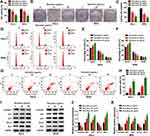
The effects of baicalein on CC cellular behaviors were explored after treatment of different concentrations in HeLa and SiHa cells. The results of CCK-8 and colony formation assays suggested that cell viability (Figure 1A) and colony formation ability (Figure 1B–C) were repressed in baicalein 20 μg/mL and 40 μg/mL groups compared to 0 μg/mL group, in a dose-dependent way. The inhibition of cell transition from G0/G1 phase to S phase indicated that baicalein treatment resulted in cell cycle retardation of HeLa and SiHa cells (Figure 1D–F). In addition, cell apoptosis was found to be enhanced by baicalein through Annexin V-FITC/PI-flow cytometry (Figure 1G–H). Western blot was performed to measure the proteins associated with cell cycle (positive marker cyclinD1 and negative regulators p21/p27) and cell apoptosis (negative Bcl-2 and positive Bax). As the protein presentation in Figure 1I, baicalein stimulation decreased the levels of cyclinD1 and Bcl-2 but upregulated p21, p27 and Bax (Figure 1J–K). Taken together, baicalein inhibited cell growth, cell cycle progression and promoted apoptosis of CC cells.
Baicalein Downregulated the Expression of miR-19a-3p in CC Cells
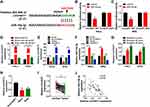
In comparison to the 0 μg/mL group, miR-19a-3p expression was significantly declined by baicalein treatment of 20 μg/mL and 40 μg/mL groups in HeLa and SiHa cells (Figure 2A). The qRT-PCR data revealed that miR-19a-3p level was much higher in HeLa or SiHa cells (Figure 2B) and CC tissue samples (Figure 2C) than that in normal End1/E6E7 cell line and normal tissue samples. The expression of miR-19a-3p was inhibited by baicalein in CC cells.
Baicalein-Induced Inhibition of CC Progression Was Attenuated by miR-19a-3p Overexpression
Due to the downregulation of miR-19a-3p by baicalein, miR-19a-3p mimic was used for miR-19a-3p overexpression to study the influence of miR-19a-3p on baicalein in CC cells. As shown in Figure 3A, the expression of miR-19a-3p was increased by more than 10-fold changes in miR-19a-3p-transfected HeLa and SiHa cells relative to miR-NC transfection. After 40 μg/mL baicalein treatment, miR-19a-3p or miR-NC was transfected into HeLa and SiHa cells and DMSO+miR-NC acted as the negative control. Through a series of cellular experiments, we found that baicalein-induced repressive effects on cell viability (Figure 3B), colony formation (Figure 3C–D) and cell cycle progression (Figure 3E–F), but the accelerative effect on cell apoptosis (Figure 3G–H) was assuaged following the overexpression of miR-19a-3p. Consistently, the transfection of miR-19a-3p also rescued all these influences of baicalein on cyclinD1, p21, p27, Bcl-2 and Bax protein levels in Western blot assay (Figure 3I–K). These results implied that the inhibition of baicalein in CC progression was associated with miR-19a-3p downregulation.
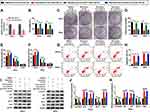
CircHIAT1 Was a Natural Sponge for miR-19a-3p in CC Cells
Bioinformatic analysis of circBank showed that circHIAT1 (hsa_circHIAT1_007) contained the complementary binding sites (UUGCAC) for miR-19a-3p (AACGUG) (Figure 4A). After these binding sites were mutated as AACGUG in circHIAT1 sequence, the dual-luciferase reporter assay was performed. The results manifested that circHIAT1-WT and miR-19a-3p co-transfection signally suppressed the relative luciferase activity but circHIAT1-MUT and miR-19a-3p co-transfection made no difference in the luciferase activity, in contrast with their co-transfection with miR-NC (Figure 4B–C). Pull-down assay also exhibited that the capture of circHIAT1 by Biotin-miR-19a-3p-WT, not Biotin-miR-19a-3p-MUT and Biotin-miR-NC (Figure 4D). The qRT-PCR affirmed that circHIAT1 expression was interfered by sh-circHIAT1 and elevated by circHIAT1 transfection respectively, compared to sh-NC or vector group (Figure 4E). Negatively, miR-19a-3p level was shown to be upregulated by knockdown of circHIAT1 while circHIAT1 overexpression caused the downregulation of miR-19a-3p in HeLa and SiHa cells (Figure 4F). Contrary to miR-19a-3p, circHIAT1 level was promoted after the treatment of baicalein (20 μg/mL and 40 μg/mL) by comparison to baicalein 0 μg/mL (Figure 4G). Relative to the control End1/E6E7 cells and normal tissues, the expression of circHIAT1 was obviously lower in CC cells (Figure 4H) and tissues (Figure 4I). The relation between circHIAT1 and miR-19a-3p was analyzed to be negative (r=−0.57, p<0.05) in CC tissue samples (Figure 4J). The above data identified circHIAT1 as a sponge of miR-19a-3p in CC cells.
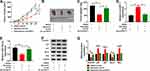
Knockdown of circHIAT1 Alleviated the Inhibition of Baicalein on CC Progression via Upregulating miR-19a-3p
Given that circHIAT1 targeted miR-19a-3p, the potential regulation of circHIAT1 on baicalein was further investigated. MiR-19a-3p expression was evidently inhibited by anti-miR-19a-3p transfection in contrast with anti-miR-NC transfection (Figure 5A). 40 μg/mL baicalein-treated HeLa and SiHa cells were transfected with sh-NC+anti-miR-NC, sh-circHIAT1+anti-miR-NC or sh-circHIAT1+anti-miR-19a-3p, then the functional assays were carried out using DMSO+sh-NC+anti-miR-NC as the control group. The introduction of sh-circHIAT1 abrogated the baicalein-mediated cell viability (Figure 5B) and colony formation (Figure 5C and D) repression, cell cycle blockage (Figure 5E and F) and the increase of apoptotic rate (Figure 5G–H), whereas these alleviate effects were eliminated by miR-19a-3p inhibitor. Similarly, miR-19a-3p inhibition counterbalanced the circHIAT1-regulated reversal on the protein expression downregulation of cyclinD1 and Bcl-2, as well as upregulation of p21, p27, and Bax caused by baicalein (Figure 5I–K). Briefly, knockdown of circHIAT1 upregulated miR-19a-3p to abate the baicalein-induced effects on cell growth, cell cycle and apoptosis.
Baicalein Blocked the AKT/mTOR Pathway by Upregulating circHIAT1 to Inhibit miR-19a-3p
To analyze whether baicalein could affect AKT/mTOR pathway via regulating circHIAT1 and miR-19a-3p, we detected the protein expression of p-AKT/AKT and p-mTOR/mTOR in HeLa and SiHa cells. As the Western blot results illustrated in Figure 6A–B, the protein levels of p-AKT/AKT and p-mTOR/mTOR were significantly downregulated in Baicalein+sh-NC+anti-miR-NC group than these in DMSO+sh-NC+anti-miR-NC group while the inhibition of baicalin on AKT/mTOR pathway was assuaged by the transfection of sh-circHIAT1, and it was interesting that miR-19a-3p inhibitor subsequently eliminated the promotion of AKT/mTOR pathway by sh-circ-HIAT1. Collectively, baicalin inhibited the AKT/mTOR signal pathway through the regulation of circHIAT1/miR-19a-3p axis.
CC Tumor Growth Was Suppressed by Baicalein via the circHIAT1/miR-19a-3p Axis in vivo
After mice were injected with transfected cells and DMSO or baicalein for 30 days, the measurement of tumor volume (Figure 7A) and weight (Figure 7B–C) indicated that baicalein treatment reduced CC growth while sh-circHIAT1 weakened this inhibition. The qRT-PCR analysis in tumor tissues exhibited that baicalein-induced circHIAT1 upregulation (Figure 7D) and miR-19a-3p downregulation (Figure 7E) were returned in Baicalein+sh-circHIAT1 group. Also, the protein level of cyclinD1 and Bcl-2 were decreased but p21, p27 and Bax were facilitated in the Baicalein+sh-NC group relative to the DMSO+sh-NC group, which was then abolished in the Baicalein+sh-circHIAT1 group (Figure 7F–G). Therefore, baicalein restrained CC tumor growth in vivo via increasing circHIAT1 to inhibit miR-19a-3p.
Discussion
It is unclear whether the function of baicalein on the development of CC is achieved by regulating some circRNAs and miRNAs. Our exploration in this study discovered that baicalein worked as a tumor inhibitor in CC through upregulating circHIAT1 to sequester the expression of miR-19a-3p to inhibit AKT/mTOR pathway, proving that the regulation of baicalein was associated with the circHIAT1/miR-19a-3p/AKT/mTOR axis in CC.
Baicalein is known as an effective inhibitor in the tumor progression. For instance, Chen et al reported that baicalein blocked cell proliferative activity and cell cycle in colorectal cancer;23 Yang et al declared that cell viability in bladder cancer was impeded while apoptosis was induced by baicalein;24 Ma et al found that baicalein treatment resulted in cell proliferation inhibition and cell cycle arrest of prostate cancer.25 In this chapter, cellular experiments have shown that CC cell viability, colony formation, cell cycle progression were all reduced but apoptotic cells were increased by baicalein. CyclinD1 is a key protein in the process of cell cycle progression.26 In addition, p21 and p27 are correlated to Cdk/cyclin complexes and inhibit the activities of kinases at the G1/S and G2/M checkpoints.27 Our Western blot results suggested that baicalein led to the downregulation of cyclinD1 and upregulation of p21/p27. Treatment of baicalein also inhibited the protein expression of anti-apoptotic Bcl-2 and enhanced the level of pro-apoptotic Bax in CC cells. Judging from these experimental data, baicalein was affirmed to play an inhibitory role in the malignant progression of CC.
MiRNAs are a class of commonly researched regulatory RNAs in cancer research, and various miRNAs have been identified as employable biomarkers for diagnosis, treatment and prognosis in CC.28 Juan et al have shown that miR-489 served for an anti-proliferative and pro-apoptotic maker in CC cells,29 and miR-148a was used as a novel target to suppress the progression of CC.30 Interestingly, we found that miR-19a-3p was highly expressed in CC tissues and cells but its expression was reduced after baicalein treatment in CC cells, which demonstrated that the biological function of baicalein in CC might be related to the downregulation of miR-19a-3p. Our reverted assays subsequently exhibited that the overexpression of miR-19a-3p recovered the regulation of baicalein in CC cells, validating that baicalein functioned in CC cells via the expression inhibition of miR-19a-3p.
Usually, circRNAs are used as another specific regulators in the progression of human cancers via targeting miRNAs. CircRNA-000911 was reported as an anti-oncogenic factor in breast cancer through the sponge effect on miR-449a31 and circRNA-100338 facilitated tumor progression in hepatitis B-related hepatocellular carcinoma by sequestering miR-141-3p.32 Through the online target binding prediction, miR-19a-3p might be a target of circHIAT1. Dual-luciferase reporter and pull-down assays further verified that circHIAT1 directly interacted with miR-19a-3p. Moreover, circHIAT1 was found to negatively affect the miR-19a-3p level as a miRNA sponge. The current studies manifested that circHIAT1 was a repressive regulator in some cancers (clear cell renal cell carcinoma, hepatocellular carcinoma and gastric cancer).19,20,33 CircHIAT1 was also differentially downregulated in CC tissues and cells, but baicalein promoted its expression. The influences of baicalein on CC cells were offset after knockdown of circHIAT1, suggesting that the upregulation of circHIAT1 was responsible for baicalein as an inhibitor in CC progression. Furthermore, the regulation of circHIAT1 in baicalein-induced CC cells was achieved by the negative effect on miR-19a-3p. In vivo assay, baicalein also reduced tumorigenesis of CC by regulating circHIAT1 and miR-19a-3p.
Guo et al have shown that baicalein suppressed the AKT/mTOR pathway to reduce cell proliferation and metastasis in prostate cancer.34 Li et al also found the inhibition of baicalein on gastric cancer cell growth via decreasing the activities of AKT/mTOR signaling.35 The same signal blockage of AKT/mTOR pathway was caused by baicalein in anaplastic thyroid cancer cells.36 Herein, the levels of p-AKT/AKT and p-mTOR/mTOR were decreased by baicalein treatment in CC cells, whereas the circHIAT1/miR-19a-3p axis was further exhibited to be responsible for the signal inhibition of AKT/mTOR by baicalein. Thus, baicalein could regulate AKT/mTOR pathway via targeting the circHIAT1/miR-19a-3p axis.
The current research is always interested in targeting cancer therapy through the inhibition of mTOR. Rapamycin is a macrolide antibiotic with antiproliferative property as an mTOR inhibitor, and it cannot directly reduce the kinase activity of mTOR instead of the conformational changes in mTOR.37 However, the dose of rapamycin is difficult to be controlled. The low dose of rapamycin only induces the partial dissociation of mTOR and Raptor (involving in the mTORC1 substrate recognition), while high dose of rapamycin causes the complete dissociation to trigger the off-target effect.38 In our study, we found that baicalein exerted the anticancer function in CC cells both at 20 μg/mL and 40 μg/mL. The issued report also revealed that baicalein inhibited proliferation of HeLa cells in a dose-dependent manner (from 25 μg/mL to 200 μg/mL).22 In comparison to rapamycin, baicalein could be a better drug for preventing mTOR signaling in CC progression. A previous study has indicated that the inhibition of mTOR regulated the Gln deprivation to affect the glutamine (Gln)-mediated G1 checkpoint in cell cycle arrest.39 There are a series of metabolic checkpoints in the late G1 phase that prevent cells’ entry into S-phase, and mTOR inhibition arrests cells in the late G1 because it may limit the nutrient sufficiency in replicating the genome.40 Due to the repression of baicalein on AKT/mTOR pathway, it is likely that baicalein inhibits the necessary nutrients to block the metabolic checkpoints by inactivating the AKT/mTOR pathway. This matter remains to be studied in the future.
In conclusion, this research unraveled that baicalein targeted the circHIAT1/miR-19a-3p axis to inactivate the AKT/mTOR pathway to inhibit the oncogenesis in CC (Figure 8). The circHIAT1/miR-19a-3p/AKT/mTOR signal network will contribute to understanding the functional mechanism of baicalein in CC progression. CircHIAT1 and miR-19a-3p can be used as available targets in the baicalein-mediated treatment of CC.
 |
Figure 8 Baicalein inactivated the AKT/mTOR pathway to inhibit the carcinogenesis of CC by upregulating circHIAT1 to sponge miR-19a-3p. |
Highlights
- Baicalein downregulates miR-19a-3p expression but upregulates circHIAT1 level in cervical cancer cells.
- CircHIAT1 is a sponge for miR-19a-3p.
- Baicalein inhibits AKT/mTOR pathway via regulating circHIAT1 and miR-19a-3p.
- Baicalein impedes cervical cancer cell progression via the circHIAT1/miR-19a-3p axis.
Data Sharing Statement
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the ethical review committee of Suizhou Hospital, Hubei University of Medicine. Written informed consent was obtained from all enrolled patients.
Consent for Publication
Patients agree to participate in this work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi:10.1016/S0140-6736(07)61416-0
2. Small W, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017;123(13):2404–2412. doi:10.1002/cncr.30667
3. Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet. 2019;393(10167):169–182. doi:10.1016/S0140-6736(18)32470-X
4. Liu ZH, Yang CX, Zhang L, et al. Baicalein, as a prooxidant, triggers mitochondrial apoptosis in MCF-7 human breast cancer cells through mobilization of intracellular copper and reactive oxygen species generation. Onco Targets Ther. 2019;12:10749–10761. doi:10.2147/OTT.S222819
5. Liu H, Dong Y, Gao Y, et al. The fascinating effects of baicalein on cancer: a review. Int J Mol Sci. 2016;17(10). doi:10.3390/ijms17101681.
6. Cathcart MC, Useckaite Z, Drakeford C, et al. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer. 2016;16:707. doi:10.1186/s12885-016-2740-0
7. Bie B, Sun J, Guo Y, et al. Baicalein: a review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed Pharmacother. 2017;93:1285–1291. doi:10.1016/j.biopha.2017.07.068
8. Yu X, Cao Y, Tang L, et al. Baicalein inhibits breast cancer growth via activating a novel isoform of the long noncoding RNA PAX8-AS1-N. J Cell Biochem. 2018;119(8):6842–6856. doi:10.1002/jcb.26881
9. Han SE, Park CH, Nam-Goong IS, et al. Anticancer effects of baicalein in FRO thyroid cancer cells through the Up-regulation of ERK/p38 MAPK and Akt pathway. Vivo. 2019;33(2):375–382. doi:10.21873/invivo.11484
10. Lian H, Hui Y, Xiaoping T, et al. Baicalein suppresses the proliferation of human cervical cancer cells via Notch 1/Hes signaling pathway. J Cancer Res Ther. 2019;15(6):1216–1220. doi:10.4103/0973-1482.204899
11. Xia X, Xia J, Yang H, et al. Baicalein blocked cervical carcinoma cell proliferation by targeting CCND1 via Wnt/beta-catenin signaling pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):2729–2736. doi:10.1080/21691401.2019.1636055
12. Yu X, Liu Y, Wang Y, et al. Baicalein induces cervical cancer apoptosis through the NF-kappaB signaling pathway. Mol Med Rep. 2018;17(4):5088–5094. doi:10.3892/mmr.2018.8493
13. Zhang J, Yang W, Zhou YB, et al. Baicalein inhibits osteosarcoma cell proliferation and invasion through the miR183/Ezrin pathway. Mol Med Rep. 2018;18(1):1104–1112. doi:10.3892/mmr.2018.9036
14. Jiang L, Song H, Guo H, et al. Baicalein inhibits proliferation and migration of bladder cancer cell line T24 by down-regulation of microRNA-106. Biomed Pharmacother. 2018;107:1583–1590. doi:10.1016/j.biopha.2018.08.107
15. Bie B, Sun J, Li J, et al. Baicalein, a natural anti-cancer compound, alters MicroRNA expression profiles in Bel-7402 human Hepatocellular Carcinoma cells. Cell Physiol Biochem. 2017;41(4):1519–1531. doi:10.1159/000470815
16. Xu XM, Wang XB, Chen MM, et al. MicroRNA-19a and −19b regulate cervical carcinoma cell proliferation and invasion by targeting CUL5. Cancer Lett. 2012;322(2):148–158. doi:10.1016/j.canlet.2012.02.038
17. Wang Y, Wang Y, Zhong W, et al. Correlation between miR-19a inhibition and radiosensitivity in SiHa cervical cancer cells. J BUON. 2017;22(6):1505–1508.
18. Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi:10.1016/j.pharmthera.2018.01.010
19. Wang Z, Zhao Y, Wang Y, et al. Circular RNA circHIAT1 inhibits cell growth in hepatocellular carcinoma by regulating miR-3171/PTEN axis. Biomed Pharmacother. 2019;116:108932. doi:10.1016/j.biopha.2019.108932
20. Quan J, Dong D, Lun Y, et al. Circular RNA circHIAT1 inhibits proliferation and epithelial-mesenchymal transition of gastric cancer cell lines through downregulation of miR-21. J Biochem Mol Toxicol. 2020;34(4):e22458. doi:10.1002/jbt.22458
21. Wu X, Yang Z, Dang H, et al. Baicalein inhibits the proliferation of cervical cancer cells through the GSK3beta-dependent pathway. Oncol Res. 2018;26(4):645–653. doi:10.3727/096504017X15031557924141
22. Peng Y, Guo C, Yang Y, et al. Baicalein induces apoptosis of human cervical cancer HeLa cells in vitro. Mol Med Rep. 2015;11(3):2129–2134. doi:10.3892/mmr.2014.2885
23. Chen Z, Hou R, Gao S, et al. Baicalein inhibits proliferation activity of human colorectal cancer cells HCT116 through downregulation of ezrin. Cell Physiol Biochem. 2018;49(5):2035–2046. doi:10.1159/000493714
24. Yang Y, Liu K, Yang L, et al. Bladder cancer cell viability inhibition and apoptosis induction by baicalein through targeting the expression of anti-apoptotic genes. Saudi J Biol Sci. 2018;25(7):1478–1482. doi:10.1016/j.sjbs.2017.03.014
25. Ma SC, Chen R, Yang TN, et al. Baicalein inhibits the proliferative activity of human prostate cancer cell line PC3 by downregulating Ezrin. J Biol Regul Homeost Agents. 2020;34(3):885–892. doi:10.23812/20-145-A-44
26. Xie M, Zhao F, Zou X, et al. The association between CCND1 G870A polymorphism and colorectal cancer risk: a meta-analysis. Medicine (Baltimore). 2017;96(42):e8269. doi:10.1097/MD.0000000000008269
27. Yoon MK, Mitrea DM, Ou L, et al. Cell cycle regulation by the intrinsically disordered proteins p21 and p27. Biochem Soc Trans. 2012;40(5):981–988. doi:10.1042/BST20120092
28. Nahand JS, Taghizadeh-Boroujeni S, Karimzadeh M, et al. microRNAs: new prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. J Cell Physiol. 2019;234(10):17064–17099. doi:10.1002/jcp.28457
29. Juan C, Hua Q, Ruping Z, et al. miRNA-489 as a biomarker in diagnosis and treatment of cervical cancer. Bratisl Lek Listy. 2018;119(5):278–283. doi:10.4149/BLL_2018_052
30. Zhang Y, Sun B, Zhao L, et al. Up-regulation of miRNA-148a inhibits proliferation, invasion, and migration while promoting apoptosis of cervical cancer cells by down-regulating RRS1. Biosci Rep. 2019;39(5). doi:10.1042/BSR20182193.
31. Wang H, Xiao Y, Wu L, et al. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-000911/miR-449a pathway in breast carcinogenesis. Int J Oncol. 2018;52(3):743–754. doi:10.3892/ijo.2018.4265
32. Huang X-Y, Huang Z-L, Xu Y-H, et al. Comprehensive circular RNA profiling reveals the regulatory role of the circRNA-100338/miR-141-3p pathway in hepatitis B-related hepatocellular carcinoma. Sci Rep. 2017;7(1):5428. doi:10.1038/s41598-017-05432-8
33. Wang K, Sun Y, Tao W, et al. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. doi:10.1016/j.canlet.2016.12.036
34. Guo Z, Hu X, Xing Z, et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 2015;406(1–2):111–119. doi:10.1007/s11010-015-2429-8
35. Li P, Hu J, Shi B, et al. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem Biophys Res Commun. 2020.
36. Park CH, Han SE, Nam-Goong IS, et al. Combined effects of baicalein and docetaxel on apoptosis in 8505c anaplastic thyroid cancer cells via downregulation of the ERK and Akt/mTOR pathways. Endocrinol Metab (Seoul). 2018;33(1):121–132. doi:10.3803/EnM.2018.33.1.121
37. Yang H, D G R, J D K, et al. mTOR kinase structure, mechanism and regulation. Nature. 2013;497(7448):217–223.
38. Mukhopadhyay S, Frias MA, Chatterjee A, et al. The enigma of rapamycin dosage. Mol Cancer Ther. 2016;15(3):347–353.
39. Mukhopadhyay S, Saqcena M, Foster DA. Synthetic lethality in KRas-driven cancer cells created by glutamine deprivation. Oncoscience. 2015;2(10):807–808. doi:10.18632/oncoscience.253
40. Saqcena M, Menon D, Patel D, et al. Amino acids and mTOR mediate distinct metabolic checkpoints in mammalian G1 cell cycle. PLoS One. 2013;8(8):e74157. doi:10.1371/journal.pone.0074157
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.