Back to Journals » Drug Design, Development and Therapy » Volume 16
Baicalein Inhibits the Progression and Promotes Radiosensitivity of Esophageal Squamous Cell Carcinoma by Targeting HIF-1A
Authors Guo D, Jin J, Liu J, Wang Y, Li D, He Y
Received 21 April 2022
Accepted for publication 16 July 2022
Published 29 July 2022 Volume 2022:16 Pages 2423—2436
DOI https://doi.org/10.2147/DDDT.S370114
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Dongli Guo, Jing Jin, Jianghui Liu, Yingying Wang, Daojuan Li, Yutong He
Cancer Institute, Fourth Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China
Correspondence: Yutong He, Fourth Hospital of Hebei Medical University, No. 12, Jiankang Road, Zhongshan East Road Street, Changan District, Shijiazhuang, People’s Republic of China, Tel +8613903398303, Email [email protected]
Purpose: To explore the mechanism of the effect of baicalein on radioresistance of esophageal cancer, and to provide ideas for the treatment of patients with poor radiotherapy effect of esophageal cancer.
Methods: The glycolytic rate assay kit was used to detect the changes in glycolytic metabolism in esophageal cancer cells after treatment with baicalein, and mass spectrometry was used to detect whether baicalein could affect the level of glycolysis-related metabolites in esophageal cancer cells. The binding of baicalein to the target protein was simulated by molecular docking technique, the protein expression level was detected by Western Blot, and the changes in the cell cycle were detected by flow cytometry.
Results: Radiation combined with baicalein could significantly inhibit the proliferation and migration of esophageal cancer cells compared with that of 6 Gy rays alone. The results of the glycolytic rate assay showed that baicalein could inhibit the glycolysis of esophageal cancer cells. Metabonomic studies showed that baicalein could affect the expression levels of glycolysis-related metabolites. The results of network pharmacology showed that baicalein could target several key glycolysis enzymes and glycolysis-related proteins, such as HIF-1A. The results of the WB experiment showed that glycolysis-related proteins and cycle-related proteins were down-regulated after baicalein treatment.
Conclusion: The main mechanism of baicalein inhibiting radiation resistance of esophageal cancer cells is that targeting HIF-1A protein regulates glucose metabolism and then regulates Cyclin D1/CDK4 axis to change the cell cycle.
Keywords: ESCC, glycolysis, metabonomic, HIF-1A, CyclinD1
Introduction
At present, esophageal cancer is a serious threat to human health, and the incidence of esophageal cancer is on the rise. According to GLOBOCAN, there were about 604, 000 new cases and about 544, 000 deaths of esophageal cancer worldwide in 2020, Morbidity and mortality ranked 8th and 6th among global cancers.1 China is the highest-risk area, including Hebei, Guangdong, and so on. In 2015, China estimated that there were 477,900 new cases and 375,500 deaths of esophageal cancer.2 Although Current treatment options for patients with esophageal cancer include surgical resection, chemotherapy, postoperative radiotherapy, and immunotherapy. Tumor radiotherapy is a local treatment for tumors using radiotherapy. However, studies have shown that the 5-year survival rate of patients with esophageal cancer after radiotherapy is less than 20%.3 This is due to the existence of individual differences so that some patients will have radiotherapy resistance, making radiotherapy insensitive, radiotherapy treatment is not complete, so the regulation of tumor radiotherapy resistance targeting drugs is our future research direction.
The main factors affecting the effect of radiotherapy are “6R” factors: Repair, Redistribution, Reoxygenation, Repopulation, intrinsic Radiosensitivity, and Reactivation of Anti-Tumor Immune Response.4 For patients who are not sensitive to radiotherapy, radiosensitizers should be used to improve the effect of radiotherapy. Sodium glycididazole has been used as a radiosensitizer in the clinic,5 and its principle is Repair in “6R”. It can fix the DNA damage of tumor hypoxic cells and inhibit the repair of DNA damage, so as to improve the sensitivity of tumor hypoxic cells to radiation.6 However, it has been discontinued due to serious side effects such as vomiting and rash. With the progress of traditional Chinese medicine research, the extracts of traditional Chinese medicine are widely used for anti-tumor therapy. Among them, baicalein has great potential in the treatment of tumors. Baicalein, a flavonoid, is extracted from the root of Scutellaria baicalensis (SB). It can induce DNA damage without producing chromosomal alterations and mutagenesis that improves in severe side effects in cancer therapy, which is an attractive candidate for improved therapeutic drugs.7 Studies showed that baicalein can inhibit HIF-1A-mediated aerobic glycolysis and mitochondrial dysfunction, which overcome TAM resistance by sensitizing resistant cells to TAM-induced growth inhibition and apoptosis.8 The antitumor activities of baicalein have been extensively studied in various tumors, whose mechanisms underlying the antitumor involve the inhibition of cell proliferation, tumor migration and invasion, induction of apoptosis and autophagy, and arrest of cell cycle. But there are few studies in esophageal cancer. Studies have shown that baicalein can change the morphology of esophageal cancer cells, inhibit proliferation of esophageal cancer cells, induce apoptosis, and suppress tumor migration and invasion.9–11 Therefore, on this basis, we further explored the mechanism of baicalein changing the function of esophageal cancer cells by studying the cycle and metabolites, and provided a theoretical basis for exploring the application of baicalein in the treatment of esophageal cancer.
Materials and Methods
Cell Culture
The human esophageal cancer cell line KYSE150 was purchased from Shanghai Zhongqiao Xinzhou Biotechnology and cultured in RPMI-1640 medium (HyClone, Logan, Utah, USA) containing 10% fetal bovine serum (FBS: PAN, Adenbach, Germany) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37 °C in a 5% CO2 humidified incubator. The baicalein was prepared in DMSO (Concentration is less than 1/1000) and used to regulate the baicalein levels.
Cell Irradiation
When the cells were up to about 60%, the baicalein-treated and untreated groups were irradiated with a 6Gy ray at a medical linear accelerator and were cultured regularly (The source-to-axis distance was 100 cm).
Flow Cytometry Assays
Cell cycle was determined by flow cytometry. The collected cells were washed twice with PBS, fixed in 70% ethanol solution at 4 °C for 24 h, and then washed once with PBS and re-suspended. The suspension was incubated with propidium iodide (PI; 50mg/mL) of 500µL for 30min and measured by flow cytometry.
Quantitative Real-Time PCR
Total RNA was extracted from cell samples as described by Trizol. According to the manufacturer’s plan, RNA is reverse transcribed into cDNA using a reverse transcription kit (Takara). QRT-PCR was performed using the SYBR Green PCR kit (Thermo Fisher Scientific). The data were analyzed by the comparative Ct method (2−ΔΔ Ct). The sequences of genes were as follows:
GAPDH forward primer: 5´-AGGTGAAGGTCGGAGTCAACG-3´;
GAPDH reverse primer: 5´-AGGGGTCATTGATGGCAACA-3´;
HIF-1A forward primer: 5´-TCACCACAGGACAGTACAGGATGC-3´;
HIF-1A reverse primer: 5´-CCAGCAAAGTTAAAGCATCAGGTTC-3´;
PKM2 forward primer: 5´-TGACGAGAACATCCTGTGGC-3´;
PKM2 reverse primer: 5´-AGCACAGATGACAGGCTTCC-3´;
Cyclin D1 forward primer: 5´-TCCTACTACCGCCTCACA-3´;
Cyclin D1 reverse primer: 5´-ACCTCCTCCTCCTCCTCT-3´;
CDK4 forward primer: 5´-GCCCTCAAGAGTGTGAGAGTC-3´;
CDK4 reverse primer: 5´-GACGTCCATCAGCCGGA-3´;
Transwell Assays
1× 105 cells treated with baicalein for 48 hours were plated onto the Transwell chamber (Corning) containing RPMI-1640 medium and matrix glue, and the lower part was filled with RPMI-1640 medium containing 10% FBS. Incubated at 37 °C for 48 hours, 4% paraformaldehyde was fixed and 0.1% crystal violet staining.
Wound-Healing Assays
8 × 105 cells treated with baicalein for 48 hours cells were plated into six-well dishes. After incubating overnight, the plastic tip was used to cross the monolayer of cells in each hole vertically and then washed twice with PBS. Images were taken at 0 and 24 hours to measure the wound distance.
Colony-Forming Unit Assays
1 × 103 cells treated with baicalein for 48 hours per well were plated into six-well dishes and cultured for 7–10 days until a visible clone was formed and stained with crystal violet solution. Clones was recorded by camera and counted by ImageJ. Repeat three times for each group.
Molecular Docking
We downloaded the protein molecule using the PDB database, selected the protein crystal structure with low resolution, ligand, and relatively complete structure, and downloaded its PDB format file. PyMOL was used to remove the ligands, and AutoDock4.2 was used to delete the water molecules of the target protein. Total hydrogen was added, and the samples were saved as a file in PDBQ format. Then, the selected main active ingredients were downloaded through the TCMSP platform, and the compounds in MOL2 format were introduced into AutoDock4.2. Total hydrogen was added, which was set as ligands (automatic charge distribution). All flexible bonds can be rotated by default and saved as a PDBQ lattice. AutoDockvina software was run to dock and calculate the free binding energy. Among them, affinity < −4.25 kcal/mol means that the ligands and receptors have the possibility of combination, affinity < −5.00 kcal/mol indicates good binding strength, and affinity < −7.00 kcal/mol suggests satisfactory binding strength. Finally, PyMOL software was used to calculate the RMSD (less than 2 indicates stable binding),12 and PyMOL and Ligplot were used for visualization.
Extracellular Acidification Rate (ECAR)
Assays were performed using a Seahorse XFe96 analyzer (Seahorse Bioscience, Agilent) according to the manufacturer’s instructions. The ECAR was measured using a Seahorse XF Glycolytic Rate Assay Kit (Seahorse Bioscience, Agilent, 103344-100). The contribution of mitochondria/CO2 to extracellular acidification was calculated by measuring cell oxygen consumption at the same time, and the acidification contributed by CO2 was subtracted from the total proton outflow rate (PER).
Sample Preparation for Metabolomics and Lipidomics Study
Cell samples: Grind the frozen cell sample with a grinder, add 300 μL of ice-cold extraction solvent methanol (4°C), vortex, sonicate for 10 min, kept at −4°C temperature for 20 min, and then centrifuged at 12,000 r /min at 4℃ for 10 min. Aspirate the supernatant, filter it with a microporous membrane, and put samples on the machine.
QC sample preparation: Take 10 μL of each processed sample and mix it as a quality control sample (QC), which is used to determine the state of the instrument before injection and to balance the chromatography-mass spectrometry system, and to evaluate the stability of the system during the experiment.
Instrumental conditions: Chromatography conditions, chromatographic separations were performed using an Acquity HSS T3 (1.8 m, 100×2.1 mm; Waters), the column temperature was maintained at 40 °C, flow rate 300 μL/min, and injection volume 15 μL. Linear gradient elution of A (0.1% formic acid water) and B (0.1% acetonitrile) was used with the gradient procedure as follows: an isocratic elution of 2% B for the initial 1 min, followed by a linear gradient elution of 2–15% B from 1 to 3 min, followed by a linear gradient elution of 15–50% B from 3 to 6 min, and then followed by a linear gradient elution of 50–95% B from 6 to 9 min, after holding the composition of 95% B for the next 3 min, followed by a linear gradient elution of 95%–2% B from 12 to 12.01 min, last holding the composition of 2% B for the next 3 min. The sample was placed in the 4°C autosamplers during the entire analysis. In order to avoid the influence caused by the fluctuation of the instrument detection signal, a random order is adopted for the continuous analysis of samples. QC samples are set in the sample queue to monitor and evaluate the stability of the system and the reliability of test data.
Mass spectrometry conditions: The samples were injected using the heated electrospray ionization source (HESI), and the capillary voltage was set to 2.5 kV in positive and negative ionization mode. The relevant parameters are as listed: capillary temperature 250°C, heater temperature 400°C, sheath gas, auxiliary gas, and sweep gas were 50.0, 13.0 and 3.0 L/min. The scan range of m/z from 80 m/z to 1200 m/z. The isolation window was set to 2 m/z, dynamic exclusion was set as 10s. Nitrogen gas was used for spray stabilization and high energy collision dissociation in the higher-energy collision dissociation (HCD), and the detection was performed in full scan mode (FM). The resolution of the primary MS scan is set to 70,000, and the resolution of the secondary MS/MS scan is 17,500. The relevant parameters are as listed: The number of ions filled with C-Trap is 1e6, the maximum ion fill time (IT) at 30 ms, Normalized Collision Energy was 12.5, 25 and 37.5 eV, Isolation window was set to 3, and Loop was set to 2.
Data processing: Import the raw data into Compound Discoverer 3.0 (Thermo Fisher Scientific, USA) for data preprocessing. The blanks were used to eliminate background noise. The pre-processing steps of the MS data are the following: Chromatographic peak extraction, retention time alignment, blank value filling, QC-based peak area correction and normalization (CV<30%), the minimum intensity threshold at 1e6, mass tolerance was set to 5 ppm, The intensity tolerance was 30%, The S/N threshold was 3, The preferred adduct ion was set to[M+H]+1 and [M-H]-1.
The selected metabolites were identified by matching the accurate mass number and fragment information with the standard mass spectra of online databases METLIN (http://metlin.scripps.edu) and HMDB (http://www.hmdb). The metabolic pathway was analyzed by the online website MetaboAnalyst3.0 (http://www.metaboanalyst.ca). Multivariate statistical analysis methods include the principal component analysis (PCA) model, which is used to reflect the distribution of population samples, natural aggregation, and abnormal samples. And the aggregation degree of QC samples is used to examine the quality of data collection. The partial least squares discriminant analysis (PLS-DA) model was used to maximize the distinction between groups. Before the multivariate analysis, the variables were transformed by log10 and Paretoscaling normalization. We select the most important metabolites based on a Variable Importance in Projection (VIP) value of each variable based on PLS-DA, and further analyze the metabolites with a VIP value greater than 1.0. In this study, compounds with VIP > 1, P < 0. 05, FC ≥ 1.5 and p ≤ 0. 05 were considered to be differential compounds.
Results
Baicalein Inhibited the Proliferation, Migration, Invasion and Cell Cycle of KYSE150 Cells
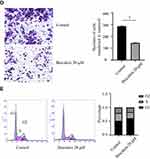
To explore the effect of baicalein on the proliferation of ESCC cells, we used MTS assays. The results showed that baicalein significantly inhibited the proliferation of KYSE150 cells in a dose-and time-dependent manner. It was more significant when the concentration of baicalein was 20μM (Figure 1A and B, t=17.77, p<0.05). The results showed that baicalein inhibited the proliferation of KYSE150 cells with 6.7μM, but the difference is not statistically significant (p>0.05). The results of colony formation assays were consistent with those of MTS assays. The results of the scratch experiment showed that after 48 hours of baicalein treatment, the scratch spacing was 3.25 times higher than that of the control (Figure 1C). The results of the cell invasion assay showed that the number of cell metastasis decreased after treatment with baicalein (Figure 1D, t= 33.52, p< 0.05). In addition, in order to evaluate the effect of baicalein on the cell cycle of esophageal cancer cells, we detected the cells treated with 20μM baicalein by flow cytometry and found that baicalein arrested cells in the G1 phase of the cell cycle, which changed from 51.2% to 60.8% (Figure 1E). Figure 1 Continue.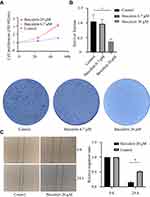
Baicalein Reduces Radiotherapy Resistance to Esophageal Cancer
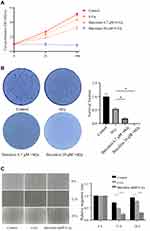
To further explore the clinical application of baicalein in the treatment of esophageal cancer, we reduced the concentration of baicalein and combined it with X-ray irradiation. We studied whether esophageal cancer cells could increase the effect of radiotherapy when cultured at 6.7 μM/L baicalein for 48 hours and then irradiated with 6 Gy rays. It was found that compared with the radiation alone group, the proliferation of esophageal cancer cells was significantly inhibited after baicalein treatment (Figure 2A). The results of colony formation assays were consistent with those of MTS assays (Figure 2B). And Cells treated with 20 μM baicalein did not form clones. The number of cell proliferation decreased and the migration distance decreased by 2.14 times compared with the radiation group (Figure 2C, p < 0.05).
Baicalein Inhibited the Glycolysis of KYSE150 Cells
To further investigate the mechanism of baicalein on radiation therapy in esophageal cancer. We first investigated whether baicalein may affect glycolysis and mitochondrial metabolism were measured by Seahorse XF96. As expected, The results showed that baicalein decreases mitochondrial metabolism in EC cells, but the difference was not statistically significant (Figure 3A and B). The picture showed that 20μM baicalein reduced glycolysis in KYSE150 cells (Figure 3C). The compensatory glycolysis level of cells treated with baicalein was 76.95% of that of the control group (Figure 3D).
Baicalein Regulated Glycolysis-Related Metabolites
We used UHPLC-Orbitrap-MS to analyze and detect pretreated samples in positive and negative ion switching mode. After preprocessing the original data, there were 130 metabolites were obtained in the sample (Table S1). Then we carried out the PCA analysis, and the score chart showed that the QC samples have a good aggregation phenomenon, indicating that the result has good repeatability and system stability (Figure 4A). Figure 4B showed that there was an obvious trend of separation between the control group and the baicalein group. Then we calculated the VIP score by the PLS-DA method and combined it with the analysis of the difference between the two groups of metabolites (Figure 4C and D). We found that Luteolin, Chrysin, Hispidulin, L-Glutamine, L-Glutamine, Indoleacrylic acid, g-Guanidinobutyrate, L-(-)-Methionine, L-Pyroglutamic acid, L-Glutamine, CLOFAZIMINE, 3-(Sulfooxy)-L-tyrosine, L-Methionine-2, L-Phenylalanine, L-Methionine, Sphingosine, Estazolam, Ecgonine, Pyridoxa et al were the main differences in metabolism between the two groups. Then we conducted KEGG analysis and found that baicalein mainly functions in the following 15 pathways: Alanine, aspartate and glutamate metabolism, Arginine biosynthesis, Purine metabolism, Pyrimidine metabolism, D-Glutamine and D-glutamate metabolism, Glyoxylate and dicarboxylate metabolism, Nitrogen metabolism, Aminoacyl-tRNA biosynthesis, Arginine and proline metabolism, Glutathione metabolism.
Phenylalanine, tyrosine and tryptophan biosynthesis, Phenylalanine metabolism, Cysteine and methionine metabolism, Sphingolipid metabolism, and Vitamin B6 metabolism (Figure 4E).
Baicalein Targeted Glycolysis Related Protein
We searched the target gene of baicalein through TCMSP and PharmMappe website. It contains 318 protein targets (Table S2). Then we searched the Msigdb database with “glycolysis” as the keyword, including 14 pathways and 308 glycolysis-related genes (Table S3). Among them, baicalein can target 19 glycolysis-related genes: ANG, AURKA, CDK1, EGFR, GALE, GALK1, ISG20, ME2, MET, MIF, PPIA, PYGL, ADH1C, ADH5, ALDH2, HK1, GPI, ALDOA, PCK1. HK1 (4QS9),13 ALDH2, GPI and ALDOA are the key enzymes in the process of glycolysis. In addition, Bacalein can also target HIF-1A (3HQU).14 Then, we created a protein-protein interaction (PPI) network of the glycolysis-related proteins to find a protein that interacts with baicalein, and we can find that HIF-1A plays a central role (Figure 5). Molecular docking is a technique that simulates the interaction between ligand small molecules and receptor proteins, and the binding energy between conjugates can be calculated to predict their affinity. The binding energy below-4.5 kcal/mol indicates that the two molecules can bind stably spontaneously, and the smaller binding can lead to a more stable conformation. Figure 6 showed in detail some local structures of baicalein docking with HK1 and HIF-1A molecules using pymol and ligplot software. Baicalein can form four hydrogen bonds with 105,106,195 residues in HK1 (Figure 6A), and the binding energy is −7.1 kcal/mol. Baicalein can also form two hydrogen bonds with ASP residues at position 570 in HIF-1A, and the binding energy is −4.9 kcal/mol (Figure 6B).
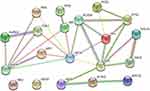 |
Figure 5 Protein-protein interaction (PPI) network of the glycolysis-related proteins. |
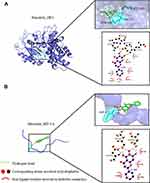 |
Figure 6 Molecular docking of the baicalein with the core targets. (A) Molecular docking of baicalein with HK1. (B) Molecular docking of baicalein with HIF-1A. |
Baicalein Regulated Glycolysis and Cycle-Related Proteins
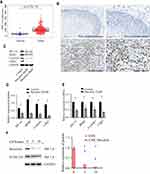
As the main regulator of aerobic glycolysis, HIF-1A plays an important role in hypoxia adaptation of solid tumors, and PKM2, as the rate-limiting enzyme of glycolysis, also plays an important role in glycolysis. Then we downloaded HIF-1A gene expression data for 171 esophageal cancers from the TCGA database. We found that HIF-1A was expressed at a significantly higher level in tumor tissues than in normal tissues (Figure 7A). We also collected 20 pairs of tumor tissues and para-carcinoma tissues from esophageal cancer patients. The immunohistochemical results showed that HIF-1A was highly expressed in tumor tissues, and HIF-1A positive cells accounted for 55%. In the results of 20 pairs of tumor tissues and para-carcinoma tissue, para-carcinoma tissue samples did not show HIF-1A positive, and 13 (65%) tumor tissues showed positive (Figure 7B). To evaluate whether baicalein regulates aerobic glycolysis by regulating HIF-1A and PKM2, we first measured the protein levels of HIF-1A and PKM2 in KYSE150 cells treated with 20μM baicalein. As shown in Figure 7C and D, the protein levels of HIF-1A and PKM2 decreased significantly after baicalein treatment. Next, we evaluated the effect of baicalein on the transcriptional activity of HIF-1A and PKM2 in KYSE150 cells. As shown in Figure 7E, baicalein decreased the expression of HIF-1A and PKM2 mRNA. Then we detected the expression level of G1-related proteins and found that the levels of genes and proteins related to G1-related decreased both at the transcriptional level and protein level (Figure 7C–E). In order to further determine that baicalein can specifically inhibit the expression of HIF-1A protein, we used CHX (50 μg/mL) combined with baicalein to treat cells. The results showed that baicalein treatment accelerated protein degradation (Figure 7F).
Discusion
Baicalein is a flavonoid compound derived from the root of Scutellaria baicalensis and has broad application prospects. It plays an important role in a variety of cancer. However, there are few studies on esophageal cancer. Drugs need to reach an effective concentration to function but tend to become cytotoxic at high concentrations. Our results showed that baicalein at the concentration of 20μM could significantly inhibit cell proliferation, and baicalein (20 μM) can also significantly enhance the radiosensitivity when combined with radiotherapy. Considering the enhancement of radiosensitivity may be due to the inhibition of proliferation by the high concentration of baicalein, We choose low-dose baicalein (6.7 μM) combined with radiotherapy, which did not affect cell proliferation. This concentration of baicalein combined with radiotherapy can further enhance the radiosensitivity, and this concentration is a non-toxic dose, indicating that the sensitizing effect of radiotherapy is the effect of the drug itself. In our experiment of baicalein combined with radiotherapy, a low concentration of baicalein (6.7 μM) promoted the effect of radiotherapy for esophageal cancer and inhibited the proliferation and migration of cells after radiotherapy. Therefore, we further explored the mechanism of baicalein inhibiting radioresistance in esophageal cancer.
Due to the specific energy supply mode of the tumor. First, we used the Seahorse instrument to examine the glycolysis process of esophageal cancer cells, which is called the Warburg effect. As expected, we found that treatment of the cells with the baicalein inhibited lactate production but did not change mitochondrial metabolism in KYSE150 cells. Unlike most normal tissues, cancer cells tend to “ferment” glucose into lactic acid even when oxygen is sufficient to support mitochondrial oxidative phosphorylation, which causes lactate and protons to accumulate outside the cell. Baicalein treatment attenuated the glycolysis process, that is, the energy supply of esophageal cancer cells, as well as the raw materials needed for cell synthesis.
Then we analyzed the metabolites of esophageal cancer cells treated with baicalein by the UHPLC-Orbitrap-MS instrument. The results showed that the expression of L-phenylpropionamide was up-regulated after baicalein treatment. Consistent with Suzuki’s study, phenylpropionamide reduced the extracellular acidification rate and inhibited the glycolysis process.15 In addition, we also found that the metabolite L-glutamine is up-regulated. Glutamine converts to glutamate by glutaminase, and glutamate can also be converted to α-ketoglutarate under the action of glutaminase, which can enter the tricarboxylic acid (TCA) cycle to provide energy for cells.16,17 In a word, the level of cell metabolism changed after baicalein treatment, which affected the glycolysis process of esophageal cancer cells.
Then we evaluated the effects of baicalein on the glycolysis process by network pharmacology analysis. We found that baicalein can affect the progression of esophageal cancer cells by targeting 19 glycolysis-related proteins and HIF-1A proteins searched in the Msigdb database. Then we further simulated the relationship between baicalein and target protein by calculating the binding energy of molecular docking, which directly revealed the interaction between drug molecules and targets to clarify their structure-activity relationship. Baicalein could be linked with HIF-1A through 2 hydrogen bonds located at the 570th ASP. The binding energy was −4.9 kcal/mol, which indicates that it could bind spontaneously and that the binding is stable Studies have found that HIF-1A is closely related to tumor radiosensitivity.18 Radiation can induce DNA breakage, and the outcome is apoptosis. HIF-1 can enhance the phosphorylation of p53 in irradiated cells, promote the function of caspase protein, and finally promote apoptosis. In addition, HIF-1A affects the expression of many glycolysis-related proteins, which maintains energy, so the expression level of HIF-1A is crucial for the effect of radiotherapy. Therefore, baicalein binds HIF-1A protein through a hydrogen bond to further regulate the process of glycolysis and improve the effect of radiotherapy. In addition, our network pharmacological analysis results showed that baicalein can also bind to glycolysis-related enzymes HK1, GPI, ALDH2, ALDOA to regulate the glycolysis process by regulating related enzymes. The target protein HK1 can catalyze the phosphorylation of various hexoses to mediate the initial step of glycolysis, which is the rate-limiting process of glycolysis.19–21 In addition, the target protein EGFR, which is a member of the epidermal growth factor receptor (HER) family, has been widely used in the clinic. EGFR can target PKM2, PKM2 with high activity tetramer form and low activity dimer form, which is more stable than other types of pyruvate kinase form.22 It is an indispensable rate-limiting catalase in the last step of the glycolysis pathway.23 It also can participate in the positive and negative feedback regulation of metabolism and promote cancer progression.24,25
Then we verified the expression of HIF-1A and PKM2 decreased at the transcriptional and protein level after baicalein treatment. That is, baicalein inhibits glycolysis by reducing the expression levels of HIF-1A and PKM2. This is consistent with the results of our network pharmacology that baicalein can directly target the HIF-1A protein to regulate its expression level. It has been mentioned above that HIF-1A plays an important role in glycolysis because baicalein inhibits the expression level of HIF-1A and PKM2, which in turn inhibits the glycolysis process, resulting in hampering cellular energy supply. It is well known that the main function of G1 is to synthesize RNA and ribosomes, which require a large amount of energy and materials. Baicalein can weaken the energy supply of cells in terms of cycle and metastasis, and then reduce the expression level of CyclinD1/CDK4. It reduced the transition of the cell cycle from the G1 phase to the S phase, that is, the cell is blocked in the G1 phase. The study showed that the radiosensitivity of cells being most radiosensitive in the G2-M phase, less sensitive in the G1 phase, and least sensitive during the latter part of the S phase.26 This also explains that combined radiotherapy with a low dose of baicalein can enhance the radiotherapy effect of esophageal cancer.
At present, there are relatively few studies on the mechanism of baicalein on esophageal cancer. In the experiment of the combined effect of low-dose baicalein and radiation, our results showed that low dose baicalein can effectively increase the radiosensitivity of esophageal cancer cells, suggesting the prospect of clinical application of baicalein. By combining molecular docking analysis and metabonomics, we found that baicalein has a further effect on esophageal cancer by regulating the process of glycolysis. Baicalein can connect HIF-1A, glycolysis-related rate-limiting enzyme HKI, and glycolysis-related genes (19) through hydrogen bonds, and then regulate the process of glycolysis and affect the energy supply of esophageal cancer cells. Therefore, the cells were blocked in the G1 phase to regulate the cell cycle and inhibit the radioresistance of esophageal cancer.
Conclusions
The main mechanism of baicalein inhibiting radiation resistance of esophageal cancer cells is that targeting HIF-1A protein regulates glucose metabolism and then regulates Cyclin D1/CDK4 axis to change the cell cycle, and baicalein is expected to be used in the treatment of esophageal cancer.
Ethics Approval and Consent to Participate
The current study was approved by the Institutional Human Ethics Committee of Hebei Medical University Fourth Hospital (Shijiazhuang, China).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Natural Scientific Foundation of China (81871922) and the Hebei Province Health Department (20170745).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi:10.3322/caac.21349
4. Boustani J, Grapin M, Laurent PA, Apetoh L, Mirjolet C. The 6th R of radiobiology: reactivation of anti-tumor immune response. Cancers. 2019;11(6):860. doi:10.3390/cancers11060860
5. Wu P, Liu J, Sun X, Li X, Xing L, Yu J. Enhanced radiosensitizing by sodium glycididazole in a recurrent esophageal carcinoma tumor model. Oncotarget. 2017;8(38):63871–63880. doi:10.18632/oncotarget.19151
6. Min X, Huang F, Huang H, et al. The radiosensitization of sodium glycididazole on nasopharyngeal carcinoma cells via enhancing DNA damage and promoting apoptosis. J Cancer. 2019;10(2):305–312. doi:10.7150/jca.25941
7. Fox JT, Sakamuru S, Huang R, et al. High-throughput genotoxicity assay identifies antioxidants as inducers of DNA damage response and cell death. Proc Natl Acad Sci U S A. 2012;109(14):5423–5428. doi:10.1073/pnas.1114278109
8. Chen Y, Zhang J, Zhang M, et al. Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1alpha. Clin Transl Med. 2021;11(11):e577. doi:10.1002/ctm2.577
9. Mi S, Qu Y, Chen X, Wen Z, Chen P, Cheng Y. Radiotherapy increases 12-LOX and CCL5 levels in esophageal cancer cells and promotes cancer metastasis via THP-1-derived macrophages. Onco Targets Ther. 2020;13:7719–7733. doi:10.2147/OTT.S257852
10. Zhang HB, Lu P, Guo QY, Zhang ZH, Meng XY. Baicalein induces apoptosis in esophageal squamous cell carcinoma cells through modulation of the PI3K/Akt pathway. Oncol Lett. 2013;5(2):722–728. doi:10.3892/ol.2012.1069
11. Qu Y, Wen Z, Mi S, et al. 12-lipoxygenase promotes tumor progress by TGF-beta1-mediated epithelial to mesenchymal transition and predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Manag Res. 2019;11:8303–8313. doi:10.2147/CMAR.S212478
12. Hsin KY, Ghosh S, Kitano H, Fraternali F. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8(12):e83922. doi:10.1371/journal.pone.0083922
13. Feng J, Zhao S, Chen X, et al. Biochemical and structural study of Arabidopsis hexokinase 1. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt2):367–375. doi:10.1107/S1399004714026091
14. Chowdhury R, McDonough MA, Mecinovic J, et al. Structural basis for binding of hypoxia-inducible factor to the oxygen-sensing prolyl hydroxylases. Structure. 2009;17(7):981–989. doi:10.1016/j.str.2009.06.002
15. Suzuki R, Sato Y, Fukaya M, Suzuki D, Yoshizawa F, Sato Y. Energy metabolism profile of the effects of amino acid treatment on hepatocytes: phenylalanine and phenylpyruvate inhibit glycolysis of hepatocytes. Nutrition. 2021;82:111042. doi:10.1016/j.nut.2020.111042
16. Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–634. doi:10.1038/nrc.2016.71
17. Gao W, Liu F, Zhang W, et al. Mutations in genes encoding antibiotic substances increase the synthesis of poly-γ-glutamic acid in Bacillus amyloliquefaciens LL3. Microbiologyopen. 2017;6(1):e00398. doi:10.1002/mbo3.398
18. Nagao A, Kobayashi M, Koyasu S, Chow C, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20(2):238. doi:10.3390/ijms20020238
19. Wang F, Wang Y, Zhang B, et al. A missense mutation in HK1 leads to autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2014;55(11):7159–7164. doi:10.1167/iovs.14-15520
20. Magnani M, Bianchi M, Casabianca A, et al. A recombinant human ‘mini’-hexokinase is catalytically active and regulated by hexose 6-phosphates. Biochem J. 1992;285(Pt 1):193–199. doi:10.1042/bj2850193
21. Wolf AJ, Reyes CN, Liang W, et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166(3):624–636. doi:10.1016/j.cell.2016.05.076
22. Gupta V, Bamezai RN. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010;19(11):2031–2044. doi:10.1002/pro.505
23. Filipp FV. Cancer metabolism meets systems biology: pyruvate kinase isoform PKM2 is a metabolic master regulator. J Carcinog. 2013;12:14. doi:10.4103/1477-3163.115423
24. Zahra K, Dey T, Ashish Mishra SP, Pandey U, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol. 2020;10:159. doi:10.3389/fonc.2020.00159
25. Icard P, Simula L. Metabolic oscillations during cell-cycle progression. Trends Endocrinol Metab. 2022;33(7):447–450. doi:10.1016/j.tem.2022.04.006
26. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi:10.1016/j.ijrobp.2004.03.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.