Back to Journals » OncoTargets and Therapy » Volume 12
Bag-1 Silence Sensitizes Non-Small Cell Lung Cancer Cells To Cisplatin Through Multiple Gene Pathways
Authors Lv J, Zhang F , Zhai C, Wang G, Qu Y
Received 3 June 2019
Accepted for publication 27 September 2019
Published 31 October 2019 Volume 2019:12 Pages 8977—8989
DOI https://doi.org/10.2147/OTT.S218182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
Jiling Lv,1,2 Fang Zhang,3 Congying Zhai,2 Gejin Wang,4 Yan Qu1
1Department of Intensive Care Unit, Affiliated Qingdao Municipal Hospital of Qingdao University, Qingdao 266071, Shandong, People’s Republic of China; 2Department of Respiratory Medicine, The First Hospital of Zibo, Zibo 255200, Shandong, People’s Republic of China; 3Department of Radiotherapy, Yantai Affiliated Hospital of Binzhou Medical University, Yantai 26400, Shandong, People’s Republic of China; 4Department of Nursing, Zibo Vocational Institute, Zibo 255314, Shandong, People’s Republic of China
Correspondence: Yan Qu
The Intensive Care Unit, Qingdao Municipal Hospital Affiliated to Qingdao University, 1 Jiaozhou Road, Qingdao 266071, Shandong, People’s Republic of China
Tel +8613668892506
Email [email protected]
Purpose: B-cell lymphoma-2 (Bcl-2) associated athanogene 1 (Bag-1) is a multifunctional protein, and Bag -1 overexpression is associated with progression, metastasis, and drug resistance in lung cancer. This study assessed the effects of Bag-1 siRNA on sensitization of cisplatin on non-small cell lung cancer (NSCLC) cells.
Material and methods: NSCLC A549 cell line was transfected with Bag-1 or negative control siRNA and then treated with cisplatin for cell viability, CCK-8, LDH, and flow cytometry assays. The Ca2+ levels were analyzed using Fluo-3/AM fluorescence staining, and the protein levels were assessed using Western blot analysis.
Results: Bag-1 siRNA significantly knocked down Bag-1 expression and inhibited cell invasion versus the negative control siRNA, while Bag-1 silence sensitized cisplatin to induce A549 cells to apoptosis by induction of cell cycle G1 arrest. At protein level, Bag-1 silence reduced the expression ratio of Bcl-2 to Bcl-2 associated X protein (Bax), downregulated activity of the PI3K/AKT and mitogen-activated protein kinase (MAPK) pathways, and potently upregulated the calcium signaling-mediated pathway.
Conclusion: This study demonstrated that Bag-1 silencing sensitized A549 to cisplatin to enhance A549 cell apoptosis by modified multiple gene pathways. Further study will evaluate the usefulness of Bag-1 siRNA as a potential targeting therapy for NSCLC.
Keywords: bag-1, apoptosis, non-small cell lung cancer, chemotherapy
Introduction
Lung cancer is the leading cause of cancer-related deaths,1 while lung cancer has become the first ranked cause of cancer death in People’s Republic of China since the beginning of the twenty-first century.2,3 70–80% of lung cancer patients are diagnosed at an advanced stage of disease and lose the opportunity for curative surgery.2,3 Thus, platinum-based chemotherapy is the standard regimen to control non-small-cell lung cancer (NSCLC), prolonging survival of these patients. However, drug resistance (e.g., cisplatin-induced therapeutic resistance in lung cancer patients) causes a significant obstacle to satisfactory overall survival of NSCLC patients. Thus, it is urgent to understand lung carcinogenesis and drug resistance mechanisms in order to develop novel strategies to effectively control lung cancer.
Apoptosis is essential for maintaining the cell and tissue physiological state and B-cell lymphoma-2 (Bcl-2) associated athanogene 1 (Bag-1) is a multi-functional protein4 and can bind to Bcl-2 to inhibit cell apoptosis. For the different initiation sites during bag-1 protein translation, Bag-1 has four isoforms, including Bag-1L (52 kDa), Bag-1 (34 kDa), Bag-1M (46–48 kDa), and Bag-1S (29 kDa),5 expressed indifferent nuclear and cytoplasmic localization patterns.6 Bag-1 was overexpressed in various human cancers, including lung cancer.7 The association between expression of Bag-1 and a prognosis in lung cancer remains controversial. For example, Wang YD et al.7 showed that Bag-1 expression was associated with prolonged survival of patients with NSCLC. Differently, previous studies showed that high expression of BAG1 associated with poor outcome of NSCLC.8,9 Additional studies reported that Bag-1 expression was associated with a favorable prognosis of NSCLC after chemotherapy.10,11 Thus, in this study, we assessed the effects of Bag-1 silencing on regulation of cisplatin sensitivity to NSCLC cells in vitro and explored the underlying molecular events. We expected to provide useful information regarding the role of Bag-1 in mediation of chemotherapy efficacy for future control of NSCLC.
Materials And Methods
Cell Culture
A human NSCLC A549 cell line was provided by the Teaching and Research Section of Microbiology of Qingdao University Medical College (Qingdao, People’s Republic of China) and the use of this cell line was approved by the institutional review board of Qingdao University. A549 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Hyclone), penicillin (100 U/mL), and streptomycin (100 μg/mL) in a humidified atmosphere with 5% CO2 at 37°C, and the culture media was replaced every 2–3 days.
Bag-1 siRNA And Cell Infection
Lentivirus carrying Bag-1 and negative control siRNA was purchased from Shanghai Genechem Company (Shanghai, People’s Republic of China). A549 cells were grown and divided into three groups: 1) a group of non-treatment cells without any gene transfection, 2) a control siRNA group where the cells were infected with the negative control siRNA, and 3) a Bag-1 siRNA group where the cells were infected with Bag-1 siRNA. The cells were then infected with these lentiviruses using the manufacturer’s instructions. The infected cells were then selected with 1 µg/mL puromycin to reach 80% confluency, and the cell culture medium was changed to DMEM containing various doses of cisplatin (Kocak Pharma, Turkey) and cultured for 24 hrs for the following experiments.
Transwell Cell Invasion Assay
A Transwell invasion assay was performed to assess tumor cell invasion with Transwell filters that were precoated with Matrigel (Corning, Corning, NY, USA). Specifically, 200 μl transfected cells (7 × 104/mL) were seeded into the upper chamber of 24-well plate inserts with serum-free medium, while 600 μl of medium supplemented with 20% FBS was added to the lower chamber as a chemoattractant. The cells were then grown for 48 hrs at 37°C with 5% CO2. At the end of the experiment, the cells that invaded the reverse side of the filters were fixed in methanol for 30 mins and stained with crystal violet (0.1%) for 20 mins at 25°C. The number of cells was counted in five randomly selected fields under a 400-fold magnification and summarized.
Cell Viability Assay
The changed cell viability was measured using the Cell Counting Kit-8 (CCK-8; Boster, Wuhan, People’s Republic of China). Briefly, 5 × 103 cells/well were seeded into 96-well plates and incubated for 24 hrs and then treated with various doses (0, 0.625, 1.25, 2.5, 5, 10, 20, 30, or 40 μg/mL) of cisplatin for 24 hrs. Subsequently, 10 μl CCK-8 reagent was added to the cell culture medium, and the cells were further incubated for 1 hr. At the end of the experiment, the absorbance rate of each well was measured at 450 nm using a microplate reader (Bio-Tek, Winooski, VT, USA), while 100 μl DMEM was used as a blank control. The experiments were in triplicate and repeated at least three times. To calculate theIC50, the optical density (OD) values were measured after the cells were treated with different doses (0, 0.625, 1.25, 2.5, 5, 10, 20, 30, or 40 μg/mL) of cisplatin for 24 hrs.
Flow Cytometry Cell Cycle Assay
A549 cells were seeded into 6-well plates at a density of 2× 105 cells/well and incubated for 24 hrs and infected with Bag-1 or negative control siRNA for 48 hrs and then treated with 5 μg/mL cisplatin for an additional 24 hrs. After that, the cells were measured for cell cycle distribution using the Propidium Iodide (PI) flow cytometry kit (Abcam, Cambridge, UK) using the manufacturer’s recommendations. In brief, the cells were washed and incubated with trypsin for 3 mins at 37°C and then fixed with 66% ethanol on ice. 1×106 cells per group were stained with 5% PI in phosphate-buffered saline (PBS) containing 0.5% RNase A and subjected to DNA content analysis using FACStar Plus flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). The mean value of three independent experiments was analyzed by using FlowJo (BD Biosciences).
Flow Cytometry Apoptosis Assay
A549 cells were seeded into 6-well plates at a density of 2×105 cells/well and incubated for 24 hrs and infected with Bag-1 or negative control siRNA for 48 hrs and then treated with 5 μg/mL cisplatin for an additional 24 hrs. After that, the cells were harvested using trypsin without EDTA and resuspended in 1× binding buffer to a concentration of 1-5×106 cells/mL from the Annexin V-APC and propidium iodide (PI) apoptosis detection kit (eBioscience, San Diego, CA, USA) using the manufacturer’s protocol. Next, the apoptotic cells were quantified with a FACStar Plus flow cytometer (Becton Dickinson analyzed using BD Accuri C6 software). The mean value of triplicate experiments was used to analyze data.
Lactate Dehydrogenase (LDH) Activity And The Intracellular Ca2+ Concentration Assays
To measure the LDH activity in cells, five parallel wells were set for each experimental group and infected with Bag-1 or negative control siRNA for 48 hrs and then treated with 5 μg/mL cisplatin for an additional 24 hrs. Next, the culture medium was collected for detection of the LDH activity using an LDH activity assay kit (Nanjing Jian Cheng Biotechnology, Nanjing, People’s Republic of China) using the manufacturer’s protocol. Each sample was run in triplicate.
To assay the intracellular Ca2+ concentration, the duplicate cells were subjected to the Fluo-3/AM kit (Jiangsu Biyuntian Biological Technology Research Institute, Jiangsu, People’s Republic of China) analysis using the manufacturer’s protocol. Each sample was run in triplicate.
Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
Total cellular RNA was isolated from the cells by using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) using the manufacturer’s instructions and quantified by UV spectrophotometry (Implen, Germany) and then reversely transcribed into cDNA using a M-MLV Reverse Transcriptase Kit (Roche, Swiss) using the manufacturer’s protocol. These cDNA samples were then mixed with FastStart Essential DNA Green Master Mix (Roche, Basel, Switzerland) and amplified in a Light Cycler 96 (Roche, Indianapolis, IN, USA) using the manufacturer’s protocol. All gene primers were purchased from Genetech Co., Ltd, People’s Republic of China, and the Bag-1 primer sequences were 5ʹ-CTTCATGTTACCTCCCAGCA-3ʹ and 5ʹ-ACGGTGTTTCCATTTCCTTC-3ʹ, and the GAPDH primer sequences were 5ʹ-ATCTCTGCCCCCTCTGCTGA-3ʹ and 5ʹ-GATGACCTTGCCCACAGCCT-3ʹ. Each experiment was repeated in triplicate. The relative level of Bag-1 mRNA was calculated using the 2-ΔΔCt method.
Western Blot
The experiment used the methods described previously.12 Briefly, A549 cells were collected and washed three times with ice-cold PBS, and the total cellular protein was extracted with a lysis buffer containing 200 μl ice-cold RIPA lysis buffer, 2 μl ice-cold PMSF lysis buffer, and 2 μl ice-cold phosphatase inhibitors for 30 mins. The supernatant fraction was collected after the mixture was centrifuged for 15 mins at 12,000 rpm. The protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit (TransGen Biotech, Beijing, People’s Republic of China), and the protein samples were then denaturated at 100°C for 5 mins in the loading buffer and separated in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and blotted onto a PVDF membrane (Bio-Rad, Hercules, CA, USA) and Western blot procedures.11 The primary antibodies used were an anti-Bag-1 (1:1000; Cell Signaling Technology, Danvers, MA, USA), anti-Hsp70 (1:1000; Abcam), anti-Bcl-2 (1:1000; Cell Signaling Technology), anti-Bax (1:1000; Cell Signaling Technology), anti-Cleaved-Caspase-3 (1:1000; Cell Signaling Technology), anti-IP3R (1:1000; Cell Signaling Technology), anti-P-IP3R (1:1000; Cell Signaling Technology), anti-AKT (1:10,000; Abcam), anti-P-AKT (1:5000; Abcam), anti-ERK (1:1000; Abcam), anti-P-ERK (1:1000; Abcam), and anti-β-Actin antibodies (1:1000; Boster, Wuhan, People’s Republic of China). The secondary antibodies (the goat anti-mouse for Bag-1, while the goat anti-rabbit antibody for other primary antibodies were from Boster (Wuhan, People’s Republic of China), and the positive bands were visualized using the Fusion FX Imaging System (Vilber Lourmat, Paris, France) and quantified by using the Bio-Rad quantity one software (Hercules, CA, USA).
Statistical Analysis
All values were expressed as a mean plus or minus the standard deviation (SD) and analyzed with the SPSS 17.0 statistical software (SPSS, Chicago, IL, USA). A One-way analysis of variance (ANOVA) followed by a Tukey’s test was performed to compare differences in multiple comparisons. A p-value less than 0.05 was considered to be statistically significant.
Results
Effect Of Bag-1 Silencing On Regulation Of Tumor Cell Invasion
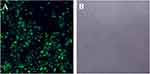
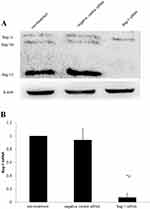
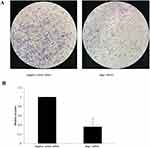
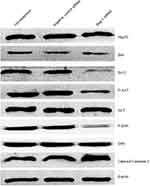
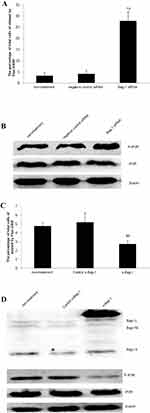
After 48 hrs of infection with lentivirus carrying Bag-1 siRNA, the fluorescence microscopic data showed that A549 cells were successfully infected by the lentivirus (Figure 1). Western blot results showed that expression of Bag-1 protein isoforms was significantly downregulated in Bag-1 siRNA group vs negative control siRNA group (Figure 2A), while qRT-PCR confirmed a dramatic decrease in Bag-1 mRNA level in Bag-1 siRNA-infected A549 cells vs the negative control siRNA group and the non-treatment cells (Figure 2B). As shown in Figure 3A and B, Bag-1 knockdown inhibited A549 cell invasion vs the cells infected with negative control siRNA (p =0.011).
Bag-1 Silence Increased A549 Cell Cytotoxicity After Cisplatin Treatment
After that, we first assessed the effect of Bag-1 silencing on regulation of cell viability. With increase in cisplatin concentrations, the cell viability of each group was decreased, but viability of Bag-1 siRNA-infected cells was even lower than that of the negative control siRNA group and non-treatment group. There was no statistical difference between the non-treatment and the negative control siRNA groups, whereas a lower IC50 was observed in Bag-1 siRNA-infected A549 cells (Figure 4A). After cisplatin concentration reached 5 μg/mL, the cell viability of Bag-1 siRNA group was significantly lower than that of non-treatment group (p=0.005) and the negative control siRNA group (p=0.003; Figure 4B). We, therefore, used this 5 μg/mL of cisplatin as a choice for our further experiments.
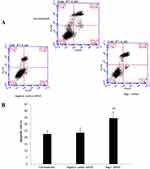
Next, we performed the flow cytometric assay to assess the changed cell apoptosis in Bag-1 silencing cells after 5 µg/mL cisplatin treatment. Our data showed that Bag-1 silencing enhanced the levels of both early and late apoptotic cells compared to that of non-treatment group (p=0.007) and the negative control siRNA group (p=0.01), while there was no difference occurred between the non-treatment and negative control siRNA groups (p=0.74; Figure 5). Cell cycle distribution assay showed that Bag-1 silencing decreased the percentage of cells at the S phase of the cell cycle but significantly increased the percentage of cells in the G1 phase of the cell cycle compared to those of the non-treatment and negative control siRNA cells (Figure 6).
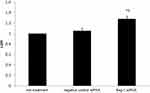
Furthermore, the LDH, a glycolytic enzyme, leaks from the cytoplasm to extracellular during apoptosis-induced cell membrane permeability and damage. Thus, the LDH is widely used for cell cytotoxicity detection.13,14 We found that bag-1 silencing of A549 cells increased LDH (p=0.000 vs that of the non-treatment group and p=0.001 vs.that of the negative control siRNA group; Figure 7). Therefore, these data suggested that Bag-1 silencing promoted cisplatin-induced A549 cell apoptosis in vitro.
Downregulation Of Bcl-2, PI3K/AKT, And The Mitogen-Activated Protein Kinase (MAPK) Pathway And Upregulation Of Caspases-3 Activity After Bag-1 Silencing
We then investigated the molecular events underlying Bag-1 silencing in A549 cells and cisplatin treatment. Our data showed that Bag-1 silencing lowered Bcl-2 levels and downregulated the phosphorylation of AKT and extracellular signal-regulated kinase (ERK1/2). However, Bag-1-silenced cells did not alter the expression of 70 kDa heat shock proteins (HSP70) and Bcl-2-associated x protein (Bax) compared to those of the non-treatment cells and negative control siRNA cells (Figure 8). To examine the effect of Bag-1 silencing on the regulation of caspase activity, we assayed the levels of caspase 3 and observed an increase in cleaved caspase-3 (17 kDa) in Bag-1 siRNA cells compared to the non-treatment and negative control siRNA cells (Figure 8).
Effect Of Bag-1 Silencing On Regulation Of The Intracellular Concentration Of Ca2+ And Inositol 1,4,5-Trisphosphate Receptor (IP3R) Phosphorylation In A549 Cells
As a second messenger in cells, Ca2+ plays an extremely important role during cell injury.15,16 To investigate whether Bag-1 silencing increased the sensitivity of cisplatin in the induction of A549 cell apoptosis through calcium overload, we performed Fluo-3/AM staining. Our data showed that Bag-1 silencing significantly elevated intracellular Ca2+ levels versus the non-treatment and negative control siRNA cells (both p-values were less than 0.001; Figure 9A), while phosphorylated IP3R levels were also enhanced in the Bag-1 siRNA group versus the non-treatment and negative control siRNA cells (Figure 9B). To further confirm whether calcium signaling is involved, we overexpressed Bag-1 in A549 cells using lentivirus carrying Bag-1 cDNA (e-Bag-1) or vector-only (control e-Bag-1). Our data showed the opposite results and changes the intracellular Ca2+ and phosphorylated IP3R levels (Figure 9C and D).
Discussion
Activity of the apoptosis signaling pathways is frequently disturbed in lung cancer cells, resulting in infinite tumor cell growth and proliferation but resistance to chemotherapy. Thus, better understanding of the molecular mechanism by which agents promote tumor cells to undergo apoptosis becomes an important direction of research in lung cancer treatment. In the current study, we investigated the pro-apoptotic effects of Bag-1 silencing in combination with cisplatin treatment in NSCLC A549 cells. Cisplatin is a well established and effective chemotherapeutic agent against various human cancers and induces DNA damage and apoptosis in highly proliferating cancer cells.17 Thus, “the background apoptotic effect” of cisplatin was quite substantial (>20%) in the “non-treatment” and “control siRNA treated” cells. We first performed lentivirus infection carrying Bag-1 or negative control siRNA into A549 cells and found that Bag-1 siRNA effectively silenced Bag-1 expression in A549 cells and inhibited cell invasion. After that, these infected A549 cells were subjected to various doses of cisplatin treatment. Our data showed that Bag-1 silencing reduced A549 cell viability and triggered them to undergo apoptosis. The caspase family is a group of proteolytic enzymes that execute cell apoptosis and Caspase-3 is the final step of the apoptosis cascades of proteolytic reactions responding to the apoptotic signals; thus, Caspase-3 is the key executor of apoptotic events.18 In our current study, Bag-1 silencing led to cleavage of caspases-3, indicative of activation of caspase 3 in cells. This phenomenon was in line with the cytotoxic effects induced by Bag-1 silencing and cisplatin.
To further identify the potential mechanisms of Bag-1 silencing, we assessed the therapeutic potential of Bag-1 silencing and explored the mechanism by which Bag-1 silencing sensitized A549 cells to cisplatin-induced apoptosis. Oltvai et al revealed that Bcl-2 can bind to Bax to form Bcl-1/Bax heterodimer19 and the ratio of Bcl/Bax determines the survival of cells after receiving the apoptotic signals. In other words, when Bax is dominant, cell apoptosis is accelerated. In the current study, we showed the decrease in ratio of Bcl to Bax, although the Bax levels did not show significant change in A549 cells after Bag-1 silencing and cisplatin treatment. Furthermore, the Bag family is one of those known cochaperones to Hsp70.20–22 Bag-1 binds to Hsp70 to modulate its chaperone activity in vitro,23 which is closely related to the anti-apoptotic function, tumorigenesis, and cell cycle redistribution in different physiological and diseased processes.24 Bag-1 can positively or negatively regulate the function of Hsp70. For example, Bag-1L overexpression could elevate HSP70 level in neuroblastoma cells,25 while Liu et al11 reported that Bag-1 siRNA increased the levels of Hsp70 protein in A549 cells. In our current study, we were unable to show change in Hsp70 expression in A549 cells after Bag-1 silencing, thus, further study of Bag-1 and its interaction with Hsp70 is warranted to explore the underlying mechanisms for NSCLC resistance to cisplatin.
In physiological conditions, the PI3K/AKT pathway plays a role in the regulation of cell growth proliferation and angiogenesis, and dysregulation of the PI3K/AKT pathway promotes cancer development.26 The P-AKT can activate the transcription factors FOXO1, FOXO 3, and FOXO4 to promote NSCLC cell progression to the S phase of the cell cycle.27 Ali et al observed that the PI3K inhibitors were able to delay tumor growth in an animal model.28 The PI3K/AKT pathway was highly activated in approximately 90% of NSCLC cell lines and involved in drug resistance.29,30 In our current study, AKT phosphorylation was potently suppressed after Bag-1 silencing, accompanied by G1 phase cell cycle arrest. As we know, cells in the G0/G1 phase are insensitive to chemotherapy, suggesting that Bag-1 silencing may downregulate the PI3K/AKT pathway to promote tumor cell apoptosis after cisplatin. In addition, the MAPK signal transduction pathway is the most important cell division regulatory system and plays an important role in the process of cell division and proliferation.31 The ERK1/2 are the main and classical pathway of the MAPK families and involved in regulation of cell cycle progression and cell proliferation.32 Thus, targeting of the MAPK pathway could enhance the efficiency of chemotherapy. In our current study, we found that the level of phosphorylated ERK1/2 was affected by Bag-1 silencing.
Normally, intracellular calcium is mainly stored in the endoplasmic reticulum (ER)/sarcoplasmic reticulum. To maintain an intracellular Ca2+ dynamic homeostasis, Ca2+ in the ER is mainly released by IP3R and RyR into the cell cytoplasm. At the same time, Ca2+ in the cytoplasm is ingested into the ER through SERCA.33 A previous study showed that calcium can be released from the ER to induce calcium overload, which, in turn, initiates mitochondrial-dependent apoptosis.34 As a universal intracellular calcium release channel, the IP3R is considered to be activated by its phosphorylation.35 In our current study, we found that the Ca2+ concentration was significantly elevated in the Bag-1 siRNA group, and, meanwhile, IP3R was activated by phosphorylation, while Bag-1 overexpression had the opposite change. Thus, we speculate that P-IP3R may be involved in Bag-1 silence–induced A549 cell apoptosis. Furthermore, Bcl-2 protein can block the release of calcium from the ER and decrease the activity of calcium-dependent endonuclease, thereby blocking cell apoptosis.36,37 A previous study revealed that Bcl-2 was able to dephosphorylate IP3R and suppress the channel activity.38,39 In our current study, we found that Bag-1 silencing enhanced IP3R phosphorylation and downregulated Bcl-2 expression in the cisplatin-treated A549 cells, suggesting that Bcl-2 downregulation could upregulate IP3R phosphorylation to improve the effectiveness of cisplatin treatment of A549 cells. In the present study, Bag-1 silencing inhibited A549 cell invasion and sensitized them to cisplatin-induced apoptosis via multiple pathways. Silencing of Bag-1 expression using Bag-1 siRNA like some tumor suppressors40 might represent a potential targeting therapy for NSCLC.
Conclusions
Bag-1 silencing sensitized NSCLC A549 cells to cisplatin-induced apoptosis through multiple pathways, i.e., decrease in the Bcl to Bax ratio, downregulation of the PI3K/AKT and MAPK pathway activity, and activation of IP3R.
Acknowledgments
We would like to thank Dr. Bing Luo of Microbiology Department, Qingdao University Medical College (Qingdao, People’s Republic of China) for his suggestions and support. This work was supported in part by grants from the National Natural Science Foundation of China (#81571938 and #81501706).
Author Contributions
Jiling Lv designed the study. Jiling Lv, Fang Zhang, Congying Zhai, Gejin Wang and Yan Qu carried out the study. Jiling Lv and Congying Zhai participated in the experiments and statistical analysis. Jiling Lv wrote the manuscript. Jiling Lv, Fang Zhang and Yan Qu revised the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Zheng R, Zeng H, Zhang S, et al. Lung cancer incidence and mortality in China, 2010. Throac Cancer. 2014;5(4):330–336. doi:10.1111/1759-7714.12098
4. Takayama S, Sato T, Krajewski S, et al. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 1995;80(2):279–284. doi:10.1016/0092-8674(95)90410-7
5. Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’?–A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188(1–2):25–32. doi:10.1016/s0304-3835(02)00456-1
6. Packham G, Brimmell M, Cleveland JL. Mammalian cells express two differently localized Bag-1 isoforms generated by alternative translation initiation. Biochem J. 1997;328(Pt 3):807–813. doi:10.1042/bj3280807
7. Wang YD, Ha MW, Cheng J, et al. The role of expression and polymorphism of the BAG-1 gene in response to platinum-based chemotherapeutics in NSCLC. Oncol Rep. 2012;27(4):979–986. doi:10.3892/or.2011.1591
8. Lin PL, Wu TC, Wu DW, Wang L, Chen CY, Lee H. An increase in BAG-1 by PD-L1 confers resistance to tyrosine kinase inhibitor in non-small cell lung cancer via persistent activation of ERK signalling. Eur J Cancer. 2017;85:95–105. doi:10.1016/j.ejca.2017.07.025
9. Liu H, Bai Y, Liu B, et al. [The expression of BAG-1 and its clinical significance in human lung cancer.]. Zhongguo Fei Ai Za Zhi. 2008;11(4):489–494. doi:10.3779/j.issn.1009-3419.2008.04.040
10. Leng XF, Chen MW, Xian L, Dai L, Ma GY, Li MH. Combined analysis of mRNA expression of ERCC1, BAG-1, BRCA1, RRM1 and TUBB3 to predict prognosis in patients with non-small cell lung cancer who received adjuvant chemotherapy. J Exp Clin Cancer Res. 2012;31:25. doi:10.1186/1756-9966-31-25
11. Liu H, Liang Y, Li Y, et al. Gene silencing of BAG-1 modulates apoptotic genes and sensitizes lung cancer cell lines to cisplatin-induced apoptosis. Cancer Biol Ther. 2010;9(10):832–840. doi:10.4161/cbt.9.10.11589
12. Hu D, Chen F, Guan C, Yang F, Qu Y. Anti-hypoxia effect of adenovirus-mediated expression of heat shock protein 70 (HSP70) on primary cultured neurons. J Neurosci Res. 2013;91(9):1174–1182. doi:10.1002/jnr.23240
13. Srivastava RK, Pant AB, Kashyap MP, et al. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line-A549. Nanotoxicology. 2011;5(2):195–207. doi:10.3109/17435390.2010.503944
14. Chen T, Gao F, Feng S, Yang T, Chen M. MicroRNA-134 regulates lung cancer cell H69 growth and apoptosis by targeting WWOX gene and suppressing the ERK1/2 signaling pathway. Biochem Biophys Res Commun. 2015;464(3):748–754. doi:10.1016/j.bbrc.2015.07.021
15. Nakayama H, Fujio Y, Yamaguchi O. [Calcium dependent signaling in cardiac hypertrophy and cell death]. Clin Calcium. 2013;23(4):505–515. CliCa1304505515
16. Seo SR, Seo JT. Calcium overload is essential for the acceleration of staurosporine-induced cell death following neuronal differentiation in PC12 cells. Exp Mol Med. 2009;41(4):269–276. doi:10.3858/emm.2009.41.4.030
17. Li QQ, Wang G, Liang H, et al. beta-Elemene promotes cisplatin-induced cell death in human bladder cancer and other carcinomas. Anticancer Res. 2013;33(4):1421–1428.
18. Ozaki K, Hanazawa S. Porphyromonas gingivalis fimbriae inhibit caspase-3-mediated apoptosis of monocytic THP-1 cells under growth factor deprivation via extracellular signal-regulated kinase-dependent expression of p21 Cip/WAF1. Infect Immun. 2001;69(8):4944–4950. doi:10.1128/IAI.69.8.4944-4950.2001
19. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi:10.1016/0092-8674(93)90509-o
20. Hohfeld J, Jentsch S. GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. Embo J. 1997;16(20):6209–6216. doi:10.1093/emboj/16.20.6209
21. Kabbage M, Dickman MB. The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci. 2008;65(9):1390–1402. doi:10.1007/s00018-008-7535-2
22. Rampelt H, Mayer MP, Bukau B. Nucleotide exchange factors for Hsp70 chaperones. Methods Mol Biol. 2011;787:83–91. doi:10.1007/978-1-61779-295-3_7
23. Takayama S, Bimston DN, Matsuzawa S, et al. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. Embo J. 1997;16(16):4887–4896. doi:10.1093/emboj/16.16.4887
24. Takayama S, Reed JC. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 2001;3(10):E237–E241. doi:10.1038/ncb1001-e237
25. Wang Y, Jia C, Li QS, Xie CY, Zhang N, Qu Y. BAG-1L protects SH-SY5Y neuroblastoma cells against hypoxia/re-oxygenation through up-regulating HSP70 and activating PI3K/AKT signaling pathway. Neurochem Res. 2017;42(10):2861–2868. doi:10.1007/s11064-017-2304-y
26. Juric D, Krop I, Ramanathan RK, et al. Phase I dose-escalation study of taselisib, an oral PI3K inhibitor, in patients with advanced solid tumors. Cancer Discov. 2017;7(7):704–715. doi:10.1158/2159-8290.CD-16-1080
27. Jin G, Kim MJ, Jeon HS, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer. 2010;69(3):279–283. doi:10.1016/j.lungcan.2009.11.012
28. Ali K, Soond DR, Pineiro R, et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–411. doi:10.1038/nature13444
29. Cidado J, Park BH. Targeting the PI3K/Akt/mTOR pathway for breast cancer therapy. J Mammary Gland Biol Neoplasia. 2012;17(3–4):205–216. doi:10.1007/s10911-012-9264-2
30. Zhao R, Chen M, Jiang Z, et al. Platycodin-D induced autophagy in non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathways. J Cancer. 2015;6(7):623–631. doi:10.7150/jca.11291
31. Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137(2):481–492. doi:10.1083/jcb.137.2.481
32. Kiyokawa E, Takai S, Tanaka M, et al. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994;54(14):3645–3650.
33. Bravo-Sagua R, Parra V, Lopez-Crisosto C, Diaz P, Quest AF, Lavandero S. Calcium transport and signaling in mitochondria. Compr Physiol. 2017;7(2):623–634. doi:10.1002/cphy.c160013
34. Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–6418. doi:10.1038/onc.2008.308
35. Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793(6):959–970. doi:10.1016/j.bbamcr.2008.12.003
36. Hockenbery DM, Zutter M, Hickey W, Nahm M, Korsmeyer SJ. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991;88(16):6961–6965. doi:10.1073/pnas.88.16.6961
37. Rong YP, Bultynck G, Aromolaran AS, et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc Natl Acad Sci U S A. 2009;106(34):14397–14402. doi:10.1073/pnas.0907555106
38. Chen R, Valencia I, Zhong F, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166(2):193–203. doi:10.1083/jcb.200309146
39. Hanson CJ, Bootman MD, Distelhorst CW, Wojcikiewicz RJ, Roderick HL. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium. 2008;44(3):324–338. doi:10.1016/j.ceca.2008.01.003
40. Leone V, Langella C, Esposito F, et al. Ccdc6 knock-in mice develop thyroid hyperplasia associated to an enhanced CREB1 activity. Oncotarget. 2015;6(17):15628–15638. doi:10.18632/oncotarget.3858
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.