Back to Journals » Biologics: Targets and Therapy » Volume 15
Baculoviruses in Gene Therapy and Personalized Medicine
Authors Schaly S, Ghebretatios M , Prakash S
Received 26 November 2020
Accepted for publication 22 February 2021
Published 28 April 2021 Volume 2021:15 Pages 115—132
DOI https://doi.org/10.2147/BTT.S292692
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Shein-Chung Chow
Sabrina Schaly, Merry Ghebretatios, Satya Prakash
Biomedical Technology and Cell Therapy Research Laboratory, Department of Biomedical Engineering, Faculty of Medicine, McGill University, Montreal, Quebec, H3A 2B4, Canada
Correspondence: Satya Prakash
Biomedical Technology and Cell Therapy Research Laboratory Department of Biomedical Engineering, Faculty of Medicine, McGill University, 3775 University Street, Montreal, Quebec, H3A 2B4, Canada
Tel +1 514 398 3676
Fax +1 514 398 7461
Email [email protected]
Abstract: This review will outline the role of baculoviruses in gene therapy and future potential in personalized medicine. Baculoviruses are a safe, non-toxic, non-integrative vector with a large cloning capacity. Baculoviruses are also a highly adaptable, low-cost vector with a broad tissue and host tropism due to their ability to infect both quiescent and proliferating cells. Moreover, they only replicate in insect cells, not mammalian cells, improving their biosafety. The beneficial properties of baculoviruses make it an attractive option for gene delivery. The use of baculoviruses in gene therapy has advanced significantly, contributing to vaccine production, anti-cancer therapies and regenerative medicine. Currently, baculoviruses are primarily used for recombinant protein production and vaccines. This review will also discuss methods to optimize baculoviruses protein production and mammalian cell entry, limitations and potential for gene therapy and personalized medicine. Limitations such as transient gene expression, complement activation and virus fragility are discussed in details as they can be overcome through further genetic modifications and other methods. This review concludes that baculoviruses are an excllent candidate for gene therapy, personalized medicine and other biotherapeutic applications.
Keywords: baculovirus, gene therapy, personalized medicine
Introduction to Gene Therapy Using Viral Vectors
Gene therapy can adapt to each person to treat a variety of illnesses including cancer, rare diseases, and to promote wound repair. Currently, adeno-associated vectors, lentivirus, and retrovirus have been successfully implemented accounting for 19 FDA approved gene therapy products.1 Nine patients infused with AAV5-hFVIII-SQ, an adeno-associated vector serotype 5 (AAV5) that delivers exogenous factor VIII, were cured of Hemophilia B.2 This novel gene delivery system effectively treats Hemophilia A by producing blood-clotting proteins leading to fewer bleeding issues and cured patients with Hemophilia B. However, AAV vectors are difficult to scale-up and have been associated with toxicity and inflammation limiting their use in gene therapy.3 Comparatively, the use of a lentiviral vector for gene transfer cured a young boy of sickle cell anemia.4 While retroviral transduction of COL7A1 cDNA cured dystrophic epidermolysis bullosa by restoring C7 synthesis encoded by OL7A1 cDNA without host integration.5 However, lentiviral and retroviral vectors have limitations such as a low cloning capacity and integration into the host genome creating the potential for insertional mutagenesis. Moreover, there are potential safety concerns for the development of replication-competent retroviruses.6 The high cost, low scalability and biosafety concerns associated with current viral vectors, outlined in Table 1, highlight the large potential use of baculoviruses in gene therapy. Baculoviruses provide a relatively safe, scalable, and cost-effective vector for gene therapy.7
 |
Table 1 Viral Vector Comparison for Gene Therapy |
Baculoviruses in Gene Therapy
Baculoviruses, naturally known to infect Lepidoptera, have been exploited for their recombinant protein expression since 1983, enabling the development of a diverse range of therapeutics.8 Baculovirus gene delivery systems enable site-specific delivery, mitigating adverse effects, and improving therapeutics.9 This easily modifiable gene therapy system may be the cost-effective and efficient backbone needed for gene therapy. Following genomic sequencing of the individual, baculoviruses can be used to deliver the deficient genes or promote a proper biological response. Baculovirus vectors have already been implemented in several successful studies including cancer treatment, vaccines and regenerative medicine demonstrating their potential.10–12 The diverse applicable use of baculoviruses generates a promising future for personalized medicine and gene therapy. Here we review the mechanism of baculovirus gene therapy and focus on optimizing it for individual treatments.
Biphasic Infection Cycle of Baculoviruses
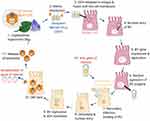
There are several types of baculoviruses that possess a high specificity to their natural insect hosts such as arthropods and Lepidoptera. Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) and Bombyx mori MNPV (BmMNPV) strains, ranging from 80–180 kbp, are the most extensively studied in gene therapy.13,14 During baculovirus transcription and replication there are three main phases termed early, late, and very late. The early phase commences upon attachment, injection of the viral genome, uncoating, viral gene expression, and finally halting host transcription. Host transcription factors recognize and transcribe early viral genes within 0.5 to 6 hours post-infection.15 The activation of these genes allows for DNA synthesis and late gene production which are mostly structural proteins.15 During the late phase, the nucleocapsid structural protein with gp64 is produced enabling horizontal infection.16 The nucleocapsid then interacts with the nuclear membrane and becomes enveloped. Finally, viral promoters, polyhedrin and p10, are transcribed and hyper-expressed.17 The polyhedron then crystalizes around ODV forming occlusion bodies that fill the nucleus and fibrillar structures.17 Meanwhile, viral proteins, chitinase and cathepsin, assist with host cuticle breakdown.18 This cycle continues until there are many occlusion bodies (OBs) causing the insect to liquefy and rupture. The OBs account for 30% of an infected larvae’s dry weight, and 25% of the cell protein produced is polyhedral capsules.19,20 This large and natural amplification feature makes baculoviruses an attractive potential for gene therapy where large scale gene production is necessary. The potential exploitation of the baculovirus life cycle for gene therapy can be seen in Figure 1. Following insect cell replication, the baculovirus vectors can be purified from the culture supernatant using heparin affinity chromatography.21 Purification concentrates the extracted baculovirus by 500-fold with a 25% infectious particle recovery rate. This can be scaled-up in a closed-system suspension culture generating sufficient clinical-grade vector levels for gene therapy.21 Alternative methods of purification include size-exclusion chromatography, monolithic ion-exchange chromatography, ion-exchange membrane chromatography, high-speed batch centrifugation, sucrose gradient centrifugation, and tangential flow ultrafiltration.
Baculoviruses as Gene Delivery Systems
Upon the discovery that baculoviruses could transduce mammalian cells, their therapeutic potential has rapidly expanded.22 The viral genome has since been modified and manipulated to improve the transduction efficiency and ease of production. Correspondingly, several vector systems have been developed including BacMam, Bac-to-Bac, MultiBac, and derivatives of these AcMNPV transfer vectors.23–25
BacMam Systems
For foreign genes to be expressed, the viral or mammalian promoter must be recognized. Viral promoters p10 and polyhedrin have been most commonly used to promote transcription due to their high expression activity.14,26 However, a mammalian promoter can also be used to drive heterogeneous gene expression following viral transduction, termed a BacMam.23 BacMam’s can support gene insertions up to 40 kb but have a transient expression of four days without a selection force. Some mammalian promoters used to initiate gene transcription include Rous-sarcoma virus long terminal repeats (RSV-LTR), cytomegalovirus (CMV), simian virus 40 (SV40), chicken beta-actin (CAG), hepatitis B virus (HBV), human a-fetoprotein/ubiquitin C promoter, and drosophila heat shock protein 70 (hsp70) promoter.27 Viral and mammalian promoters can be used in conjugation with genomic enhancers to promote transgene transcription. Specifically, the insertion of an additional homologous region 1 (hr1) into baculoviruses has been used to activate mammalian promoters and results in improved stability, overexpression of the transgene, and prolonged transgene expression.13 A dual expressing BacMam vector (BV-Dual-s1) has since been produced. This system fuses s1 glycoprotein of avian infectious bronchitis virus with AcMNPV gp64 glycoprotein displaying the S1-gp64 on the viral surface.28 Moreover, vesicular stomatitis virus G (VSVG) glycoprotein has been incorporated under p10 promoter control allowing for viral surface display, enhanced transduction, and prolonged expression.26 However, this system can induce a strong humoral and cell-mediated immunity. The BacMam system also led to the development of BacMaM derivatives such as pFastBac1 and pFastBacmam.29 Specifically, pFASTBacMam-1 is driven by an SV40 promoter and a neomycin resistance marker, which allows for stable cell line selection after BacMam transduction.29 Promoter selection facilitates transcription and permits more strict controls over transgene expression.
Homologous Recombination and Transposition
Recombinant baculoviruses (rBVs) were first generated using homologous recombination in insect cells. This led to the development of the Bacmid system which uses bacterial artificial chromosomes containing E. coli fertility factor replicon maintained as a circular supercoiled extrachromosomal single-copy plasmid.23 The Bacmid system can accept 300 Kb gene inserts and can be modified using site-specific recombination.23 Homologous recombination can also delete background parental genes while repairing an essential gene like the orf1629 gene, essential for viral replication, or p10 genes allowing for purification.30,31 However, this technique only has a 1% transduction efficiency.32 This led to the development of flashBAC.33 The flashBAC method contains a partially deleted orf1629 gene so that homologous recombination can restore orf1629’s function while eliminating bacterial sequences.33 Only rBVs have a functional orf1629 gene and can replicate allowing for easier purification. Other baculovirus genes have also been eliminated to improve foreign protein quality and yield.
New methods using primarily transposition also improved transduction efficiency. One of the first and most used systems is the Bac-to-Bac system.30 This system consists of three antibiotic selection markers (ampicillin, kanamycin, and gentamycin) and an intermediary transfer plasmid to insert foreign genes via targeted transposition. Specifically, Tn7-mediated site-specific transposition in E. coli is used to direct cassette integration and expression producing recombinant baculoviruses.30 This is still the only system that generates 100% pure recombinant baculoviruses (rBVs) without further purification. A similar system, Bac-2-the-Future (B2F), was developed based upon this Tn7 transposition method.24 However, the gentamycin resistance marker was replaced with pDP1381 reducing the number of false positives and vector size.24 These baculovirus systems provide the bases for site-specific gene delivery, within personalized medicine, compared to the standard systemic administration of common drugs.
Enhancing Insect Cell Baculovirus Production for Gene Therapy
Baculovirus production can be enhanced in insect cells by altering the chromatin state and media supplements. A more relaxed chromatin state facilitates accessibility for more efficient transcription. Sodium butyrate, trichostatin A and valproic acid all induce histone acetylation promoting chromatin accessibility and transgene expression.29,34 Similarly, histone deacetylation inhibitors induce histone hyperacetylation, relaxing the chromatin structure, and improving gene transcription and delivery.35 Media supplements also affect baculovirus transgene expression. Monteiro et al demonstrated that the addition of cholesterol to the media results in a 2.5-fold increase in baculovirus production and a 6-fold increase in virus-like particle (VLP) production.36 Similarly, the addition of glutathione, antioxidants, and polyamines resulted in a 3-fold increase in baculovirus production.36 These simple yet effective modifications can significantly enhance the efficiency and feasibility of baculovirus production for gene therapy.
Post-Translational Modifications Using Baculovirus Expression Vector Systems (BEVS)
A large advantage to BEVS is that they naturally generate proteins with proper phosphorylation and post-translational modification.37 Human-like glycosylation can also easily be achieved through genetic engineering enabling efficient treatment between individuals.38 Specifically, the N-terminal signal peptides are essential for directing the protein destination and fate. Native baculovirus signal peptides can be replaced by insect proteins like honeybee melittin or baculovirus proteins like gp64 to alter the protein fate.39,40 However, the difference in protein glycosylation between lepidopteran and higher eukaryotes can affect protein folding, degradation, location, and immunological response.38 N-glycosylation in insects also involves the transfer of preassembled oligosaccharide (Glucose3Mannose9N-acetylglucosamine2) from a lipid complex to an aspartate residue in the endoplasmic reticulum (ER) lumen.38 The protein then moves from the ER to the Golgi where enzymes trim and add sugar moieties to the glycan molecules. Comparatively, mammalian cells differ in that complex sugars with terminal sialic acids are added instead of sugar moieties. This led to the development of Sf9 and High five cells which encode bovine β-1, 4-galactosyl transferase and rat α-2, 6-sialyltransferase which enable proper addition of galactosyl and sialyl into proteins.37,41 Recently, Moremen et al developed an expression vector library encoding all known human glycosyltransferases, glycoside hydrolases and other glycan-modifying enzymes to enable proper glycosylation disease and person-specific use.42
Other baculovirus modifications for optimal human use include gene deletions or insertions to prevent proteolytic cleavage or assist with protein folding. Specifically, genes such as chitinase and cathepsin, responsible for breaking down the insect cuticle, are not necessary for human therapeutic applications and can be replaced with genes of interest.31 Beneficially, the deletions of both of these bacculovirus genes results in increased levels of transgene proteins and ensures the transmission of viral occlusion bodies.18 Chaperone proteins often assist with protein modification, directing location and folding which corresponds to function. Cytosolic chaperones, like hsp70 and hsp40, prevent polypeptide aggregation and can be incorporated into the baculovirus genome to promote proper protein folding.43 Similarly, other chaperones such as binding immunoglobin protein, calnexin, calreticulin and protein disulfide isomerase can all assist with folding proteins produced from BEVS.44,45 A list of modifications that can enhance BEVS protein production, for therapeutic use, is outlined in Table 2.
 |
Table 2 Enhancing Insect Cell Baculovirus Production |
Enhancing Baculovirus Cell Entry for Gene Therapy
An essential step for gene delivery is the ability of the viral vector to enter the intended cell type. Advantageously, baculoviruses are capable of transducing both dividing and non-dividing cells. This includes common cell lines like HeLa, Huh-7, HepG2, bone marrow fibroblasts, PK1 cells, and human neural cells.8,46,47 However, transduction efficiency varies depending on cell type; 30% in undifferentiated human neural progenitor cells and 55% in differentiated cells.47 Specifically, gp-64 and heparan sulfate are required for mammalian cell entry.48,49 Several factors contribute to baculovirus production efficiency including cell type, chromatin state, promoter type, and protein expression. The ability of engineered baculoviruses to transduce specific mammalian cells reveals its potential for site-specific gene therapy and extension into personalized medicine.
Baculovirus Promoter Selection for Mammalian Cell Entry in Gene Therapy
Optimizing the virus’ method of cell entry and viral protein production is essential for therapeutic applications. Baculoviruses are capable of entering both permissive and nonpermissive cells, eliminating a common barrier to gene therapy.50 Specifically, the viral surface protein, gp64, is critical for efficient virus entry and endosomal escape in mammalian cells.51 The addition of another gp64 gene results in a 10 to 100-fold increase in reporter gene expression.39 Gp64 has also been fused to short peptide motifs of gp350/220 on Epstein-Barr virus (EBV) for enhanced gene delivery to B cells.52 Alternatively, co-expression of glycoproteins from thogotoviruses with gp64 improves virus-endosome fusion and endosomal escape resulting in a 4 to 12-fold increase in transduction efficiency.53 The high adaptability of baculoviruses elucidate its potential role in treating diseases in a person-specific manner.
Baculovirus Surface Modifications for Enhancing Transduction Efficiency
The addition of several other molecules to the surface of baculoviruses has also enhanced transduction efficiency. Some of these additions into the baculovirus envelope include VSVG, influenza virus neuraminidase, single-chain antibody fragment, Spodoptera exigua MNPV (SeMNPV) F protein, endogenous retrovirus, and single antibody chains.26,54–57 Specifically, Fc regions of antibodies enable antigen-presenting cells (APC) specificity.55 Similarly, the addition of VSVG demonstrated a 10 to 100-fold increase in transduction in human hepatoma and rat neuronal cells and broadened baculovirus tropism.58 VSVG has also been fused to tumor-homing peptides (LyP-1, F3, and CGKRK) on the baculovirus surface improving tumor binding 2-5-fold.59 Moreover, the strong attraction between avidin and biotin was exploited in avidin-displaying baculoviruses to increase transduction efficiency and correspondingly gene delivery.60 Chen et al fused a cytoplasmic transduction peptide to gp64 producing a cytoplasmic membrane penetrating baculovirus (vE-CTP).61 Simultaneously, the HIV Tat protein transduction domain was fused to the baculovirus’ capsid protein VP39 forming a nuclear membrane penetrating baculovirus (vE-PTD) improving transduction efficiency.61 Alternatively, cationic amino-functional poly (amidoamine) dendrimers complexed with baculoviruses enabled the binding of the cationic viral particles to the cell membrane.12 This strong interaction assisted with virus internalization and improved angiogenic vascular endothelial growth factor (VEGF) gene transfer and expression.12 Malaria proteins, three circumsporozoite protein variants and a thrombospondin-related anonymous protein, have also been added to the baculovirus envelope to enhance transduction efficiency in hepatocytes.62 Overall, the incorporation of diverse foreign proteins, into the baculovirus envelope, can be chosen to optimize transduction efficiency based on the disease and personalized needs.
Promoter Effect on Baculovirus Transgene Expression
As previously mentioned, the promoters used in baculovirus gene delivery systems can dictate transduction efficiency in gene therapy. The most commonly used viral promoters include polyhedron and p10. The fusion of heterologous genes at the 5ʹ end of the gp64 gene, placed under the control of the polyhedrin or p10 promoter, allows viral envelope incorporation. Other viral promoters include p6.9, viral promoter 39, immediate early gene (IE1) promoter, and pB2, which have improved expression levels, particularly in early phases.63,64 Comparatively, in human mesenchymal cells, often the focus of regenerative medicine, human cytomegalovirus, ubiquitin C, phosphoglycerate kinase, and elongation factor-1 alpha (EF1α) promoters have been incorporated into the Bac-to-Bac system.65 Particularly, EF1α demonstrated the highest transgene expression indicating the efficiency of the promoter is largely dependent upon cell type and more importantly revealing the potential for stem cell gene therapy. Moreover, promoters can be used in combination with transcriptional enhancers to increase transgene expression. For example, Gwak et al generated a baculovirus expression system with p6.9 promoter and transcriptional enhancers, homologous region 3 and repeated burst sequences, resulting in a 94-fold increase in foreign gene expression.66 Moreover, the stage of promoter expression can also alter gene expression. A 20-fold increase in transgene expression can be achieved using a very late promoter compared to an early promoter, in Drosophila melanogaster.50 The numerous combinations of viral and mammalian promoters enable adaptability and customization within baculovirus gene delivery.
Prolonging Baculovirus Transgene Expression for Gene Therapy
rBVs have a relatively short transgene expression window of 7–14 days which can be optimized or extended based on the disease.67 Specifically, baculoviruses activate both the classical and alternative complement pathway leading to viral degradation and transient gene expression.68 Several methods have been employed to prevent complement activation and prolong gene expression. Activation of the alternative and classic complement pathway can be prevented through the display of decay-accelerating factor (DAF), factor H-like protein-1, C4b-binding protein, and membrane cofactor protein on the baculovirus envelope.69,70 Another study concluded that fusion of cluster of differentiation 46 and 59 with DAF (CD46-DAF-CD59) provides complement protection in HepG2 cells.71 Alternative envelope displays include VSVG, complement antibody C5, cobra venom factor, soluble, complement inhibitor I, compstatin and complement regulatory proteins.26,51,68 Moreover, Liu et al recently demonstrated that the BmNPV vector is more stable in human serum than AcMNPV.72 Hindering complement activation, through the above-mentioned methods, can effectively prolong gene expression and dampen the associated immune response for personalized approaches. Alternatively, the short baculovirus gene expression can be optimized for wound repair whereas genetically prolonged gene expression can be beneficial in anticancer therapy.
The addition of proteins onto the baculovirus envelope can be optimized for each individual and therapeutic use. Specifically, the insertion of VSVG extended gene expression to 178 days in DBA/2J mice and 35 days in BALC/c mice.26 Moreover, the incorporation of vankaryin (an anti-apoptotic gene) into a baculovirus vector increased cell viability and length of protein production.73 Similarly, BV-AAV hybrids have shown promise whereby gene expression lasted 90 days in rat brains.74 Similarly, Luo et al constructed a baculovirus with inverted terminal repeats (ITRs), the origin of plasmid replication (oriP)/EBV-expressed nuclear antigen 1 (EBNA1) and Sleeping Beauty (SB) transposon.75 They found that the SB system enabled gene expression for 77 days without antibiotic selection.75 Moreover, the incorporation and expression of an antiangiogenic fusion protein comprising endostatin and angiostatin (hEA) inhibited prostate and human ovarian xenograft tumor growth.75 More recently, Wang et al generated a bivalent hybrid baculovirus that displayed DAF and eGFP mediated by SB transposon system which prolonged the expression of hEA genes to 90 days.76 Moreover, the hEA genes exhibited antitumor effects in hepatocellular carcinoma xenograft mouse models as well as complement resistance.76 Alternatively, two baculovirus vectors have been used to generate a self-replicative episome providing constant gene expression for 48 days.77 Here, one vector encoding flippase recombinase cleaves and activates the other encoding oriP/EBNA1 from EBV and gene of interest within the Frt flanking region.77 Alternatively, viral components can be combined with non-viral such as fibrin gels to further prevent bleeding and promote wound healing. Previously, fibrin gels and BacMam-mediated gene delivery modulated gene release, enhanced transduction efficiency and prolonged gene expression in vivo.78 Methods of baculovirus optimization for gene therapy are described in Table 3, below.
 |
Table 3 Optimizing Baculoviruses in Mammalian Cells for Gene Therapy |
Optimizing Therapeutic Protein Production using Baculovirus Experession Vector Systems (BEVS)
With the basis of BEVS established, more systems worked on improving protein quality and yield for therapeutics. Top-Bac was able to increase protein yield by 300%.80 Top-Bac uses several promoters some of which are hybrid sequences formed from late and very late AcMNPV genes. Moreover, Steele et al were able to generate a cell line with vankryin directly incorporated improving yield.73 Several other studies have looked into the genetic makeup of baculoviruses to better understand which genes can be manipulated or even removed. It was found that the combination of PCR and transformation-associated recombination, in yeast, generated a synthetic baculovirus genome based upon AcMNPV (AcMNPV-WIV-Syn1).81 The synthetic baculovirus omitted baculovirus genes enhancing recombinant protein production.81
Multi-Complex Protein Synthesis for Gene Therapy
Another barrier to viral gene therapy is the complexity and cooperation of native proteins. Beneficially, the large cloning capacity of BEVS allows for the production of several proteins or complex structures like virus-like particles (VLPs). Berger et al incorporated an array of small synthetic DNA plasmids termed acceptors and donors.25 The acceptors can be loaded with several genes to produce eukaryotic protein complexes with many subunits, termed MultiBac.25 This system enabled the discovery, understanding and treatment of complex molecules which was previously inaccessible. Similarly, Weissmann et al were able to assemble a rBV producing 25 individual genes in just 6 days.82 This method uses Gibson assembly reaction along with concepts from MultiBac earning the name biGBac.82 Comparatively, Zhang et al used a Uracil-specific Excision Reagent ligation-free cloning method.28 This enabled the targeted expression of multi-subunit anaphase-promoting complex within MultiBac, under the polyhedrin or chitinase gene loci, producing 13 proteins.28 The expression of multi-complex or multi-subunit proteins is essential for proper protein function and can be tailored to each individual’s treatment providing a functional pathway, not just a protein.
Advantageously, the large cloning capacity of baculoviruses allows for large gene insertions (proteins, viral particles and more). The prolonged gene expression of AAV vectors can be combined in BEVS to prolong transgene expression. The first recombinant AAV (rAAV) treatment, derived from baculoviruses, successfully treated familial lipoprotein lipase deficiency (LPLD), Glybera.83 Although successful, the large $1-million cost led to the treatment’s withdrawal from the market. OneBac appears to be a more affordable option by using a stable insect Sf9 cell line with silent copies of inducible AAV1012 Rep and Cap genes.84 The combination of AAV vectors with OneBac increases the yield of genomic particles and functional particles by 6-fold and 20-fold, respectively.85 Similar beneficial results were seen in hypopharyngeal carcinoma gene therapy where Bac-Adeno-Associated viral vectors with Luc-P2A-eGFP or sodium iodide symporter (NIS), under CMV promoter control, infected bone marrow mesenchymal cells (BMSCs).86 The BMSCs effectively took up radioactive iodine demonstrating its potential to act as a targeted-delivery vehicle in mice.86 More recently, Wu et al developed a new combination vector using ribosome leaky-scanning to express AAV Rep and Cap proteins downstream polh and p10 promoters, respectively.87 The rAAV genome can be inserted between two Bac promoters yielding 105 vector rAAV2/8/9 genomes from Sf9 baculovirus-infected cells.87 This indicated that BEVS may be suitable for large-scale rAAV production as well as targeted cell therapy. This is particularly useful in treating diseases like cancer with high heterogeneity.
Application of Baculoviruses in Gene and Other Therapies
Baculoviruses can also be exploited within vaccines and treatments for immune diseases through immunological modifications. Cytoplasmic sensors like retinoic acid-inducible gene 1 (RIG-1) and melanoma differentiation-association protein 5 (MDA5) recognize dsRNA activating the interferon-beta promoter stimulator (IPS-1) mediated signal pathway resulting in interferon type 1 (IFN-1) production.34 This is accompanied by activation of toll-like receptors 3/7/9 which are endosomal sensors that recognize viral DNA, RNA and intermediate RNA, respectively.34 This leads to the activation of IRF3/7 and NF-kβ (nuclear factor kappa light chain enhancer of activated B cells) in macrophages and dendritic cells.34 Ultimately this leads to the production of IFN-1, inflammatory cytokines, and inflammatory chemokines, all of which promote inflammation, and viral DNA degradation. This immune activation can be exploited in vaccine candidates providing a safe, personalized and scalable vector.
Moreover, the incorporation of foreign proteins into the baculovirus envelope or nucleocapsid core can be used in gene therapy. Baculovirus proteins expressed on the viral surface or nucleocapsid core can elicit a humoral immune response or activate MHC I leading to activation of CD8+ T cells, respectively.88,89 Baculovirus surface peptide display demonstrated a strong adjuvant activity protecting against lethal viruses like influenza and encephalomyocarditis.34,90 Influenza immunity has been induced by Hemagglutinin (HA) expression on baculovirus using Bmg64HA HA fragment of H5N1 fused to the gp64 gene.91 Alternatively, baculoviruses can be used for VLP production like in severe acute respiratory syndrome (SARS), human immunodeficiency virus (HIV), Sudan virus, Ebola virus, Marburg virus, rabbit hemorrhagic disease virus (RHDV) and Rous sarcoma virus.92–97 More recently, Hinke successfully constructed a BEVS with a recombinant 65 kDa glutamate decarboxylate, Diamyd, to treat type 1 diabetes.98 Evidently, BEVS surface display and VLP production can be customized for personalized vaccines and treating heterogeneous diseases.
The display of surface proteins can also direct cell-specific uptake of baculoviruses. Currently, Fc receptors, folate, and epidermal growth factor (EGF) have been used to dictate baculovirus selectivity.99 Räty et al exploited the avidin-biotin interaction to increase transduction efficiency while expressing biotinylated EGF causing the system to target EGF displaying cells.60 Polyethylene glycol (PEG)-folate has also been displayed on the baculovirus surface to target the Fc receptors displayed specifically on malignant cells enabling targeted gene delivery.100 In comparison, rBVs displaying human epidermal growth factor-2 (HER2) single-chain variable domain fragments (scFV) while expressing Apoptin bind specifically HER2 positive SK-BR-3 breast cancer cells reducing cancer cell viability.101 Similarly, a rBV expressing BIMs, a strong apoptosis inducer, resultedin selective death of HCV-positive cells only further proving BV’s potential for selective gene therapy.102 The selective treatment of an individual’s malfunctioning or impaired cells can mitigate the systemic and adverse effects seen in traditional medical treatments, significantly improving the quality of treatment, care, and life. Consequently, baculoviruses can be exploited in regenerative medicine (Table 4), anti-cancer treatments (Table 5), and vaccine vectors.
 |
Table 4 Baculoviruses in Therapeutics and Regenerative Medicine |
 |
Table 5 Baculoviruses in Cancer Treatment |
Baculovirus Expression Vector System (BEVS)
The large cloning capacity of baculoviruses enables transgene expression of large multi-complex proteins both in vivo and ex vivo. This is particularly useful for use in anticancer therapy, stem cell regeneration and in vaccine development. Specifically, a toxin vector for diphtheria toxin A has been developed to eliminate malignant glioma cells within the brain.106 Other rBVs expressing normal epithelial cell specific-1 and herpes simplex virus-1 thymidine kinase have shown similar promising results in eliminating glioblastoma and gastric cancer cells.107,108 Moreover, angiogenesis-dependent tumours have been treated with a hybrid SB-Baculovirus vector to prolong antiangiogenic fusion protein expression (endostatin and angiostatin).75 Lin et al engineered bone marrow-derived mesenchymal cells (BMSCs) to express bone morphogenetic protein 2 and VEGF enabling enhanced femoral bone repair and bone quality.109 Similarly, for myocardial infarction therapy, baculoviruses can be engineered to expressed Angiopoietin-1 to increase capillary density,reduce infarct sizes and other clinically fevaourable conditions in experimental rats.110
rBVs also have a large potential in VLP and vaccine production. One of the first vaccines using baculoviruses, called FluBlok, used the HA antigen as a subunit vaccine to elicit a protective immune response.29 This technique has been extended into other vaccines such as human papillomavirus, prostate cancer and familial lipoprotein lipase deficiency.10,111,112 The three vaccines expressed HPV-L1 protein, granulocyte macrophage colony-stimulating factor and an AAV vector with lipoprotein lipase transgene, respectively. Moreover, the administration of baculoviruses was capable of eliminating malaria parasite in mice liver and eliciting a protective humoral and cellular immune response.113 The scalability of BEVS are beneficial for mass production of molecules like VLPs. It is predicted that baculoviruses are capable of generating 415 million 10 µg/dose vials of anti-flu vaccines in one week compared to the 6 months standard using chicken embryos.114 The high protein production and efficacy supports the use of baculoviruses as a promising vaccine vector and scalable approach to personalized medicine. Current vaccines involving baculoviruses are included in Table 6, below.
 |
Table 6 Baculoviruses in VLP Production and Vaccines |
Baculoviruses; Limitations and Future Outlooks
There are a few limitations associated with baculovirus in gene therapy, hindering its wide-scale use and production. Specifically, BEVS can induce an immune response producing inflammatory cytokines and chemokines and activating the complement pathway. This can lead to an unnecessary immune response and viral genome degradation if used for non-vaccination purposes. Upon serum contact baculoviruses activate RIG-I/IPS-1 or cyclic GMP-AMP synthase/stimulator of interferon genes (cGAS/STING) pathway which can suppress transgene expression.130 Moreover, baculoviruses exhibit transient gene expression. Without selection, gene expression typically lasts 7–14 days in most cell lines, including CHO, HeLa and BHK.67 However, several gene insertions or modifications have been able to extend gene expression and prevent complement recognition.75,77,131 Transgene expression can also be prolonged by shielding the baculovirus from the immune system using a polymer coating. This prevents immune activation and prolongs gene expression and its associated therapeutic effect. Alternatively, the transient gene expression mitigates safety concerns providing potential in vaccine vector or adjuvant field. Another limitation of baculovirus vector systems is the virus fragility. The half-life of the virus is only 173 hours at 27°C and 7–8 hours at 37°C.44 Moreover, defective interfering (DI) particles accumulate during serial cell culture passages. The amount of DI particles can be reduced by using a low MOI or by removing the non-hr origin from the SeMNPV baculovirus genome preventing DI formation for 20 cell passages.132
Future outlooks of baculoviruses in therapeutics are exciting and very promising. This potential has been recently recognized worldwide such as in project Baculogene. This project focuses on developing methods for large-scale production, downstream processing, purification and analysis methods for direct baculovirus applications in gene therapy. More recently, baculoviruses have been used in four pre-clinical COVID-19 vaccines, highlighting its use and adaptability. Specifically, baculoviruses were used to produce viral S protein and receptor binding domain protein in three subunit vaccine candidates as well as for VLP production in the fourth vaccine.133 The ease of genetic manipulations to extend transgene expression, prevent complement recognition, improve transduction efficiency, increase protein yield, and include several proteins at once, promote the feasibility and implementation of personalized medicine. This simple yet cost-effective scale-up method can be used to produce the exact dose and customized based on the genetic information of each individual.
Conclusion
Baculoviruses have excellent therapeutic potential in a number of diseases. They have been sucessfully used in vaccine industry, anticancer therapy, and recombinant protein productions. Their associated limitations may be quickly overcome through further genetic engineering and other methods. Moreover, the relative ease of production, non-replicative nature in mammalian cells, large gene(s) pay load, stability of the genes, advanced delivery features, and other methods continue to make them ideal for gene therapy, personalized medicine and other applications. Baculoviruses have a large potential to be optimized for each disease and individual through targeted gene and dose modifications. The simple production, protein extraction, and easy manipulation of insect cells provide the cost-effective method needed to advance gene therapy and personalized medicine.
Acknowledgments
This work is supported by the Canadian Institute of Health Research (CIHR) (grant # 252743). The figure was created using biorender.com
Disclosure
The authors report no conflicts of interest.
References
1. Shahryari A, Saghaeian Jazi M, Mohammadi S, et al. Development and clinical translation of approved gene therapy products for genetic disorders. Front Genet. 2019;10:868. doi:10.3389/fgene.2019.00868
2. Rangarajan S, Walsh L, Lester W, et al. AAV5–factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519–2530. doi:10.1056/NEJMoa1708483
3. Hinderer C, Katz N, Buza EL, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. 2018;29(3):285–298. doi:10.1089/hum.2018.015
4. Kanter J, Walters MC, Hsieh MM, et al. Interim results from a Phase 1/2 clinical study of lentiglobin gene therapy for severe sickle cell disease. Blood. 2016;128(22):1176. doi:10.1182/blood.V128.22.1176.1176
5. Nyström A, Bruckner-Tuderman L. Gene therapy for epidermolysis bullosa: sticky business. Mol Ther. 2016;24(12):2035–2036. doi:10.1038/mt.2016.199
6. Vanin EF, Kaloss M, Broscius C, Nienhuis AW. Characterization of replication-competent retroviruses from nonhuman primates with virus-induced T-cell lymphomas and observations regarding the mechanism of oncogenesis. J Virol. 1994;68(7):4241–4250. doi:10.1128/JVI.68.7.4241-4250.1994
7. Kost TA, Condreay JP. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 2002;20(4):173–180. doi:10.1016/S0167-7799(01)01911-4
8. Volkman LE, Goldsmith PA. In vitro survey of autographa californica nuclear polyhedrosis virus interaction with nontarget vertebrate host cells. Appl Environ Microbiol. 1983;45(3):1085–1093. doi:10.1128/AEM.45.3.1085-1093.1983
9. Paul A, Elias CB, Shum-Tim D, Prakash S. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci Rep. 2013;3(1):2366. doi:10.1038/srep02366
10. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi:10.1158/1078-0432.Ccr-10-3126
11. Monie A, Hung C-F, Roden R, Wu TC. Cervarix: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics. 2008;2(1):97–105.
12. Muzzarelli RAA, El Mehtedi M, Bottegoni C, Aquili A, Gigante A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar Drugs. 2015;13(12):7314–7338. doi:10.3390/md13127068
13. Pijlman GP, van den Born E, Martens DE, Vlak JM. Autographa californica baculoviruses with large genomic deletions are rapidly generated in infected insect cells. Virology. 2001;283(1):132–138. doi:10.1006/viro.2001.0854
14. Ishiyama S, Ikeda M. High-level expression and improved folding of proteins by using the vp39 late promoter enhanced with homologous DNA regions. Biotechnol Lett. 2010;32(11):1637–1647. doi:10.1007/s10529-010-0340-7
15. Hefferon KL, Miller LK. Reconstructing the replication complex of AcMNPV. Eur J Biochem. 2002;269(24):6233–6240. doi:10.1046/j.1432-1033.2002.03342.x
16. Whitford M, Stewart S, Kuzio J, Faulkner P. Identification and sequence analysis of a gene encoding gp67, an abundant envelope glycoprotein of the baculovirus Autographa californica nuclear polyhedrosis virus. J Virol. 1989;63(3):1393–1399. doi:10.1128/JVI.63.3.1393-1399.1989
17. Van Der Wilk F, Van Lent JWM, Vlak JM. Immunogold detection of polyhedrin, p10 and virion antigens in Autographa californica nuclear polyhedrosis virus-infected Spodoptera frugiperda cells. J Gen Virol. 1987;68(10):2615–2623. doi:10.1099/0022-1317-68-10-2615
18. Hawtin RE, Zarkowska T, Arnold K, et al. Liquefaction of Autographa californica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology. 1997;238(2):243–253. doi:10.1006/viro.1997.8816
19. Miller LK, Lingg AJ, Bulla LA. Bacterial, viral, and fungal insecticides. Science. 1983;219(4585):715–721. doi:10.1126/science.219.4585.715
20. Adang MJ, Miller LK. Molecular cloning of DNA complementary to mRNA of the baculovirus Autographa californica nuclear polyhedrosis virus: location and gene products of RNA transcripts found late in infection. J Virol. 1982;44(3):782–793. doi:10.1128/JVI.44.3.782-793.1982
21. Nasimuzzaman M, van der Loo JCM, Malik P. Production and purification of baculovirus for gene therapy application. J Vis Exp. 2018;134:57019. doi:10.3791/57019
22. Via ST, Zu Altenschildesche GM, Doerfler W. Autographa californica nuclear polyhedrosis virus (AcNPV) DNA does not persist in mass cultures of mammalian cells. Virology. 1983;125(1):107–117. doi:10.1016/0042-6822(83)90067-3
23. Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci. 1996;93(6):2348–2352. doi:10.1073/pnas.93.6.2348
24. Mehalko JL, Esposito D. Engineering the transposition-based baculovirus expression vector system for higher efficiency protein production from insect cells. J Biotechnol. 2016;238:1–8. doi:10.1016/j.jbiotec.2016.09.002
25. Berger I, Garzoni F, Chaillet M, Haffke M, Gupta K, Aubert A. The multiBac protein complex production platform at the EMBL. J Vis Exp. 2013;77:e50159–e50159. doi:10.3791/50159
26. Pieroni L, Maione D, La Monica N. In vivo gene transfer in mouse skeletal muscle mediated by baculovirus vectors. Hum Gene Ther. 2001;12(8):871–881. doi:10.1089/104303401750195845
27. Fabre ML, Arrías PN, Masson T, Pidre ML, Romanowski V. Baculovirus-derived vectors for immunization and therapeutic applications. Emerging and Reemerging Viral PathogensAcademic Press Elsevier; 2020:197–224.
28. Zhang Z, Yang J, Barford D. Recombinant expression and reconstitution of multiprotein complexes by the USER cloning method in the insect cell-baculovirus expression system. Methods. 2016;95:13–25. doi:10.1016/j.ymeth.2015.10.003
29. Condreay JP, Witherspoon SM, Clay WC, Kost TA. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc Natl Acad Sci. 1999;96(1):127–132. doi:10.1073/pnas.96.1.127
30. Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67(8):4566–4579. doi:10.1128/JVI.67.8.4566-4579.1993
31. Kaba SA, Salcedo AM, Wafula PO, Vlak JM, van Oers MM. Development of a chitinase and v-cathepsin negative bacmid for improved integrity of secreted recombinant proteins. J Virol Methods. 2004;122(1):113–118. doi:10.1016/j.jviromet.2004.07.006
32. Smith GE, Fraser MJ, Summers MD. Molecular engineering of the Autographa californica nuclear polyhedrosis virus genome: deletion mutations within the polyhedrin gene. J Virol. 1983;46(2):584–593. doi:10.1128/JVI.46.2.584-593.1983
33. Hitchman RB, Possee RD, King LA. High-throughput baculovirus expression in insect cells. Methods Mol Biol. 2012;824:609-27. doi:10.1007/978-1-61779-433-9_33
34. Abe T, Kaname Y, Wen X, et al. Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner. J Virol. 2009;83(15):7629–7640. doi:10.1128/jvi.00679-09
35. Krämer OH, Göttlicher M, Heinzel T. Histone deacetylase as a therapeutic target. Trends Endocrinol Metab. 2001;12(7):294–300. doi:10.1016/S1043-2760(01)00438-6
36. Monteiro F, Bernal V, Chaillet M, Berger I, Alves PM. Targeted supplementation design for improved production and quality of enveloped viral particles in insect cell-baculovirus expression system. J Biotechnol. 2016;233:34–41. doi:10.1016/j.jbiotec.2016.06.029
37. Seo N-S, Hollister JR, Jarvis DL. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Protein Expr Purif. 2001;22(2):234–241. doi:10.1006/prep.2001.1432
38. Shi X, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell system. Curr Drug Targets. 2007;8(10):1116–1125. doi:10.2174/138945007782151360
39. Tani H, Nishijima M, Ushijima H, Miyamura T, Matsuura Y. Characterization of cell-surface determinants important for baculovirus infection. Virology. 2001;279(1):343–353. doi:10.1006/viro.2000.0699
40. Tessier DC, Thomas DY, Khouri HE, Laliberié F, Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98(2):177–183. doi:10.1016/0378-1119(91)90171-7
41. Hollister J R, Jarvis DL. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian β1, 4-galactosyltransferase and α2, 6-sialyltransferase genes. Glycobiology. 2001;11(1):1–9. doi:10.1093/glycob/11.1.1
42. Moremen KW, Ramiah A, Stuart M, et al. Expression system for structural and functional studies of human glycosylation enzymes. Nat Chem Biol. 2018;14(2):156–162. doi:10.1038/nchembio.2539
43. Yokoyama N, Hirata M, Ohtsuka K, et al. Co-expression of human chaperone Hsp70 and Hsdj or Hsp40 co-factor increases solubility of overexpressed target proteins in insect cells. Biochim Biophys Acta Gene Struct Expr. 2000;1493(1–2):119–124. doi:10.1016/S0167-4781(00)00170-6
44. Hsu T-A, Eiden JJ, Bourgarel P, Meo T, Betenbaugh MJ. Effects of co-expressing chaperone BiP on functional antibody production in the baculovirus system. Protein Expr Purif. 1994;5(6):595–603. doi:10.1006/prep.1994.1082
45. Hsu T-A, Watson S, Eiden JJ, Betenbaugh MJ. Rescue of immunoglobulins from insolubility is facilitated by PDI in the baculovirus expression system. Protein Expr Purif. 1996;7(3):281–288. doi:10.1006/prep.1996.0040
46. van Loo N-D, Fortunati E, Ehlert E, Rabelink M, Grosveld F, Scholte BJ. Baculovirus infection of nondividing mammalian cells: mechanisms of entry and nuclear transport of capsids. J Virol. 2001;75(2):961–970. doi:10.1128/jvi.75.2.961-970.2001
47. Sarkis C, Serguera C, Petres S, et al. Efficient transduction of neural cells in vitro and in vivo by a baculovirus-derived vector. Proc Natl Acad Sci U S A. 2000;97(26):14638–14643. doi:10.1073/pnas.260472897
48. Kataoka C, Kaname Y, Taguwa S, et al. Baculovirus GP64-mediated entry into mammalian cells. J Virol. 2012;86(5):2610–2620. doi:10.1128/jvi.06704-11
49. Makkonen K-E, Turkki P, Laakkonen JP, Yla-Herttuala S, Marjomaki V, Airenne KJ. 6-O- and N-Sulfated syndecan-1 promotes baculovirus binding and entry into mammalian cells. J Virol. 2013;87(20):11148–11159. doi:10.1128/jvi.01919-13
50. Morris TD, Miller LK. Promoter influence on baculovirus-mediated gene expression in permissive and nonpermissive insect cell lines. J Virol. 1992;66(12):7397–7405. doi:10.1128/JVI.66.12.7397-7405.1992
51. Hofmann C, Strauss M. Baculovirus-mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998;5(4):531–536. doi:10.1038/sj.gt.3300607
52. Ge J, Huang Y, Hu X, Zhong J. A surface-modified baculovirus vector with improved gene delivery to B-lymphocytic cells. J Biotechnol. 2007;129(3):367–372. doi:10.1016/j.jbiotec.2007.01.037
53. Hu L, Li Y, Deng F, Hu Z, Wang H, Wang M. Improving baculovirus transduction of mammalian cells by incorporation of thogotovirus glycoproteins. Virol Sin. 2019;34(4):454–466. doi:10.1007/s12250-019-00133-0
54. Borg J, Nevsten P, Wallenberg R, et al. Amino-terminal anchored surface display in insect cells and budded baculovirus using the amino-terminal end of neuraminidase. J Biotechnol. 2004;114(1–2):21–30. doi:10.1016/j.jbiotec.2004.05.014
55. Martyn JC, Cardin AJ, Wines BD, et al. Surface display of IgG Fc on baculovirus vectors enhances binding to antigen-presenting cells and cell lines expressing Fc receptors. Arch Virol. 2009;154(7):1129–1138. doi:10.1007/s00705-009-0423-8
56. Yu IL, Lin YC, Robinson JH, Lung O. Transduction of vertebrate cells with Spodoptera exigua multiple nucleopolyhedrovirus F protein-pseudotyped gp64-null Autographa californica multiple nucleopolyhedrovirus. J Gen Virol. 2009;90(\(Pt 9)):2282–2287. doi:10.1099/vir.0.012138-0
57. Lee HJ, Park N, Cho HJ, et al. Development of a novel viral DNA vaccine against human papillomavirus: acHERV-HP16L1. Vaccine. 2010;28(6):1613–1619. doi:10.1016/j.vaccine.2009.11.044
58. Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997;8(17):2011–2018. doi:10.1089/hum.1997.8.17-2011
59. Mäkelä AR, Matilainen H, White DJ, Ruoslahti E, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80(13):6603–6611. doi:10.1128/JVI.00528-06
60. Räty JK, Airenne KJ, Marttila AT, et al. Enhanced gene delivery by avidin-displaying baculovirus. Mol Ther. 2004;9(2):282–291. doi:10.1016/j.ymthe.2003.11.004
61. Chen HZ, Wu CP, Chao YC, Liu CY. Membrane penetrating peptides greatly enhance baculovirus transduction efficiency into mammalian cells. Biochem Biophys Res Commun. 2011;405(2):297–302. doi:10.1016/j.bbrc.2011.01.032
62. Tamura T, Kawabata C, Matsushita S, Sakaguchi M, Yoshida S. Malaria sporozoite protein expression enhances baculovirus-mediated gene transfer to hepatocytes. J Gene Med. 2016;18(4–6):75–85. doi:10.1002/jgm.2879
63. Jarvis DL, Fleming J-AGW, Kovacs GR, Summers MD, Guarino LA. Use of early baculovirus promoters for continuous expression and efficient processing of foreign gene products in stably transformed lepidopteran cells. Bio/Technology. 1990;8(10):950–955. doi:10.1038/nbt1090-950
64. López-Vidal J, Gómez-Sebastián S, Sánchez-Ramos I, Escribano JM. Characterization of a Trichoplusia ni hexamerin-derived promoter in the AcMNPV baculovirus vector. J Biotechnol. 2013;165(3):201–208. doi:10.1016/j.jbiotec.2013.03.012
65. Sprick G, Weidner T, Salzig D, Czermak P. Baculovirus-induced recombinant protein expression in human mesenchymal stromal stem cells: a promoter study. N Biotechnol. 2017;39:161–166. doi:10.1016/j.nbt.2017.08.006
66. Gwak W-S, Kim H-S, Bae J-S, Kim T-H, Bae S-M, Woo S-D. Development of a novel enhanced baculovirus expression vector via promoter combination. J Asia Pac Entomol. 2020;23(4):909–914. doi:10.1016/j.aspen.2020.07.016
67. Hu YC, Tsai CT, Chang YJ, Huang JH. Enhancement and prolongation of baculovirus-mediated expression in mammalian cells: focuses on strategic infection and feeding. Biotechnol Prog. 2003;19(2):373–379. doi:10.1021/bp025609d
68. Hoare J, Waddington S, Thomas HC, Coutelle C, McGarvey MJ. Complement inhibition rescued mice allowing observation of transgene expression following intraportal delivery of baculovirus in mice. J Gene Med. 2005;7(3):325–333. doi:10.1002/jgm.671
69. Hüser A, Rudolph M, Hofmann C. Incorporation of decay-accelerating factor into the baculovirus envelope generates complement-resistant gene transfer vectors. Nat Biotechnol. 2001;19(5):451–455. doi:10.1038/88122
70. Kaikkonen MU, Maatta AI, Ylä-Herttuala S, Airenne KJ. Screening of complement inhibitors: shielded baculoviruses increase the safety and efficacy of gene delivery. Mol Ther. 2010;18(5):987–992. doi:10.1038/mt.2010.25
71. Kawai Y, Kawabata C, Sakaguchi M, Tamura T. Protection of baculovirus vectors expressing complement regulatory proteins against serum complement attack. Biol Pharm Bull. 2018;41(10):1600–1605. doi:10.1248/bpb.b18-00451
72. Liu X, Li Y, Hu X, Yi Y, Zhang Z. Gene delivery and gene expression in vertebrate using baculovirus Bombyx mori nucleopolyhedrovirus vector. Oncotarget. 2017;8(62):106017–106025. doi:10.18632/oncotarget.22522
73. Steele KH, Stone BJ, Franklin KM, et al. Improving the baculovirus expression vector system with vankyrin-enhanced technology. Biotechnol Prog. 2017;33(6):1496–1507. doi:10.1002/btpr.2516
74. Palombo F, Monciotti A, Recchia A, Cortese R, Ciliberto G, La Monica N. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus–adeno-associated virus vector. J Virol. 1998;72(6):5025–5034. doi:10.1128/JVI.72.6.5025-5034.1998
75. Luo WY, Shih YS, Hung CL, et al. Development of the hybrid sleeping beauty-baculovirus vector for sustained gene expression and cancer therapy. Gene Ther. 2012;19(8):844–851. doi:10.1038/gt.2011.129
76. Wang Z, Li M, Ji Y, et al. Development of a novel bivalent baculovirus vectors for complement resistance and sustained transgene expression and its application in anti-angiogenesis gene therapy. Biomed Pharmacother. 2020;123:109765. doi:10.1016/j.biopha.2019.109765
77. Lo W-H, Hwang S-M, Chuang C-K, Chen C-Y, Hu Y-C. Development of a hybrid baculoviral vector for sustained transgene expression. Mol Ther. 2009;17(4):658–666. doi:10.1038/mt.2009.13
78. Whitlow J, Pacelli S, Walston T, Paul A. Bioactive hydrogel platforms for spatiotemporal delivery of baculoviruses in biomedical applications. Adv Ther. 2020;3(1):1900103. doi:10.1002/adtp.201900103
79. Georgopoulos LJ, Elgue G, Sanchez J, et al. Preclinical evaluation of innate immunity to baculovirus gene therapy vectors in whole human blood. Mol Immunol. 2009;46(15):2911–2917. doi:10.1016/j.molimm.2009.07.008
80. López-Vidal J, Gómez-Sebastián S, Bárcena J, et al. Improved production efficiency of virus-like particles by the baculovirus expression vector system. PLoS One. 2015;10(10):e0140039. doi:10.1371/journal.pone.0140039
81. Shang Y, Wang M, Xiao G, et al. Construction and rescue of a functional synthetic baculovirus. ACS Synth Biol. 2017;6(7):1393–1402. doi:10.1021/acssynbio.7b00028
82. Weissmann F, Petzold G, VanderLinden R, et al. biGBac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proc Natl Acad Sci. 2016;113(19):E2564. doi:10.1073/pnas.1604935113
83. Rip J, Nierman MC, Sierts JA, et al. Gene therapy for lipoprotein lipase deficiency: working toward clinical application. Hum Gene Ther. 2005;16(11):1276–1286. doi:10.1089/hum.2005.16.1276
84. Mietzsch M, Grasse S, Zurawski C, et al. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1–12 vectors for gene therapy. Hum Gene Ther. 2014;25(3):212–222. doi:10.1089/hum.2013.184
85. Joshi PRH, Cervera L, Ahmed I, et al. Achieving high-yield production of functional AAV5 gene delivery vectors via fedbatch in an insect cell-one baculovirus system. Mol Ther Clin Dev. 2019;13:279–289. doi:10.1016/j.omtm.2019.02.003
86. Wang J, Zhu L, Chen X, Huang R, Wang S, Dong P. Human bone marrow mesenchymal stem cells functionalized by hybrid baculovirus-adeno-associated viral vectors for targeting hypopharyngeal carcinoma. Stem Cells Dev. 2019;28(8):543–553. doi:10.1089/scd.2018.0252
87. Wu Y, Jiang L, Geng H, et al. A recombinant baculovirus efficiently generates recombinant adeno-associated virus vectors in cultured insect cells and Larvae. Mol Ther Methods Clin Dev. 2018;10:38–47. doi:10.1016/j.omtm.2018.05.005
88. Abe T, Hemmi H, Miyamoto H, et al. Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79(5):2847–2858. doi:10.1128/jvi.79.5.2847-2858.2005
89. Molinari P, Crespo MI, Gravisaco MJ, Taboga O, Morón G. Baculovirus capsid display potentiates OVA cytotoxic and innate immune responses. PLoS One. 2011;6(8):e24108. doi:10.1371/journal.pone.0024108
90. Gronowski AM, Hilbert DM, Sheehan KC, Garotta G, Schreiber RD. Baculovirus stimulates antiviral effects in mammalian cells. J Virol. 1999;73(12):9944–9951. doi:10.1128/JVI.73.12.9944-9951.1999
91. Jin R, Lv Z, Chen Q, et al. Safety and immunogenicity of h5n1 influenza vaccine based on baculovirus surface display system of Bombyx mori. PLoS One. 2008;3(12):e3933. doi:10.1371/journal.pone.0003933
92. Ho Y, Lin P-H, Liu CYY, Lee S-P, Chao Y-C. Assembly of human severe acute respiratory syndrome coronavirus-like particles. Biochem Biophys Res Commun. 2004;318(4):833–838. doi:10.1016/j.bbrc.2004.04.111
93. Buonaguro L, Buonaguro FM, Tornesello ML, et al. High efficient production of Pr55gag virus-like particles expressing multiple HIV-1 epitopes, including a gp120 protein derived from an Ugandan HIV-1 isolate of subtype A. Antiviral Res. 2001;49(1):35–47. doi:10.1016/S0166-3542(00)00136-4
94. Warfield KL, Dye JM, Wells JB, et al. Homologous and heterologous protection of nonhuman primates by Ebola and Sudan virus-like particles. PLoS One. 2015;10(3):e0118881–e0118881. doi:10.1371/journal.pone.0118881
95. Warfield KL, Posten NA, Swenson DL, et al. Filovirus-like particles produced in insect cells: immunogenicity and protection in rodents. J Infect Dis. 2007;196(Supplement_2):S421–S429. doi:10.1086/520612
96. Zheng X, Liu G, Opriessnig T, Wang Z, Yang Z, Jiang Y. Development and validation of a multiplex conventional PCR assay for simultaneous detection and grouping of porcine bocaviruses. J Virol Methods. 2016;236:164–169. doi:10.1016/j.jviromet.2016.06.014
97. Deo VK, Tsuji Y, Yasuda T, et al. Expression of an RSV-gag virus-like particle in insect cell lines and silkworm larvae. J Virol Methods. 2011;177(2):147–152. doi:10.1016/j.jviromet.2011.07.012
98. Hinke SA. Diamyd, an alum-formulated recombinant human GAD65 for the prevention of autoimmune diabetes. Curr Opin Mol Ther. 2008;10(5):516–525.
99. Lu L, Ho Y, Kwang J. Suppression of porcine arterivirus replication by baculovirus-delivered shRNA targeting nucleoprotein. Biochem Biophys Res Commun. 2006;340(4):1178–1183. doi:10.1016/j.bbrc.2005.12.133
100. Kim Y-K, Choi JY, Yoo M-K, et al. Receptor-mediated gene delivery by folate-PEG-baculovirus in vitro. J Biotechnol. 2007;131(3):353–361. doi:10.1016/j.jbiotec.2007.07.938
101. Meysami P, Rezaei F, Marashi SM, Amiri MM, Bakker E, Mokhtari-Azad T. Antitumor effects of a recombinant baculovirus displaying anti-HER2 scFv expressing Apoptin in HER2 positive SK-BR-3 breast cancer cells. Future Virol. 2019;14(3):139–152. doi:10.2217/fvl-2018-0187
102. Ono C, Ninomiya A, Yamamoto S, et al. Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells. J Virol. 2014;88(4):2157–2167. doi:10.1128/jvi.03055-13
103. Cheshenko N, Krougliak N, Eisensmith RC, Krougliak VA. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8(11):846–854. doi:10.1038/sj.gt.3301459
104. Chen HC, Sung LY, Lo WH, et al. Combination of baculovirus-expressed BMP-2 and rotating-shaft bioreactor culture synergistically enhances cartilage formation. Gene Ther. 2008;15(4):309–317. doi:10.1038/sj.gt.3303087
105. Gottardo MF, Pidre ML, Zuccato C, et al. Baculovirus-based gene silencing of Humanin for the treatment of pituitary tumors. Apoptosis. 2018;23(2):143–151. doi:10.1007/s10495-018-1444-0
106. Wang CY, Li F, Yang Y, Guo HY, Wu CX, Wang S. Recombinant baculovirus containing the diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006;66(11):5798–5806. doi:10.1158/0008-5472.Can-05-4514
107. Balani P, Boulaire J, Zhao Y, Zeng J, Lin J, Wang S. High mobility group box2 promoter-controlled suicide gene expression enables targeted glioblastoma treatment. Mol Ther. 2009;17(6):1003–1011. doi:10.1038/mt.2009.22
108. Huang W, Tian XL, Wu YL, et al. Suppression of gastric cancer growth by baculovirus vector-mediated transfer of normal epithelial cell specific-1 gene. World J Gastroenterol. 2008;14(38):5810–5815. doi:10.3748/wjg.14.5810
109. Lin C-Y, Chang Y-H, Lin K-J, et al. The healing of critical-sized femoral segmental bone defects in rabbits using baculovirus-engineered mesenchymal stem cells. Biomaterials. 2010;31(12):3222–3230. doi:10.1016/j.biomaterials.2010.01.030
110. Paul A, Binsalamah ZM, Khan AA, et al. A nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapy. Biomaterials. 2011;32(32):8304–8318. doi:10.1016/j.biomaterials.2011.07.042
111. Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi:10.1056/NEJMoa021641
112. Ferreira V, Petry H, Salmon F. Immune responses to AAV-vectors, the glybera example from bench to bedside. Front Immunol. 2014;5:82. doi:10.3389/fimmu.2014.00082
113. Emran TB, Iyori M, Ono Y, et al. Baculovirus-induced fast-acting innate immunity kills liver-stage Plasmodium. J Immunol. 2018;201(8):2441. doi:10.4049/jimmunol.1800908
114. Fedson DS, Dunnill P. New approaches to confronting an imminent influenza pandemic. Perm J. 2007;11(3):63. doi:10.7812/TPP/07-044
115. Caballero S, Guix S, Ribes E, Bosch A, Pintó RM. Structural requirements of astrovirus virus-like particles assembled in insect cells. J Virol. 2004;78(23):13285. doi:10.1128/JVI.78.23.13285-13292.2004
116. Liu Q, Yan K, Feng Y, et al. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine. 2012;30(47):6642–6648. doi:10.1016/j.vaccine.2012.08.071
117. Metz SW, Gardner J, Geertsema C, et al. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl Trop Dis. 2013;7(3):e2124. doi:10.1371/journal.pntd.0002124
118. Chung C-Y, Chen C-Y, Lin S-Y, et al. Enterovirus 71 virus-like particle vaccine: improved production conditions for enhanced yield. Vaccine. 2010;28(43):6951–6957. doi:10.1016/j.vaccine.2010.08.052
119. Mohana Subramanian B, Madhanmohan M, Sriraman R, et al. Development of foot-and-mouth disease virus (FMDV) serotype O virus-like-particles (VLPs) vaccine and evaluation of its potency. Antivir Res. 2012;96(3):288–295. doi:10.1016/j.antiviral.2012.09.019
120. Suzuki H, Tamai N, Habu Y, Chang MOO, Takaku H. Suppression of hepatitis C virus replication by baculovirus vector-mediated short-hairpin RNA expression. FEBS Lett. 2008;582(20):3085–3089. doi:10.1016/j.febslet.2008.07.056
121. Wang Y, Ouyang W, Liu X, et al. Virus-like particles of hepatitis B virus core protein containing five mimotopes of infectious bursal disease virus (IBDV) protect chickens against IBDV. Vaccine. 2012;30(12):2125–2130. doi:10.1016/j.vaccine.2012.01.040
122. Treanor JJ, El Sahly H, King J, et al. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok®) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011;29(44):7733–7739. doi:10.1016/j.vaccine.2011.07.128
123. Yoshida S, Kawasaki M, Hariguchi N, Hirota K, Matsumoto M. A baculovirus dual expression system-based malaria vaccine induces strong protection against Plasmodium berghei sporozoite challenge in mice. Infect Immun. 2009;77(5):1782–1789. doi:10.1128/IAI.01226-08
124. Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine. 2011;29(45):8126–8133. doi:10.1016/j.vaccine.2011.08.026
125. Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–2187. doi:10.1056/NEJMoa1101245
126. Bernstein DI, El Sahly HM, Keitel WA, et al. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine. 2011;29(43):7357–7363. doi:10.1016/j.vaccine.2011.07.080
127. Bräutigam S, Snezhkov E, Bishop DH. Formation of poliovirus-like particles by recombinant baculoviruses expressing the individual VP0, VP3, and VP1 proteins by comparison to particles derived from the expressed poliovirus polyprotein. Virology. 1993;192(2):512–524. doi:10.1006/viro.1993.1067
128. Liu L, Celma CCP, Roy P. Rift Valley fever virus structural proteins: expression, characterization and assembly of recombinant proteins. Virol J. 2008;5:82. doi:10.1186/1743-422X-5-82
129. Akinobu K, Fumio A, Makoto T, Hiroaki Y, Akihiro K, Hiroshi H. Purification and characterization of virus-like particles and pentamers produced by the expression of SV40 capsid proteins in insect cells. Biochim Biophys Acta Gen Subj. 1996;1290(1):37–45. doi:10.1016/0304-4165(95)00184-0
130. Takahama M, Fukuda M, Ohbayashi N, et al. The RAB2B-GARIL5 complex promotes cytosolic DNA-induced innate immune responses. Cell Rep. 2017;20(12):2944–2954. doi:10.1016/j.celrep.2017.08.085
131. Wang X, Ao Z, Chen L, Kobinger G, Peng J, Yao X. The cellular antiviral protein APOBEC3G Interacts with HIV-1 reverse transcriptase and inhibits its function during viral replication. J Virol. 2012;86(7):3777 LP- 3786. doi:10.1128/JVI.06594-11
132. Kool M, Voncken JW, Van Lier FLJ, Tramper J, Vlak JM. Detection and analysis of Autographa californica nuclear polyhedrosis virus mutants with defective interfering properties. Virology. 1991;183(2):739–746. doi:10.1016/0042-6822(91)91003-Y
133. Draft landscape of covid-19candidate vaccines. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.