Back to Journals » Infection and Drug Resistance » Volume 15
Bacteriological Profile and Antimicrobial Susceptibility Patterns of Gram-Negative Bloodstream Infection and Risk Factors Associated with Mortality and Drug Resistance: A Retrospective Study from Shanxi, China
Authors Shi N, Kang J, Wang S , Song Y, Yin D, Li X, Guo Q, Duan J , Zhang S
Received 11 April 2022
Accepted for publication 28 June 2022
Published 6 July 2022 Volume 2022:15 Pages 3561—3578
DOI https://doi.org/10.2147/IDR.S370326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Nan Shi,1,2,* Jianbang Kang,2,* Shuyun Wang,2 Yan Song,2 Donghong Yin,2 Xiaoxia Li,2 Qian Guo,2 Jinju Duan,2 Shuqiu Zhang1
1Department of Pharmacy, Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 2Department of Pharmacy, Second Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinju Duan; Shuqiu Zhang, Email [email protected]; [email protected]; [email protected]
Objective: The aim of this study was to analyze the epidemiological of gram-negative bloodstream infection (GNBSI) and establish a risk prediction model for mortality and acquiring multidrug resistant (MDR), the extended spectrum beta-lactamases (ESBLs) producing and carbapenem-resistant (CR) GNBSI.
Methods: This retrospective study covered five years from January 2015 to December 2019. Data were obtained from Hospital Information System (HIS) and microbiology department records. The risk factors for mortality and acquiring MDR, ESBLs-producing and CR GNBSI were analyzed by univariable and multivariable analysis.
Results: A total of 1018 GNBSI cases were collected. A majority of GNBSI patients were in hematology ward (23.77%). There were 38.61% patients who were assigned in the 41– 60 age group. Escherichia coli was the most common gram-negative organism (49.90%). Among isolates of GNBSI, 40.47% were found to be MDR strains, 34.09% were found to be ESBLs-producing strains and 7.06% were found to be CR strains. Escherichia coli was the most common MDR (71.36%) and ESBLs-producing strain (77.81%). Acinetobacter baumannii was the most common CR isolate (46.15%). Multivariate analysis indicated that diabetes mellitus, solid organ tumor, non-fermentative bacteria, MDR strain, central venous cannula, urinary catheter, therapy with carbapenems or tigecycline prior 30 days of infection were independent mortality risk factors for GNBSIs. Over all, therapy with tigecycline prior 30 days of infection was the mutual predictor for mortality of GNBSI, acquiring MDR, ESBLs-producing and CR GNBSI (OR, 8.221, OR, 3.963, OR, 3.588, OR, 9.222, respectively, all p < 0.001).
Conclusion: Collectively, our study implies that patients who were diagnosed as GNBSI had a younger age. Therapy with tigecycline was the mutual and paramount predictor for mortality of GNBSI, acquiring MDR, ESBLs-producing and CR GNBSI. Our investigation had provided a theoretical basis for the use of antibiotics and prevention and control of hospital infection in our region.
Keywords: gram-negative bloodstream infection, epidemiology, risk prediction model, MDR, ESBL, carbapenem resistance
Introduction
Bloodstream infection (BSI) is defined as laboratory-confirmed isolation of at least one gram-negative or gram-positive bacteria or other pathogens from blood samples1. At present, gram-negative bacteria had become the most common pathogen of BSI in the clinic, namely gram-negative bloodstream infection (GNBSI).2,3 GNBSI accounts for 279,000 cases and 33,500–41,900 deaths annually in the USA based on the current population.4 Although the mortality rate did not rise, increasing antimicrobial resistance rates of GNBSI isolates, especially the emerging of multidrug resistant (MDR, non-susceptible to at least one agent in ≥3 classes of antibiotics) isolates, the extended spectrum beta-lactamases (ESBLs) producing isolates and carbapenem-resistant (CR) isolates have become one of the greatest threats to global health and a common drug-resistant pattern with a quite high epidemic trend around the world.5–8
Therefore, fully understanding the epidemiological of GNBSI and drug resistance data was essential for selecting empirical antibacterial agents as well as optimizing antibiotic therapy regimen.9,10 The Ministry of Health in China indicated: (1) when the antibiotic resistance rate of target bacteria is over 40%, this antibiotic should be used with caution in empirical therapy, (2) when the antibiotic resistance rate of target bacteria is over 50%, the use of this antibiotic should be based on drug sensitivity results, (3) when the antibiotic resistance rate of target bacteria is over 75%, the clinical use of this antibiotic should be suspended. Therefore, it is crucial for health workers to summarize the bacterial distribution of GNBSI and analyze the drug resistance pattern of main isolates for early initiation of appropriate empirical antimicrobial therapy.11–13 The other important modifiable variable is to surveil the patients who are at high risk of death or infection with antibiotic resistant bacteria and conducting advance intervention and management afterwards.14,15 However, excessive prevention and control measure might lead to the waste of medical resources and take a lot of effort for little return.16 Conducting risk factor analysis by using large amounts of previous data can identify populations who need routine assessment and intervention. Therefore, these data are essential for health workers to perform precise intervention and management of patients. However, the bacterial distribution and the drug-resistant spectrum of GNBSI vary from place to place.17 The mortality risk factors of patients with GNBSI are dynamic as well.18 The experience of other countries or regions may be of little guide to this region. Therefore, the significance of contemporary local data should be highlighted.
However, there are rare reports on the epidemiological analysis of GNBSI, the mortality risk factors and the predictors of intractable antibiotic resistant GNBSI in Shanxi, China. Therefore, in this retrospective study, we attempted to analyze the epidemiological of GNBSI, drug resistance of main strains isolated from patients with GNBSI and establish a risk prediction model for mortality and acquiring MDR, ESBLs-producing or carbapenem-resistant GNBSI in a large cohort of patients in Shanxi. Furthermore, our results could provide a theoretical basis for appropriate empirical antimicrobial therapy in clinic, provide predictors for mortality in patients, improve the prognostic for patients and strengthen the control and management of the spread of MDR, ESBLs-producing and carbapenem-resistant GNBSI.
Method
Setting and Study Design
This study was performed at the second hospital of Shanxi Medical University, an educational 2700-bed inpatient center. This was a retrospective study covered five years from January 2015 to December 2019. In this study, we included the patients with positive blood culture of gram-negative bacteria and diagnosed as BSI. Also, only patients who had all clinical and laboratory data available were included. The exclusion criteria were as follows: The hospitalization was <24 hours. Blood culture samples with contamination or subjects who had insufficient data.
Data Collection
Data were obtained from Hospital Information System (HIS) and microbiology department records. This study collected general data of patients with positive blood culture and the information on the strains isolated from the blood culture including the following variables: ID number, age, gender, admitting department, smoking and drinking history, hospitalization history 90 days prior to this BSI, history of antibiotic use 30 days prior to this BSI, past medical history, name of microorganism, antimicrobial drug susceptibility results and outcome.
Microbiological Methods
All the microbiological related experimental operations were conducted in the standardized microbiology laboratory. First, blood culture bottles were incubated in the automated blood culture monitoring BACTEC (Becton Dickinson Diagnostic Instrument Systems) system. The samples were commonly incubated for 5 days, however the incubation time was increased for slow-growing microorganism. When a positive alarm occurred in the blood culture instrument, the microscopic smear was examined under microscope for an initial observation to identify whether it is gram-positive cocci or gram-negative rods. Moreover, 1 drop from each bottle was plated on standard bacteriology media for overnight incubation. And then microorganism identification and antimicrobial susceptibility test were performed via technology Vitek 2 (bioMe’rieux, Marcy l’Etoile, France). The results of antimicrobial susceptibility were interpreted as “susceptible”, “resistant”, or “intermediate” according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.
Definitions
Each patient with positive blood culture and identified from one or more blood specimens obtained by culture and at least one sign or symptom which included fever (>38°C), chills, or hypotension and not be related to an infection at another site were considered as true BSI.28 This was estimated by professional physicians. Multidrug-resistant (MDR) bacteria were defined according to the European Centre for Disease Prevention and Control (ECDC) criteria (https://www.ecdc.europa.eu/en). Enterobacteriales bacteria and non-fermenting bacteria isolates resistant to ceftazidime or cefotaxime were considered extended-spectrum beta-lactamase (ESBLs) producers. Carbapenem-resistant strains were defined as isolates intermediate or resistant to one or more carbapenems using the CLSI current breakpoints. However, not all isolates tested against all carbapenems.19
Statistical Analysis
In this study, we used the chi-squared test for a row-by-column contingency table with appropriate degrees of freedom to examine the critical factors that may influence the outcome of patients and the drug-resistant phenotype of strains. We used the survival status to represent the outcome of patient. Moreover, the drug-resistant phenotype of strains was divided into MDR strains, ESBLs-producing strains or carbapenem-resistant strains. All variables in the chi-squared test (p ≤ 0.1) and variables with clinical significance entered into a multivariable logistic regression model. We reported odds ratio values and also the confidence interval of the odds ratio for each variable. p < 0.05 was considered statistically. All the analysis was done by SPSS version 25 (IBM Corp., Armonk, NY, USA).
Result
Demographics and Epidemiology
From 2015 to 2019, a total of 1640 positive blood cultures (cases of BSIs) were isolated in the hospital. The number of gram-negative (1018, 62.07%), gram-positive (583, 35.55%) and fungus (39, 2.38%) pathogens isolated from blood culture is shown in Figure 1. The isolation rate of negative bacteria increased year by year, but the change of isolation rate of positive bacteria was not obvious. The isolation rate of negative bacteria was higher than positive bacteria each year. In this study, we include 1018 GNBSIs in the follow-up study.
 |
Figure 1 The distribution of BSI pathogens by year. |
A vast majority of patients who were diagnosed as GNBSIs were in hematology ward (n = 242, 23.77%), followed by the intensive care unit (n = 89, 8.74%), oncology department (n = 75, 7.37%), respiratory and critical department (n = 74, 7.27%), emergency department (n = 71, 6.97%), gynaecology and obstetrics department (n = 69, 6.78%) and rheumatology department (n = 59, 5.80%). The department distribution of GNBSI cases is specifically shown in Table 1.
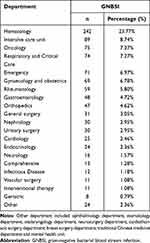 |
Table 1 The Department Distribution of GNBSI Cases |
The incidence of GNBSIs was quite similar in male and female. In particular, five hundred and nineteen patients were male (50.98%) while four hundred and ninety-nine were female (49.02%). The mean age was 54.32 ± 17.07 years old. The age distributions of the patients with BSIs are reported in Figure 2. Most of the positive patients were assigned in the 41–60 and 61–80 age group. Hematologic malignancies (251, 24.66%), diabetes mellitus (216, 21.22%) and solid tumors (119, 11.69%) were the most common underlying diseases, respectively. The mortality of GNBSIs of each year is shown in Figure 3. The average mortality rates were firstly decreased and then increased, and the variation trend of mortality rates were statistically significant (p = 0.003). The lowest death rate was in 2018 (6.37%). However, the overall average mortality was 10.61% (108/1018).
 |
Figure 2 The age distribution of GNBSI cases. Sum means the total number of patients of each age group over five years. |
 |
Figure 3 The mortality of GNBSI cases by year. |
Microbiology
As shown in Figure 4, Escherichia coli was the most common gram-negative organism (508/1018, 49.90%), followed by Klebsiella pneumoniae (160/1018, 15.72%), Brucella melitensis (89/1018, 8.74%), Pseudomonas aeruginosa (64/1018, 6.29%), Acinetobacter baumannii (38/1018, 3.73%) and Enterobacter cloacae (34/1018, 3.34%).
Overall, in GNBSI cases, 412 cases (40.47%) were found to be MDR GNBSI (ranged from 62 to 123, 31.63% to 48.18%, Figure 5). The MDR GNBSI cases decreased firstly and then increased from 2015 to 2019, the variation trend was statistically significant (p < 0.001, Figure 5). In 2019, the cases of MDR GNBSI reached its highest level. Moreover, Escherichia coli was the most common MDR strains (71.36%), followed by Klebsiella pneumoniae, Acinetobacter baumannii, and Enterobacter cloacae (11.17%, 7.77% and 4.37%, respectively). Most of them were Enterobacteriales strains (86.89%).
Overall, in GNBSI cases, 34.09% were found to be ESBLs-producing strains and 7.06% were found to be carbapenem-resistant strains. As shown in Figure 5, although the ESBLs-producing GNBSI cases decreased firstly and then increased from 2015 to 2019 (ranged from 29.08% to 38.93%), the change trend was not statistically significant (p = 0.216). The ESBLs-producing BSI cases got to the maximum in 2019. Similarly, the carbapenem-resistant BSIs were relatively stable during the study years (p = 0.289). Escherichia coli was the most common ESBLs producers followed by Klebsiella pneumoniae (77.81% and 13.26%, respectively). Acinetobacter baumannii was the most common carbapenem-resistant isolates (46.15%), followed by Klebsiella pneumoniae, Burkholderia cepacian, Escherichia coli and Pseudomonas aeruginosa (12.31%, 12.31%, 7.69%, 7.69%, respectively).
Antibiotic susceptibility results for the most common isolated bacteria are shown in Table 2. The change of drug resistance rate was not significant over the years (not shown in the Table). There were no polymyxin-resistant or tigecycline-resistant strains emerged except Acinetobacter baumannii. Escherichia coli showed higher drug resistance rates to cephalosporins of the first and second generations (82.28%), quinolones (56.30%) and cephalosporins of the third and fourth generations (55.91%). More than 99% of Escherichia coli were sensitive to carbapenems. In addition, Escherichia coli showed high sensitivity to the other drugs. Klebsiella pneumoniae had higher resistance rates to cephalosporins, especially to cephalosporins of the first and second generations (73.75%). The resistance rate of Pseudomonas aeruginosa to β-Lactam/β-lactamase inhibitor was higher as well, which reached 31.25%. The resistance rates of Acinetobacter baumannii to many drugs were over 70% which included cephalosporins of the third and fourth generations (81.58%), β-Lactam/β-lactamase inhibitor (73.68%), carbapenems (76.32%), aminoglycosides (71.05%) and quinolones (81.58%). Enterobacter cloacae was highly sensitive to aminoglycosides. However, the resistance rate of Enterobacter cloacae to cephalosporins was over 60%.
 |
Table 2 Antibiotic Resistance Results for the Most Common Isolated Bacteria |
Predictors of Mortality with GNBSIs
Univariate analyses and multivariate logistic regression comparing the clinical characteristics of patients who survived or died are shown in Table 3. Compared with patients in survivor group, there were more patients who were older than 60-year-old in non-survivor group (p = 0.088). The following factors were most frequently detected in dead patients: hypoproteinemia (p = 0.026), diabetes mellitus (p = 0.078), history of hospitalization for 90 days prior to BSI (p = 0.031), solid organ tumor (p = 0.003) and the isolated strains were Enterobacteriales (p < 0.001), non-fermentative bacteria (p < 0.001), MDR strains (p < 0.001) and carbapenem-resistant strains (p < 0.001). Moreover, the patients in the death group were more likely to have invasive procedures, such as drainage tube (p = 0.015), central venous cannula, trachea cannula, tracheotomy, urinary catheter and nasogastric tube (all p < 0.001). Compared to the survivor group, the proportion of therapy with carbapenems and tigecycline prior 30 days of GNBSIs was higher in the non-survivor group (p < 0.001). And beyond that, compared with patients in the survivor group, death group patients are more likely to have a higher proportion of agranulocytosis (29.63% versus 25.27%) and hematologic malignancy (26.85% versus 24.40%). However, there were no statistically significant difference (p = 0.328 and p = 0.576, respectively).
 |
Table 3 Predictors of Mortality with GNBSIs |
In this study, the variables with p < 0.1 in the univariate logistic regression models and factors which were considered clinically relevant were selected into the multivariate logistic regression model. Multivariate analysis indicated that diabetes mellitus (OR, 1.852; 95% Cl, 1.109–3.093; p = 0.019), solid organ tumor (OR, 3.229; 95% Cl, 1.840–5.667; p < 0.001), non-fermentative bacteria (OR, 2.867; 95% Cl, 1.665–4.936; p < 0.001), MDR strain (OR, 1.675; 95% Cl, 1.047–2.679; p = 0.031), central venous cannula (OR, 1.809; 95% Cl, 1.127–2.905; p = 0.014), urinary catheter (OR, 2.259; 95% Cl, 1.420–3.596; p = 0.001), receipt of carbapenems prior 30 days of infection (OR, 1.716; 95% Cl, 1.062–2.773; p = 0.027) and therapy with tigecycline prior 30 days of infection (OR, 8.221; 95% Cl, 4.164–16.233; p < 0.001) were independent mortality risk factors for GNBSIs when compared with the survivor group. Although the carbapenem-resistant strains were not independent mortality risk factor, compared with patients in the survivor group, the mortality rate was much higher in the non-survivor group (29.85% versus 3.96%).
Risk Factors for acquiring MDR, ESBLs-Producing, and Carbapenem-Resistant in GNBSIs Patients
Univariate analyses and multivariate logistic regression comparing the clinical characteristics of patients with or without infections caused by MDR strains are shown in Table 4. Compared with patients in non-MDR group, there were more male patients in MDR group (p = 0.005). The following factors were most frequently detected in patients with MDR GNBSI: diabetes mellitus (p = 0.023), history of hospitalization for 90 days prior to BSI (p = 0.002), solid organ tumor (p = 0.079) and the isolated strains were Enterobacteriales (p < 0.001) and non-fermentative bacteria (p = 0.006). Moreover, the patients in the MDR group were more likely to have invasive procedures, such as central venous cannula (p = 0.001), urinary catheter (p < 0.001) and nasogastric tube (p = 0.025). Compared to the non-MDR group, the proportion of therapy with 1st or 2nd generation cephalosporins (p = 0.002), 3rd or 4th generation cephalosporins (p = 0.058), β-Lactam/β-lactamase inhibitor combinations (p = 0.001), carbapenems (p = 0.095), aminoglycosides (p = 0.019) and tigecycline (p < 0.001) prior 30 days of GNBSIs were higher in the MDR group. And beyond that, compared with patients in the non-MDR group, patients with MDR were older and more likely to have a higher proportion of hypoproteinemia (44.66% versus 40.76%, 86.41% versus 82.84%, respectively). However, there were no statistically significant difference (p = 0.216 and p = 0.125, respectively).
 |
Table 4 Risk Factors for MDR in GNBSIs Patients |
Multivariate analysis indicated that Enterobacteriales (OR, 20.491; 95% Cl, 8.906–47.143; p < 0.001), non-fermentative bacteria (OR, 6.364; 95% Cl, 2.540–15.948; p < 0.001), central venous cannula (OR, 1.401; 95% Cl, 1.043–1.882; p = 0.025), therapy with 1st or 2nd generation cephalosporins prior 30 days of infection (OR, 1.936; 95% Cl, 1.333–2.812; p = 0.001) and therapy with tigecycline prior 30 days of infection (OR, 3.963; 95% Cl, 2.025–7.757; p < 0.001) were independent risk factors for MDR infection in GNBSIs patients when compared with the non-MDR group.
Univariate analyses and multivariate logistic regression comparing the clinical characteristics of patients with or without infections caused by ESBLs-producing strains are shown in Table 5. Compared with patients in non-ESBLs-producing group, there were less male patients (p = 0.004) and lower isolation rate of non-fermentative bacteria (p < 0.001). The following factors were most frequently detected in patients with ESBLs-producing GNBSI: history of hospitalization for 90 days prior to BSI (p = 0.002), solid organ tumor (p = 0.019) and the isolated strains were Enterobacteriales (p < 0.001). Moreover, the patients in the ESBLs-producing group were more likely to have invasive procedures, such as trachea cannula (p = 0.013) and urinary catheter (p = 0.061). Compared to the non-ESBLs-producing group, the proportion of therapy with 1st or 2nd generation cephalosporins (p = 0.002), 3rd or 4th generation cephalosporins (p = 0.037), β-Lactam/β-lactamase inhibitor combinations (p = 0.002) and aminoglycosides (p = 0.049) prior 30 days of GNBSIs were higher in the ESBLs-producing group. And beyond that, compared with patients in the non-ESBLs-producing group, patients with ESBLs-producing strains were more likely to have a higher proportion of diabetes mellitus (23.63% versus 19.97%) and central venous cannula (34.01% versus 30.40%). However, there were no statistically significant difference (p = 0.176 and p = 0.241, respectively).
 |
Table 5 Risk Factors for ESBLs-Producing in GNBSIs Patients |
Multivariate analysis indicated that history of hospitalization for 90 days prior to BSI (OR, 1.461; 95% Cl, 1.086–1.963; p = 0.012), Enterobacteriales (OR, 24.748; 95% Cl, 12.944–47.315; p < 0.001), therapy with 1st or 2nd generation cephalosporins prior 30 days of infection (OR, 1.964; 95% Cl, 1.315–2.934; p = 0.001), therapy with 3rd or 4th generation cephalosporins prior 30 days of infection (OR, 1.057; 95% Cl, 1.057–2.267; p = 0.025), therapy with β-Lactam/β-lactamase inhibitor combinations prior 30 days of infection (OR, 1.424; 95% Cl, 1.055–1.923; p = 0.021) and therapy with tigecycline prior 30 days of infection (OR, 3.588; 95% Cl, 1.644–7.734; p < 0.001) were independent risk factors for ESBLs-producing infection in GNBSIs patients when compared with the non-ESBLs-producing group.
Univariate analyses and multivariate logistic regression comparing the clinical characteristics of patients with or without infections caused by carbapenem-resistant strains are shown in Table 6. Compared with patients in non-carbapenem-resistant group, there were more male patients and lower isolation rate of Enterobacteriales in carbapenem-resistant group (p < 0.001). The following factors were most frequently detected in patients with carbapenem-resistant GNBSI: history of smoking (p < 0.022) and drinking (p = 0.004), hypoproteinemia (p = 0.001), solid organ tumor (p = 0.026) and the isolated strains were non-fermentative bacteria (p < 0.001). Moreover, the patients in the carbapenem-resistant group were more likely to have invasive procedures, such as drainage tube (p < 0.001), lumbar puncture (p = 0.017), arterial cannula (p = 0.003), central venous cannula (p < 0.001), trachea cannula (p < 0.001), tracheotomy (p < 0.001), urinary catheter (p < 0.001) and nasogastric tube (p < 0.001). Compared to the non-carbapenem-resistant group, the proportion of therapy with cephamycin (p = 0.006), β-Lactam/β-lactamase inhibitor combinations (p = 0.004), carbapenems (p < 0.001), aztreonam (p = 0.007) and tigecycline (p < 0.001) prior 30 days of GNBSIs were higher in the carbapenem-resistant group. And beyond that, compared with patients in the non-carbapenem-resistant group, patients with carbapenem-resistant were more likely to be over 60 years old (47.69% versus 141.97%). However, there was no statistically significant difference (p = 0.367).
 |
Table 6 Risk Factors for Carbapenem-Resistant in GNBSIs Patients |
Multivariate analysis indicated that history of drinking (OR, 5.043; 95% Cl, 1.797–14.154; p = 0.002), non-fermentative bacteria (OR, 10.176; 95% Cl, 5.149–20.111; p < 0.001), drainage tube (OR, 3.023; 95% Cl, 1.339–6.823; p = 0.008), trachea cannula (OR, 5.680; 95% Cl, 2.633–12.256; p < 0.001), therapy with aztreonam prior 30 days of infection (OR, 22.434; 95% Cl, 2.385–211.034; p = 0.007) and therapy with tigecycline prior 30 days of infection (OR, 9.222; 95% Cl, 3.873–21.956; p < 0.001) were independent risk factors for carbapenem-resistant infection in GNBSIs patients when compared with the non-carbapenem-resistant group.
Over all, therapy with tigecycline prior 30 days of infection was the mutual and paramount predictor for mortality of GNBSI, MDR, ESBLs-producing and carbapenem-resistant GNBSI.
Discussion
GNBSI represent a global problem that needs prompt action. Timely identification of causative pathogens, reasonable empirical treatment and hospital surveillance are needed to improve GNBSI management. The pathogens involved in GNBSI may vary geographically and temporally,20 which highlighted the importance of timely local data. However, the data from Shanxi in patients infected with gram-negative strains or even with intractable antibiotic resistant strains are scarce. Here, we reported the epidemiological characteristics of GNBSI, evaluated the sensitivity profile to the main antibiotics used in the treatment, the mortality predictors and the risk factors of intractable antibiotic resistant strains in the period between January 2015 and December 2019.
In this study, gram-negative bacteria were the most causative pathogens in BSI. This was similar to previous research.21 The isolation rate of negative bacteria increased year by year; however, the isolation rate of positive bacteria did not change much from year to year. This may be related to prolonged hospitalization, low overall immunity, and the use of broad-spectrum antibiotics in hospital which resulting in the increased likelihood of nosocomial bacterial infection. This result indicated that clinicians should attach great importance to BSI by gram-negative bacteria. The gender makes no difference in prevalence of GNBSI, which coincided with previous studies.22 In addition, some studies showed that male had a higher infection rate without supporting explanations.23 To our knowledge, there is no credible evidence that BSI was associated with gender.
A vast majority of patients who were diagnosis as GNBSIs were in hematology ward (n = 242, 23.77%), followed by the intensive care unit (n = 89, 8.74%). The patients in hematology ward usually undergo multiple chemotherapies, resulting in agranulocytosis and poor immunity. Therefore, these patients always have multiple site infections. Once the infection gets out of control, it can easily become a bloodstream infection. The patients in intensive care unit were usually in bad conditions with serious disease, hypoproteinemia, electrolyte disturbance and even septic shock. So they were also more likely to get bloodstream infections.
In our study, the age of GNBSI patients had a younger trend, specifically, most of the positive patients were assigned in the 41–60 age group followed by 61–80 age group. This went against our conventional wisdom and research results which highlighted the increased incidence with age.24 This may be related to the composition of GNBSI patients in the study hospital. In this study, a vast majority of GNBSI patients were in hematology ward (23.77%), which the majority of patients were diagnosed as hematologic malignancies (73.82%). The average age of the patients in hematology ward was 53.86-year-old. They were admitted to hospital with their underlying medical conditions usually accompanied by immune deficiency. This kind of patients were predisposed to developing BSI probably because of bone marrow suppression, neutropenia and mucosal injury induced by chemotherapy.21 Therefore, this result confirmed the substantial burden of these pathogens in younger patient in our region, justifying the implementation of programs to prevent GNBSI in this age group.25–27 On the other hand, the orthopedics in our hospital is a national key discipline. There were more patients who were suffering serious injuries. The orthopedic trauma patients (car accidents, falls, etc.) tend to be younger, which may account for our overall lower age.
The mortality rate of this study was 10.86% which was lower than that in the USA (vs 12–15%).4 However, this was only a result from one hospital and data were not available at the country level in China. However, the mortality rate of BSI was higher than that of other kind of infections, so preemptive treatment and optimization of treatment plan for BSI were very important to save patients’ lives. Previous study of the USA also indicated that the mortality rate would be higher than 12–15% unless clinical and antimicrobial management.6,7 In other words, this result underlined the vital importance of proper clinical and antimicrobial management.
In our current study, Escherichia coli was the most common gram-negative organism (508/1018, 49.90%), followed by Klebsiella pneumoniae (160/1018, 15.72%). This result was consistent with previous studies performed in China, Iran, Sweden and Rwanda.28–32 While an Italian study showed that the most frequently isolated pathogens were K. pneumoniae (45.3%), followed by E. coli (15.4%).33 This difference in primary bacteria might be associated with geographic area. As a study reported that K. pneumoniae was endemic in European countries bordering the Mediterranean Sea.27 Furthermore, the differences in strain prevalence between geographic area may be caused by differences in disease prevalence and different medication habits. These results indicated that these two bacteria had a worldwide popularity, thereby, fully understand the characteristics of strain and its drug resistance mechanism would play a decisive role in the control of GNBSI.
Over the past decade, lots of researches have shown an increase in the prevalence of multidrug-resistant organisms (MDROs) in the general population and in the incidence of these bacteria in hospitals.34 One report showed that the most frequent MDR bacterial isolated from gram-negative were Escherichia coli, whose frequency ranged from 10.1 to 53.6%, and Klebsiella pneumoniae (frequency ranging from 4.1% to 44.6%).35,36 In our study, the MDR GNBSI cases significantly increased over the years and the number of MDR GNBSI cases reached its highest level in 2019. In MDR GNBSI, the frequency of Escherichia coli in our study was 66.67%, which showed a little bit higher incidence than the report mentioned above and the frequency of Klebsiella pneumoniae was coincident (9.74%). The ESBLs-producing GNBSI cases increased but there was no statistical significance. The carbapenem-resistant GNBSI cases remained a steady trend. These results were consistent with previous study.3 MDR strains are more difficult to treat and some study reported crude mortality rates to reach as high as 34% to 50%, especially in MDR GNBSI.37,38 Moreover, the plasmids harbouring blaESBL gene contain resistance gene for non-beta-lactam antibiotics such as aminoglycosides, fluoroquinolones, tetracyclines, sulphonamides, etc. Therefore, ESBLs-producing isolates were always MDR isolates. In this study, we found a high proportion of the Enterobacteriales family in ESBL producers (91.07%). The resistance mechanism of Enterobacteriales isolates was relatively simple. Therefore, carbapenem antibiotics and β-Lactam/β-lactamase inhibitor combinations were effective choices. However, for severe patients with ESBL infection, carbapenem antibiotics were regarded as the most effective treatment. However, there were 17 cases of carbapenem-resistant Enterobacteriales (CRE) (2.33%). The emergence of CRE can lead to widespread drug resistance. In our antimicrobial susceptibility test in vitro, the resistant rates of Enterobacter cloacae, Klebsiella pneumoniae and Escherichia coli to carbapenem reached 20.59%, 5.00% and 0.98%, respectively. For CRE, tigecycline and polymyxin were the last resort treatment. However, for CRE BSI, tigecycline was not admitted to use for the lower serum concentration. Therefore, only polymyxin can be used in the CRE BSI. Although cefoperazone/avibatam was an available choice for serinase-producing CRE and sometimes the aminoglycosides were sensitive, the overall difficulty in treatment increased and the failure rate raised.
We also found a high proportion of non-fermenters in carbapenem-resistant isolates (53.84%). The resistant rates of Acinetobacter baumannii and Pseudomonas aeruginosa to carbapenem reached 76.32% and 6.25%, respectively. The resistance mechanisms in non-fermentative bacteria (Pseudomonas and Acinetobacter) were complex, including enzyme production, loss of pore proteins, changes in membrane permeability, high expression of efflux pump, biofilm formation, etc. For the treatment of carbapenem resistant Pseudomonas aeruginosa, aminoglycosides, quinolones, β-Lactam/β-lactamase inhibitor combinations and ceftazidime might be better choices as well. For the treatment of patients infected with carbapenem-resistant Acinetobacter baumannii, there were few antibiotics that can be used because of the higher resistance rate to many antibiotics.
Knowledge of potential mortality risk factors of GNBSI might help to better understand GNBSI and improve the control precautions. In the present study, we used the retrospective method to investigate the mortality factors. These results indicated that there were several characteristics of patients with a GNBSI that increase the risk of mortality, highlighting a subgroup of patients who may benefit from more frequent monitoring to earlier identify deterioration. The main finding of this cohort study that GNBSI caused by MDR gram-negative bacteria was a strong predictor of death. This was in accordance with some studies which have reported that mortality related to MDR organisms is higher than that related to nonresistant organisms.39 Conversely, other studies suggest that antibiotic resistance in BSI does not adversely affect the outcome for patients, representing only a small fraction of deaths.40,41 However, in this study, although the carbapenem-resistant strains were not independent mortality risk factor, the mortality rate was much higher in the non-survivor group (29.85% versus 3.96%). Patients who therapy with carbapenems prior 30 days of infection had a higher mortality rate than those who did not (63.89% versus 40.22%). Carbapenem antibiotics are very important in the treatment of negative bacteria. In our country, carbapenem antibiotics are of special use grade, with strict consultation system and use process. It is generally applied when a variety of other antibacterial drugs are ineffective and the patient has a very severe infection. Therefore, this might be the reason of higher mortality rate for those who received carbapenems prior 30 days of infection. This factor was not included in the multivariate analysis, probably because the small number of cases, especially CRE cases. This may affect the statistical results. This non-significant result may be related to the small sample size of carbapenem-resistant infections. Moreover, GNBSIs caused by ESBL bacteria did not significantly increase mortality. In addition, we found that patients with diabetes mellitus, solid organ tumor, non-fermentative bacteria, central venous cannula, urinary catheter, therapy with carbapenems or tigecycline prior 30 days of infection were more likely to die. This was consistent with previous study.32
Although ESBLs-producing and carbapenem-resistant strains were not included in the multivariate mortality risk factor model, for their further perniciousness, we analyzed the risk factors of MDR, ESBLs-producing or carbapenem-resistant GNBSI.
Numerous researches have demonstrated antibiotic exposure as a strong risk factor for infection with MDR organisms.42,43 The same results were found in our study. In the recent risk factor studies, therapy with aminoglycosides and tigecycline prior 30 days of infection were investigated as independent risk factors in MDR GNBSI. Multivariate analysis also implicated age over 60-year-old, history of hospitalization for 90 days prior to BSI, Enterobacteriales, central venous cannula, trachea cannula. These were similar to previous studies.44–46
In this study, central venous catheter was the corporate risk factors of mortality and MDR GNBSI. This has been confirmed in several studies, attributed to by the repeated need for vascular access addition to patients more likely to have a poorer baseline health status, lending to a greater risk of deterioration.47 Aminzadeh et al reported that central venous catheter related BSIs constituted as a large proportion of high-risk patients in non-ICU settings, with the first two weeks after central venous catheter insertion having the highest risk of developing a BSI and 69.2% of central venous catheter related BSIs occurred within <4 weeks of line insertion.48
In this study, therapy with cephalosporins (all generation), β-Lactam/β-lactamase inhibitor combinations and tigecycline prior 30 days of infection could induce the production of ESBLs. Our findings are consistent with previous studies concerning the global emergence of ESBLs-producing pathogens could be attributed to the overuse of antibiotics and necessitates the development of new antibiotics.49,50 Besides, this is even more emphatic that early reasonable use of antibiotics, including dosing interval and dosage, is really vital in management of resistant bacteria. As we all know, β-Lactam/β-lactamase inhibitor combinations can inhibit ESBLs. However, in this study, therapy with β-Lactam/β-lactamase inhibitor combinations prior 30 days of infection was a risk factor of ESBLs GNBSI. The specific mechanism needs to be further studied. Multivariate analysis also indicated that history of hospitalization for 90 days prior to GNBSI and Enterobacteriales infection were independent risk factors for ESBLs-producing infection in GNBSI patients when compared with the non-ESBLs-producing group. These were similar to previous studies.3
Carbapenems remain the treatment of choice for severe ESBL and MDR infections.51 Prevention of carbapenem resistance is very important. In this study, history of drinking, non-fermentative bacteria, drainage tube, trachea cannula, receipt of aztreonam prior 30 days of infection and receipt of tigecycline prior 30 days of infection were independent risk factors for carbapenem-resistant infection in GNBSI patients. These were similar to previous studies.52 Although a history of drinking is an independent risk factor of carbapenem-resistant GNBSI, our research was a retrospective study in which there were no strict and uniform standards for the drinking history of patients. The units of alcohol consumption and drinking frequency were not uniform. Therefore, this result was of little reference value and needed to be confirmed by more prospective studies. Aztreonam is a kind of antibiotic which need special class antibiotic management. Aztreonam is mainly used in combination with other drugs to treat infection due to Pseudomonas aeruginosa and metallo β-lactamases-producing CRE. Therefore, Carbapenems resistance rate was higher in patients treated with aztreonam. In this study, solid organ tumor is a protective factor for carbapenem-resistant infection in GNBSIs patients. This may be due to the low isolation rate of carbapenem-resistant strains from solid organ tumor patients in this study. We found two carbapenem-resistant strains (1.68%), similar to what has been reported at other hospitals (0.8–3.3%).53 However, the exact mechanism is unclear.
Intriguingly, therapy with tigecycline prior 30 days of infection was the mutual and paramount predictor for mortality of GNBSI, MDR, ESBLs-producing and carbapenem-resistant GNBSI (OR = 7.254, 3.701, 3.588, 9.222, respectively). This means patients whose therapy with tigecycline prior 30 days of infection were 7.254 times more likely to die, 3.701 times more likely to have MDR GNBSI, 3.588 times more likely to have ESBLs-producing GNBSI and 9.222 times more likely to have carbapenem-resistant GNBSI than those who did not. Although there were few studies on this mechanism, one study reported that tigecycline exposure increased the risk of carbapenem resistance Enterobacteriales BSI might be due to intestinal flora disorder.54 The specific mechanisms need more research. To our knowledge, tigecycline has a large volume of distribution and high concentration in gallbladder, colon and pulmonary tissue. In contrast, the serum concentrations of tigecycline are relatively low which are generally deemed not adequate. Therefore, although tigecycline has a wide antibacterial spectrum, which is active against a wide range of gram-positive and gram-negative bacteria, tigecycline is not approved for the treatment of BSI. It suggested us that more attention should be paid to avoid tigecycline exposure in the treatment of BSI. For the treatment of CRE, polymyxin or ceftazidime/avibactam should be started early according to the enzyme production, so as to better optimize the treatment plan and grasp the treatment opportunity. Overall, the risk factors for MDR, ESBLs-producing and carbapenem-resistant GNBSI in this study provide possibilities to prevent such kind of GNBSI.55
Our study has some limitations. Firstly, this research was conducted in a single center, and we used the retrospective observational study. Thus, there might be some bias and these results could not represent the condition of Shanxi, China but only the survey unit. A larger sample is necessary for further study. Secondly, we did not explore the molecular characteristics. This needs further study.
Conclusion
Collectively, our study implies that patients who were diagnosed as GNBSI had a younger age. The incidence rate of MDR GNBSI remarkably increased over the years. Therapy with tigecycline was the mutual and paramount predictor for mortality of GNBSI, acquiring MDR, ESBLs-producing and CR GNBSI. This investigation had provided a theoretical basis for the use of antibiotics and prevention and control of hospital infection in our region. The effectiveness of these investigation-guided interventions requires further research.
Ethics Statement
Our study complied with the Declaration of Helsinki. This study was approved by the Ethics Committee of the second hospital of Shanxi medical university (Code 2021 YX-183). The data of patients’ clinical variables were collected from their medical records and did not contain name, address, or other personal information. The patients’ written informed consent was exempt.
Acknowledgments
We thank the Department of Pharmacy, Second Hospital of Shanxi Medical University and Department of pharmacy, Shanxi Medical University, for supporting this research. Jinju Duan and Shuqiu Zhang are co-corresponding authors. Nan Shi and Jianbang Kang have contributed equally to this work and share first authorship.
Funding
This work was supported by the Shanxi Province Natural Science Foundation (grant number 201803D31124). The sponsor had no involvement in any of the stages from the study design to submission of the paper for publication.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Islas-Muñoz B, Volkow-Fernández P, Ibanes-Gutiérrez C, Villamar-Ramírez A, Vilar-Compte D, Cornejo-Juárez P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int J Infect Dis. 2018;71:59–64. doi:10.1016/j.ijid.2018.03.022
2. Jiang ZQ, Wang SD, Feng DD, Zhang BX, Mao SH, Wu JN. Epidemiological risk factors for nosocomial bloodstream infections: a four-year retrospective study in China. J Crit Care. 2019;52:92–96. doi:10.1016/j.jcrc.2019.04.019
3. Liang T, Xu C, Cheng Q, Tang Y, Zeng H, Li X. Epidemiology, risk factors, and clinical outcomes of bloodstream infection due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in hematologic malignancy: a retrospective study from Central South China. Microb Drug Resist. 2021;27:800–808. doi:10.1089/mdr.2020.0033
4. Al-Hasan MN Gram-negative bloodstream infection: implications of antimicrobial resistance on clinical outcomes and therapy. Antibiotics-Basel. 2020;9(12):922. doi:10.3390/antibiotics9120922
5. Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Med Chem. 2014;6:25–64.
6. Kadri SS, Adjemian J, Lai YL, et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–1814.
7. Kadri SS, Lai Y, Ricotta EE, et al. External validation of difficult-to-treat resistance prevalence and mortality risk in gram-negative bloodstream infection using electronic health record data from 140 US hospitals. Open Forum Infect Dis. 2019;6:ofz110.
8. Bassetti M, Vena A, Sepulcri C, Giacobbe DR, Peghin M. Treatment of bloodstream infections due to gram-negative bacteria with difficult-to-treat resistance. Antibiotics. 2020;9:632.
9. Kadri SS, Lai YL, Warner S, et al. Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21:241–251.
10. Cain SE, Kohn J, Bookstaver PB, Albrecht H, Al-Hasan MN Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob Agents Chemother 2015;59(1):245–250. doi:10.1128/AAC.03935-14
11. Zhu S, Kang Y, Wang W, Cai L, Sun X, Zong Z The clinical impacts and risk factors for non-central line-associated bloodstream infection in 5046 intensive care unit patients: an observational study based on electronic medical records. Crit Care 2019;23(1):52. doi:10.1186/s13054-019-2353-5
12. Obeng-Nkrumah N, Labi AK, Acquah ME, Donkor ES Bloodstream infections in patients with malignancies: implications for antibiotic treatment in a Ghanaian tertiary setting. BMC Res Notes 2015;8(1):742. doi:10.1186/s13104-015-1701-z
13. Gudiol C, Bodro M, Simonetti A, et al. Changing aetiology, clinical features, antimicrobial resistance, and outcomes of bloodstream infection in neutropenic cancer patients. Clin Microbiol Infect 2013;19(5):474–479. doi:10.1111/j.1469-0691.2012.03879.x
14. Timsit JF, Ruppé E, Barbier F, Tabah A, Bassetti M Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med 2020;46(2):266–284. doi:10.1007/s00134-020-05950-6
15. O’, Grady NP, Alexander M, Burns LA, et al. Summary of recommendations: guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis 2011;52(9):1087–1099. doi:10.1093/cid/cir138
16. See I, Mu Y, Albrecht V, et al. Trends in incidence of methicillin-resistant staphylococcus aureus bloodstream infections differ by strain type and healthcare exposure, United States, 2005–2013. Clin Infect Dis 2020;70(1):19–25. doi:10.1093/cid/ciz158
17. Song F, Zhang K, Huang J, et al. Clinical characteristics, risk factors, and outcomes of patients with polymicrobial Klebsiella pneumoniae bloodstream infections. Biomed Res Int 2021;2021:6619911. doi:10.1155/2021/6619911
18. Moghnieh R, Estaitieh N, Mugharbil A, et al. Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Front Cell Infect Microbiol 2015;5:11. doi:10.3389/fcimb.2015.00011
19. Bindschedler A, Wacker R, Egli J, et al. Plasmodium berghei sporozoites in nonreplicative vacuole are eliminated by a PI3P-mediated autophagy-independent pathway. Cell Microbiol 2021;23(1):e13271. doi:10.1111/cmi.13271
20. Liu J, Fang Z, Yu Y, et al. Pathogens distribution and antimicrobial resistance in bloodstream infections in twenty-five neonatal intensive care units in China, 2017–2019. Antimicrob Resist Infect Control 2021;10(1):121. doi:10.1186/s13756-021-00989-6
21. Chen S, Lin K, Li Q, et al. A practical update on the epidemiology and risk factors for the emergence and mortality of bloodstream infections from real-world data of 3014 hematological malignancy patients receiving chemotherapy. J Cancer 2021;12(18):5494–5505. doi:10.7150/jca.50802
22. Gubbels S, Nielsen J, Voldstedlund M, et al. Utilization of blood cultures in Danish hospitals: a population-based descriptive analysis. Clin Microbiol Infect 2015;21:
23. Santella B, Folliero V, Pirofalo GM, et al. Sepsis-A retrospective cohort study of bloodstream infections. Antibiotics 2020;9(12). doi:10.3390/antibiotics9120851
24. da Silva N, da Rocha JA, Do Valle FM, Silva A, Ehrlich S, Martins IS. The impact of ageing on the incidence and mortality rate of bloodstream infection: a hospital-based case-cohort study in a tertiary public hospital of Brazil. Trop Med Int Health 2021;26(10):1276–1284 doi:10.1111/tmi.13650
25. Serra N, Di Carlo P, D’ Arpa F, et al. Human bile microbiota: a retrospective study focusing on age and gender. J Infect Public Health 2021;14(2):206–213. doi:10.1016/j.jiph.2020.11.005
26. Licata F, Quirino A, Pepe D, Matera G, Bianco A, Group C Antimicrobial resistance in pathogens isolated from blood cultures: a two-year multicenter hospital surveillance study in Italy. Antibiotics 2020;10(10). doi:10.3390/antibiotics10010010
27. Brannon JR, Dunigan TL, Beebout CJ, et al. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat Commun 2020;11(1):2803. doi:10.1038/s41467-020-16627-5
28. Amanati A, Sajedianfard S, Khajeh S, et al. Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect Dis. 2021;21(1):636. doi:10.1186/s12879-021-06243-z
29. Nestor D, Andersson H, Kihlberg P, et al. Early prediction of blood stream infection in a prospectively collected cohort. BMC Infect Dis 2021;21(1):316. doi:10.1186/s12879-021-05990-3
30. Habyarimana T, Murenzi D, Musoni E, Yadufashije C, Niyonzima FN Bacteriological profile and antimicrobial susceptibility patterns of bloodstream infection at Kigali University Teaching Hospital. Infect Drug Resist 2021;14:699–707. doi:10.2147/IDR.S299520
31. Santoro A, Franceschini E, Meschiari M, et al. Epidemiology and risk factors associated with mortality in consecutive patients with bacterial bloodstream infection: impact of MDR and XDR bacteria. Open Forum Infect Dis 2020;7:ofaa461. 11 doi:10.1093/ofid/ofaa461
32. Ma Y, Wang S, Yang M, Bao J, Wang C. Analysis of risk factors and clinical indicators in bloodstream infections among patients with hematological malignancy. Cancer Manag Res. 2020;12:13579–13588. doi:10.2147/CMAR.S289291
33. Di Carlo P, Serra N, Lo Sauro S, et al. Epidemiology and pattern of resistance of gram-negative bacteria isolated from blood samples in hospitalized patients: a single center retrospective analysis from Southern Italy. Antibiotics 2021;10. doi:10.3390/antibiotics11010010
34. Saliba R, Ghelfenstein-Ferreira T, Lomont A, et al. Risk factors for the environmental spread of different multidrug-resistant organisms: a prospective cohort study. J Hosp Infect 2021;111:155–161. doi:10.1016/j.jhin.2021.01.029
35. Trecarichi EM, Tumbarello M Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr Opin Infect Dis 2014;27(2):200–210. doi:10.1097/QCO.0000000000000038
36. Trecarichi EM, Tumbarello M, Caira M, et al. Multidrug resistant Pseudomonas aeruginosa bloodstream infection in adult patients with hematologic malignancies. Haematologica 2011;96(1):
37. Fortún J, Sanz MÁ, Madero L, et al. Update on bacteraemia in oncology and hematology. Enferm Infecc Microbiol Clin 2011;29(Suppl 4):48–53. doi:10.1016/S0213-005X(11)70036-2
38. Cornejo-Juárez P, Pérez-Jiménez C, Silva-Sánchez J, et al. Molecular analysis and risk factors for Escherichia coli producing extended-spectrum β-lactamase bloodstream infection in hematological malignancies. PLoS One 2012;7(4):e35780. doi:10.1371/journal.pone.0035780
39. Trecarichi EM, Pagano L, Candoni A, et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin Microbiol Infect 2015;21(4):337–343. doi:10.1016/j.cmi.2014.11.022
40. Lye DC, Earnest A, Ling ML, et al. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: cohort study. Clin Microbiol Infect. 2012;18:502–508.
41. Blot S, Vandewoude K, De Bacquer D, Colardyn F. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis. 2002;34:1600–1606.
42. Papakonstantinou I, Angelopoulos E, Baraboutis I, et al. Risk factors for tracheobronchial acquisition of resistant Gram-negative bacterial pathogens in mechanically ventilated ICU patients. J Chemother. 2015;27:283–289.
43. Huang Y, Zhuang S, Du M. Risk factors of nosocomial infection with extended-spectrum beta-lactamase-producing bacteria in a neonatal intensive care unit in China. Infection. 2007;35:339–345.
44. Villafuerte D, Aliberti S, Soni NJ, et al. Prevalence and risk factors for Enterobacteriaceae in patients hospitalized with community-acquired pneumonia. Respirology. 2020;25:543–551.
45. Tian X, Huang C, Ye X, et al. Molecular epidemiology of and risk factors for extensively drug-resistant Klebsiella pneumoniae infections in Southwestern China: a retrospective study. Front Pharmacol. 2019;10:1307.
46. Ruppé E, Andremont A, Armand-Lefèvre L. Digestive tract colonization by multidrug-resistant Enterobacteriaceae in travellers: an update. Travel Med Infect Dis. 2018;21:28–35.
47. Bell T, O’ Grady NP. Prevention of central line-associated bloodstream infections. Infect Dis Clin North Am. 2017;31:551–559.
48. Aminzadeh Z, Simpson P, Athan E. Central venous catheter associated blood stream infections (CVC-BSIs) in the non-intensive care settings: epidemiology, microbiology and outcomes. Infect Dis Health. 2019;24:222–228.
49. Halaji M, Shahidi S, Ataei B, Atapour A, Feizi A, Havaei SA. Molecular epidemiology of blaCTX-M gene-producing uropathogenic Escherichia coli among Iranian kidney transplant patients: clonal dissemination of CC131 and CC10. Ann Clin Microbiol Antimicrob. 2021;20(1):65.
50. Halaji M, Shahidi S, Atapour A, Ataei B, Feizi A, Havaei SA. Characterization of extended-spectrum β-lactamase-producing uropathogenic Escherichia coli among Iranian kidney transplant patients. Infect Drug Resist. 2020;13:1429–1437.
51. Ghassabi F, Hashempour T, Moghadami M, et al. Bacterial etiology and antibiotic resistance pattern of septicemia in HIV and non-HIV patients admitted to tertiary care hospitals, Shiraz, South of Iran. Cell Mol Biol. 2017;63(9):115–121.
52. Bassetti M, Carnelutti A, Peghin M. Patient specific risk stratification for antimicrobial resistance and possible treatment strategies in gram-negative bacterial infections. Expert Rev Anti Infect Ther. 2017;15:55–65.
53. Satlin MJ, Cohen N, Ma KC, et al. Bacteremia due to carbapenem-resistant Enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect. 2016;73:336–345.
54. Wang Y, Lin Q, Chen Z, et al. Construction of a risk prediction model for subsequent bloodstream infection in intestinal carriers of Carbapenem-Resistant Enterobacteriaceae: a retrospective study in hematology department and intensive care unit. Infect Drug Resist. 2021;14:815–824.
55. Halaji M, Fayyazi A, Rajabnia M, Zare D, Pournajaf A, Ranjbar R. Phylogenetic group distribution of uropathogenic Escherichia coli and related antimicrobial resistance pattern: a meta-analysis and systematic review. Front Cell Infect Microbiol. 2022;12:790184.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


