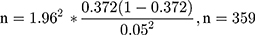Back to Journals » Infection and Drug Resistance » Volume 14
Bacterial Profile, Antibiotic Susceptibility Pattern and Associated Factors Among Patients Attending Adult OPD at Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia
Authors Mechal T, Hussen S , Desta M
Received 26 November 2020
Accepted for publication 29 December 2020
Published 14 January 2021 Volume 2021:14 Pages 99—110
DOI https://doi.org/10.2147/IDR.S287374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Tigist Mechal,1 Siraj Hussen,2 Moges Desta2
1Department of Medical Laboratory Science, Hawassa College of Health Sciences, Hawassa, South Nations and Nationalities Peoples Region, Ethiopia; 2School of Medical Laboratory Science, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Correspondence: Moges Desta
School of Medical Laboratory Science, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Tel +251911744891
Email [email protected]
Background: Urinary tract infection (UTI) is a common health problem occurring when infectious agents colonize, invade, and propagate the urinary tract including the urethra, bladder, renal pelvis, or renal parenchyma. The study aimed to determine the prevalence of symptomatic UTI, drug resistance pattern, and its associated factors among patients attending adult outpatient department (OPD) at Hawassa University Comprehensive Specialized Hospital (HUCSH).
Methods: A cross-sectional study was conducted from October 2018 to February 2019 among adults ≥ 18 years old with symptoms of UTI. Processing of specimens for culture and identification was done. Antimicrobial susceptibility was done for positive urine cultures. Data entry and analysis were performed using SPSS version 23.0 software. Bivariate and multivariate logistic regression analysis test results were used.
Results: The overall prevalence of symptomatic urinary tract infection was 32.8% (95% CI: 28.3– 37.6). The predominant isolated bacteria was E. coli 46 (36.2%) followed by S. aureus 21 (16.5%). Gram-negative bacteria were a high level of resistance to ampicillin (71.4%), and tetracycline (68.2%). Gram-positive bacteria were highly resistant to norfloxacin (77.7%). The overall prevalence of multi-drug resistant isolates was 102 (80.3%). Being female, no formal education, and self-medication history had more likely cause UTI.
Conclusion: Urinary tract infection (UTI) among adults was prevalent in the study area. Being female, educational status and self-medication history had a significant association with UTI. Resistance to ampicillin, tetracycline, and norfloxacin was high. Therefore, culture and antibiotic susceptibility testing should be routinely used for the proper management of patients with UTI.
Keywords: urinary tract infection, antimicrobial susceptibility, Hawassa, Southern Ethiopia
Background
Urinary tract infection (UTI) is some of the most common infections occurring when infectious agents colonize, invade and propagate the urinary tract including the urethra, the bladder, renal pelvis, or renal parenchyma1,2 and it is the most common infectious diseases diagnosed in outpatient as well as in hospitalized patients and can lead to significant morbidity, mortality, and high medical costs.3,4 All individuals are susceptible to UTIs with a diverse spectrum of etiological agents; however, the prevalence of infection differs with age, sex, and certain predisposing factors.5–7 Women are more vulnerable than men due to anatomical and physiological factors.8 Around 150 million peoples have been estimated to suffer from asymptomatic and symptomatic UTIs each year worldwide.9 Reporting of epidemiological data in urology and urinary tract infections are crucial to influence the development of therapeutic guidelines for UTIs and also the relevant stakeholders and government representatives often base their decisions on published evidence.10
According to the Center for Disease Control and Prevention (CDC), UTIs are the most common bacterial infection requiring medical care, resulting in 8.6 million ambulatory care visits in 2007, 23% of which occurred in the emergency department.11 An estimated 50% of women report having had a UTI at some point in their lives and 8.3 million office visits and more than 1 million hospitalizations, for an overall annual cost of more than $1 billion.12 Annual deaths from untreatable infections may rise from an estimated 700,000 in 2015 to 10 million by 2050 if antibiotic resistance is not controlled.13
Studies in a different part of Ethiopia showed UTI is among the major health problem with a prevalence range of 9.2% to 37.2%14–16 and the common pathogens responsible for UTI are Gram-negative bacteria especially E. coli which accounts for a large percentage of isolates.17,18
Other bacterial species occurring were Klebsiella pneumoniae, coagulase-negative Staphylococci, group B Streptococcus, and Enterococci.19,20 Gram-positive cocci have emerged as important causative agents of UTIs, particularly among elderly patients with predisposing conditions.21 The inappropriate overuse of antimicrobial agents can lead to the development of antibiotic resistance, increased hospital cost, morbidity, and mortality.22
Risk factors for UTI include female sex, lower educational level, low immunity, employment status, incomplete bladder emptying, bladder dysfunction, and prostate syndrome in men.23
The common risk factors reported from different studies conducted in Ethiopia among symptomatic UTI patients were sex, level of education, previous history of UTI, previous history of hospitalization.18,23,24
Treatment of UTI cases often started empirically and therapy is based on information determined from the antimicrobial resistance pattern of the urinary pathogens.25 Trimethoprim/sulfamethoxazole (SXT), ciprofloxacin, cephalosporins, nitrofurantoin, and fosfomycin are used as antibiotic drugs in the treatment of UTIs.26 UTIs are often treated with broad-spectrum antibiotics because of the invariably increased development of a resistant organism. The inappropriate overuse of antimicrobial agents can lead to the development of antibiotic resistance, increased hospital cost, morbidity, and mortality.27 Hence information on UTI and drug susceptibility patterns are very important for the proper selection and use of antibiotic agents. Therefore, this study aimed to determine the bacterial profile, antibiotic susceptibility pattern, and associated factors among patients attending adult outpatient department at Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia.
Materials and Methods
Study Area and Period
The study was conducted in Hawassa University Comprehensive Specialized Hospital for five months from October 2018–February 2019. The Hospital is found in Hawassa city which is the capital city of Sidama Regional state of Ethiopia with a total population of 319,023 based on the 2007 national census.28
The city is located 275 km to the South of Addis Ababa, the capital city of Ethiopia. Hawassa University Comprehensive Specialized Hospital is serving people of the region and patients coming from the nearby Oromia Region by different outpatient, inpatient, and emergency units (surgery, gynecology and obstetrics, internal medicine, pediatrics, ophthalmology, psychiatry, radiology, pathology, Microbiology).
Study Design and Participants
A cross-sectional study was conducted among patients with symptomatic UTI visiting adult OPD at Hawassa University Comprehensive Specialized Hospital. A systematic random sampling technique was used to select study participants until the required sample size was obtained.
The sample size was calculated by using 37.2% estimated prevalence (p) in a study conducted at Arba Minch Hospital,15 5% margin of error (0.05) and 95% level confidence (z=1.96), Hence the sample size was calculated as;
Sample size = n (sample size) + (10% non-respondent).
Sample size (N) = 359 + 35.9= 395
Total sample size was=395.
Data Collection
Socio-Demographic and Clinical Data Collection
Socio-demographic and clinical data were collected from patients using semi-structured questionnaires by nurses.
Laboratory Data Collection
Ten milliliters of clean-catch midstream urine sample was collected in a wide-mouthed sterile container from study participants.29
The collected urine sample was labeled and delivered to the Hospital microbiology laboratory within 30 minutes.
Urine Culture and Identification
Using a calibrated wire loop (0.001 mL) urine samples were inoculated into blood agar and MacConkey agar plates. Streaked culture plates were incubated at 37°C overnights. After 24 hours of incubation, the bacterial growth on the respective media was seen, and a total colony count was done to check significant growth. Colony counts of bacterial growth of ≥105 CFU/mL of urine were significant. Colony morphology and gram-stain were used for the characterization of the pure colony. Positive urine cultures were further identified by their characteristic’s appearance on their respective media and confirmed by the pattern of their biochemical reactions.30
Antibiotic Susceptibility Testing
Kirby-Bauer disc diffusion method was used for the antibiotic susceptibility test. Three to five pure colonies were transferred into a tube containing 4–5mL nutrient broth and mixed gently and then incubated at 35–37°C for 2–6hrs. The turbidity of the suspension was compared with McFarland 0.5 tubes to standardize the inoculums size.31
By using a sterile cotton swab dipping it into the suspension evenly over the entire surface of Mueller-Hinton agar (MHA) (Oxide Ltd, Hampshire, UK). The inoculated plates were left at room temperature to dry for 3–15 minutes. The following commercially available antibiotic discs were used with their respective concentrations: ampicillin (AMP) (10μg), ciprofloxacin (CIP) (5μg), cotrimoxazole (COT) (25μg), gentamycin (GEN) (10μg), meropenem (MER) (10µg), penicillin (PEN) (30µg), amoxicillin/clavulanic acid (AMC) (20/10µg), vancomycin (VAN) (30µg), ceftriaxone (CEF) (30μg), ceftazidime (CZD) (30µg), norfloxacin (NOR) (10µg), clindamycin (CLD) (2µg), erythromycin (ERY) (15μg), tetracycline (TET) (30μg), and cefoxitin (CXT) (30µg). The Zone of growth inhibition was measured by using a caliper after 18–24hour incubation at 35–37°C. The diameters were interpreted according to the clinical and Laboratory Standards Institute (CLSI) guideline of 2019 as susceptible (S), intermediate (I), or resistant (R)32
Quality Control
Completeness of the data collected by interview administered questionnaire about the socio-demographic and clinical data were checked daily by the principal investigator. The culture media, Gram staining reagents, and antibiotic discs were checked by looking at the expired date. Standard operating procedures were followed for all the methods described above. The prepared culture media, biochemical test, and antimicrobial susceptibility tests were checked by inoculating the reference strains of E. coli (ATCC-25922), S. aureus (ATCC-25923), and P. aeruginosa (ATCC-27853) which were used as quality control throughout the study. The sterility of prepared culture media was checked by overnight incubating 5% of the batch at 35–37°C and observed for bacterial growth. If there was a growth in any of the prepared culture media, the whole batch of the media was discarded and prepared again by following correct SOPs.
Data Entry and Analysis
The data was analyzed using SPSS version 23.0. Frequency distribution and percentage calculation were done to describe the socio-demographic characteristics and clinical characteristics related variables. Crude odds ratio (COR) and adjusted odds ratio (AOR) with 95% Confidence Interval (CI) was computed using bivariate and multivariate logistic regression analysis. P-value calculated for assessing the association between dependent and independent variables. The major factor associated with the prevalence of UTI was calculated and interpreted at p<0.05 and using a 95% confidence interval for statistical significance.
Results
Socio-Demographic Characteristics
Out of 395, a total of 387 study participants were interviewed with a response rate of 98%. In this study, the mean age of study participants was 25.04 (±8.20) years within the age range of 18–65. More than half of the study participants, 225 (58.1%) were 18–24 years old, 212 (54.8%) were females and 219 (56.6%) were urban dwellers with their residence. Regarding the occupational status, 139 (35.9%) were Farmer and 105 (27.1%) were employed. And 59 (15.2%) had no formal education (Table 1).
 |
Table 1 Socio-Demographic Characteristics of SUTI Patients Attending Adult OPD at HUCSH, Southern Ethiopia, 2019 (n=387) |
Clinical Characteristics
In this study, 76 (19.6%) of the study participants had smelly and cloudy urine. One hundred (25.8%) of the participants had a burning sensation during urination. One hundred forty - two (36.7%) of the study participants had a frequency of urination (Table 2).
 |
Table 2 Clinical Characteristics of SUTI Patients Attending Adult OPD at HUCSH, Southern Ethiopia, 2019 (n=387) |
Prevalence of Urinary Tract Infection
The prevalence of urinary tract infection was 32.8% (95% CI: 28.3–37.6). Of these127 positive cases, seven different types of bacteria were identified that include both Gram-negative and Gram-positive bacteria. The majority of the isolates belong to the Gram-negative bacteria 91 (71.7%). Among the isolated bacteria E. coli was predominant; 46 (36.2%), followed by S. aureus 21 (16.5%), P. aeruginosa 16 (12.6%), CoNS15 (11.8%), Klebsiella pneumonia 14 (11.0%), Proteus mirabilis 11 (8.7%) and Enterobacter aerogenes 4 (3.1%).
Antibiotic Susceptibility Pattern
Gram-negative bacteria were resistant to ampicillin (71.4%), tetracycline (68.2%), and cotrimoxazole (51.6%). While they were sensitive to cefoxitin (96.7%), AMC (91.3%), erythromycin (88%), ceftriaxone (84.6%), ceftazidime (84.6%), and gentamycin (84.6%). E. coli were resistant to ampicillin (73.9%), cotrimoxazole (56.5%), and tetracycline (65.2%), but sensitive to gentamycin (91.3%), and AMC (91.3%), meropenem (82.6%), ceftriaxone (82.6%), and ceftazidime (82.6%). Pseudomonas aeruginosa was resistant to ampicillin (81.3%), cotrimoxazole (56.3%) and tetracycline (81.3%) but showed sensitivity to cefoxitin (100.0%), ciprofloxacin (62.5%), gentamycin (81.3%), meropenem (75.0%), AMC (87.5%) and ceftriaxone (87.5%). K. pneumonia was resistant to ampicillin (78.6%) and tetracycline (71.4%). All K. pneumonia isolates showed sensitivity to meropenem, AMC, and erythromycin, and K.pneumonia were also sensitive to cefoxitin (92.9%), ceftriaxone (85.7%), ceftazidime (85.7%), ciprofloxacin (78.6%), and norfloxacin (78.6%). P.mirablis were resistant to cotrimoxazole (54.5%) and tetracycline (63.6%). All P.mirablis isolates were sensitive to AMC, cefoxitin, ceftazidime, and ceftriaxone; while 90.9% of P.mirablis were sensitive to gentamycin and meropenem each. Seventy-five percent of E. aerogenes were resistant to ampicillin, ciprofloxacin, meropenem each; and 50.0% of E. aerogenes were resistant to gentamycin, AMC, ceftriaxone, ceftazidime, norfloxacin, and tetracycline each. All E.aerogenes isolates were sensitive to tetracycline and cefoxitin and 75.0% of E. aerogenes were sensitive to cotrimoxazole. The Gram-positive bacteria were resistant to norfloxacin (66.7%) and cotrimoxazole (47.2%). Staphylococcus aureus were resistant to norfloxacin (85.7%) while sensitive to penicillin (90.5%), vancomycin (90.5%), gentamycin (81.0%), ciprofloxacin (76.2%), clindamycin (76.2%), erythromycin (76.2%) and tetracycline (76.2%). Coagulase-negative staphylococci (CoNS) were resistant to norfloxacin (66.7%) but 100.0% of CoNS were sensitive to penicillin, clindamycin, and erythromycin and 86.7% of them were sensitive to vancomycin (Tables 3 and 4).
 |
Table 3 The Antibiotic Susceptibility Pattern of Gram-Negative Bacteria Isolated from Symptomatic UTI Patients Attending Adult OPD at HUCSH, Southern Ethiopia, 2019 (n=91) |
 |
Table 4 The Antibiotic Susceptibility Pattern of Gram-Positive Bacterial Isolates from Symptomatic UTI Patients Attending Adult OPD at HUCSH, Southern Ethiopia, 2019 (n=36) |
Among the total bacterial isolates (n=127), the overall prevalence of multi-drug resistance pattern was recorded as 102 (80.3%) of which 72/91 (79.1%) of Gram-negative and 30/36 (83.3%) of Gram-positive bacterial isolates were resistant to two or more classes of antimicrobial agents (Table 5).
 |
Table 5 Multi-Drug Resistance Pattern of Bacterial Isolates from SUTI Patients Attending Adult OPD at HUCSH, Southern Ethiopia, 2019 (n=102) |
Factors Associated with Urinary Tract Infection
In bivariate logistic regression analysis variables that had an association with the prevalence of UTI at p-value less than 0.25 and considered a candidate for multivariate analysis were sex, educational status, previous history of UTI, history of hospital admission, self-medication history and interrupt or with-draw of treatment. In multivariate logistic regression analysis, female participants were 3.10 times more likely to develop UTI (AOR= 3.10, 95% CI: 1.80, 5.32 p=<0.0001), than male counterparts. Similarly, no formal education were 3.56 times (AOR= 3.56: 95% CI: 1.55, 8.21 p=0.003) and primary education 2.22 times (AOR= 2.22: 95% CI: 1.10, 4.45 p=0.025) had a statistically significant association with UTI when compared with the study participants who had college and above education. In addition, study participants who had self-medication history were about 6.17 times more likely to develop UTI with (AOR=6.17: 95% CI: 3.54, 10.74 p=0.0001) as compared to their counterparts (Table 6).
 |
Table 6 Bivariate and Multivariate Logistic Regression Analysis of Factors Associated with UTI Among Symptomatic Patients Attending Adult OPD at HUCSH, Southern Ethiopia, 2019 (n=387) |
Discussion
The overall prevalence of UTI among adult patients in Hawassa City was 32.8%. A comparable result was reported in studies conducted in Addis Ababa, Ethiopia (36%) and Arbaminch, Ethiopia (37.2%).15,33 This result was higher than the previous studies which were conducted in Jimma, Ethiopia (9.2%)25 Harar, Ethiopia (27.9%)24 and Mekele Ethiopia (21.1%).18 But it was lower than the studies reported in Nigeria (42.0%, 40.0%, and 39.0%)34–36 and with the study conducted in Harar, Ethiopia (39.6%).37 This variation might be due to the difference in sample size, educational status, and other socio-demographic characteristics.
In this study, out of 127 study participants with positive urine culture, the prevalence of UTI was higher (70.9%) in females than male participants. This result was in agreement with other studies conducted on UTI from Mekele (88.9%), Addis Ababa, (84.6%), and another study in Addis Ababa, (80.3%).18,38,39 The high prevalence among females might be due to their shorter and wider urethra, as well as proximity to the anus of female, make them highly susceptible to UTI.40
The majority of the causative agents of urinary tract infection in this study were Gram-negative bacteria 91 (71.7%) which was comparable with previous studies conducted in Shashemene, Ethiopia (59.2%).41 Arba Minch, Ethiopia (66.7%),15 Addis Ababa (60.8%),39 Harar Ethiopia (77.8%)42 and Nigeria (86.1%).35
This study opposes another study which was conducted in Nigeria which says the predominant bacterial isolates were Gram-positive bacteria (Staphylococcus aureus) (47.19%).36
The difference may be due to the sample collection method used, socio-demographic characteristics, and different study times. Escherichia coli was the predominant bacterial isolate (36.2%) it was also similar to studies conducted in Mekele, Ethiopia (63.5%),18 Harar, Ethiopia (38.1%),42 Addis Ababa, Ethiopia (49%),39 Arba Minch, Ethiopia (41.6%)15 and Shashemene, Ethiopia (39.3%).41
The second most common bacterial isolate was S. aureus (16.5%). This finding was agreed with a study conducted in Nigeria (14.9%),43 Nigeria (18.4%),34 and another study which was conducted in Addis Ababa, Ethiopia (19.6%).39 This result is higher than a study conducted in Bangladesh (13.2%),44 Nigeria (13.9%),35 three studies from Harar, Ethiopia (12.4%), (11.1%) and (7.5%).24,37,42 But the prevalence of S. aureus in this study was lower than studies conducted in Nigeria (47.19%),36 Addis Ababa, Ethiopia (30.8%)38 and Arba Minch, Ethiopia (20.8%).15 The difference in prevalence may be due to the method used and sample size.
The other isolate which was identified in this study was Pseudomonas aeruginosa with a proportion of (12.6%), this result was close to studies conducted in Metu, Ethiopia (10.35)45 and Harar, Ethiopia (10%).37 But it was higher than different studies in India (4.53%),46 Nigeria (4.4%),43 Harar Ethiopia (6.4%),42 Harar, Ethiopia (3.1%),24 Addis Ababa, Ethiopia (1.2%)33 and Hawassa, Ethiopia (3.0%).47
In this study the proportion of CoNS was (11.8%) this result is closer to two studies previously conducted in Harar, Ethiopia (7.9%) and (8.2%),24,42 this result is higher than a study conducted in India (2.7%).27 But it is lower than a study conducted in Addis Ababa, Ethiopia (18.9%)29 and Hawassa, Ethiopia (24.2%)47
The proportion of Klebsiella pneumonia in this study was (11%) close to a report from Harar, Ethiopia (15.5%),24 it was higher than Hawassa, Ethiopia (9.1%),47 but lower than India (27.40%),48 Iraq (24.5%),49 Bangladesh (22%)44 and Harar, Ethiopia (23.8%).42 Proteus mirabilis in this study was (8.7%) close to two reports in Harar, Ethiopia (9.5%) and (10.3%).24,42 It was higher than India (4.79%)48 and Bangladesh (5.5%),44 but lower than Iraq (17%).49 The proportion of Enterobacter aerogenes was (3.1%) this result agree with a study done in India (5.4%),27 Addis Ababa, Ethiopia (5.4%),29 and higher than a study in India (1.71%)48 and Mekele, Ethiopia (1.37%),18 but lower than Jimma, Ethiopia (9.5%).25 In this study, all patients UTI was caused by a single species.
Classical resistance classifications (multidrug resistance [MDR], extensive drug resistance [XDR], pan-drug resistance [PDR]) are very useful for epidemiological purposes, however, they may not correlate well with clinical outcomes, therefore, several novel classification criteria (eg, usual drug resistance [UDR] – if they were resistant to at least one tested antibiotic outside of their realm of intrinsic non susceptibility, difficult-to-treat resistance [DTR]- if an isolate showed resistance to carbapenems, extended-spectrum cephalosporins, and fluoroquinolones) were introduced in recent years.50
In the present study, a large number of Gram-negative bacterial isolates were resistant to ampicillin (71.4%), tetracycline (68.2%), and cotrimoxazole (51.6%), and this result agree with another study conducted in Harar, Ethiopia.24
The high resistance to common antibiotics was thought to be since study participants had a self-medication history without prescription to common antibiotics. The Gram-negative bacterial isolates were sensitive to cefoxitin (96.7%), AMC (91.3%), erythromycin (88%), ceftriaxone, ceftazidime, gentamycin (84.6%) each, meropenem (82.4%), norfloxacin (75.8%) and ciprofloxacin (70.3%). This result was in agreement with another study conducted in Mekele, Ethiopia which reported that the Gram-negative isolates were sensitive to ciprofloxacin (85.15), ceftriaxone (83.0%), and norfloxacin (80.8%).18 Among the Gram-negative isolates E. coli was resistant to ampicillin (73.9%), cotrimoxazole (56.5%), and tetracycline (65.2%), while sensitive to gentamycin, and AMC (91.3%) each, meropenem (82.6%), ceftriaxone, and ceftazidime (82.2%) each.
Gram-positive bacterial isolates show resistance to norfloxacin (66.7%) and high level of sensitivity to penicillin (94.4%), vancomycin (88.9%), clindamycin and erythromycin (86.1%) each gentamycin and tetracycline (69.4%) each ciprofloxacin (66.7%) and cotrimoxazole (52.8%). Staphylococcus aureus was resistant to norfloxacin (85.7%) and was sensitive to both penicillin and vancomycin (90.5%), gentamycin (81.05%), ciprofloxacin, clindamycin, erythromycin, and tetracycline (76.2%) each, and cotrimoxazole (57.1%). In the present study the overall prevalence of MDR pattern was (80.3%) this result is higher than the studies conducted in Mekele, Addis Ababa, and Harar, Ethiopia18,39,42 respectively. In this study, the prevalence of MDR pattern of Gram-negative and Gram-positive bacteria were (79.1%) and (83.3%) respectively. The MDR of Gram-negative bacteria in this study higher than the result which was reported from Harar, Ethiopia.42
Multidrug-resistant (MDR) Gram-positive bacteria in this study were higher than studies conducted in Mekele, Addis Ababa, Harar, Ethiopia respectively.18,39,42 The possible reason for this MDR might be the self-medication history of study participants.
This study reported that sex, being female was 3.10 (1.80, 5.32), p=<0.0001, education, study participants who had no formal education 3.56 (1.55, 8.21) p=0.003, participants who had only primary education 2.22 (1.10, 4.45) p=0.025 and self-medication history 6.17 (3.54, 10.74), p=0.0001 had a statistically significant association with SUTI. As reported in this study in many other studies sex had been reported as a statistically significant factor for SUTI in Uganda,51 similar studies in Ethiopia in Mekele, Ethiopia.18
Study in Metu, Ethiopia reported being female 3.56 (1.44, 8.76) and educational status, participants who were not able to read and write had 2.6 (1.19, 5.49) statistical association with SUTI,45 similarly in Nekemt, Ethiopia level of education (no formal education) had statistically significant with SUTI 9.3 (1.1–79.2).23
In another study which was conducted in Bale Zone, Southeast Ethiopia, study participants with low educational level were statistically significant with SUTI 6.617 (1.87, 9.94, p=0.024). The possible reason for the significant association of SUTI with no formal education might be due to participants who had no formal education lack knowledge about personal hygiene and contamination of bacteria from the gastrointestinal tract.52
Limitation of the Study
This study had its limitation, first, absence of novobiocin disc, this may lead differentiation of coagulase-negative Staphylococcus species, Second, the urine sample was collected by the patient itself-may not avoid contamination during sample collection. And third, a recall bias by study participants may exist.
Conclusions
Urinary tract infection among adults was prevalent in the study area. Being female, educational status and self-medication history had a significant association with urinary tract infection. Most isolated bacteria were resistant to Ampicillin and Tetracycline.
Abbreviations
AST, antibiotic susceptibility test; ATCC, American Type Culture Collection; BAP, blood agar plate; CDC, Center for Disease Control and prevention; CFU, colony forming unit; CLSI, Clinical and Laboratory Standards Institute; CSA, Central Statistical Agency; CoNs, coagulase-negative Staphylococcus; EDHS, Ethiopian Demographic Health Survey; H2O2, hydrogen peroxide; H2S, hydrogen sulfide; HUCSH, Hawassa University Comprehensive Specialized Hospital; IRB, Institutional Review Board; KB, Kirby Bauer; MDR, multi-drug resistance; MHA, Muller-Hinton agar; NB, nutrient broth; OPD, outpatient department; SGC, School of Graduate Committee; SNNPRs, Southern Nations Nationalities People Regional State; SPSS, Statistical Package for Social Science; SUTI, symptomatic urinary tract infection; TSI, triple sugar iron; UTI, urinary tract infection; WBCs, white blood cells; WHO, World Health Organization.
Data Sharing Statement
The data that support the findings of this study will be available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Ethical clearance was obtained from Hawassa University College of Medicine and Health Science Institution Review Board (IRB) Ref. No IRB/012/10. A permission and support letter was obtained from the Hospital administration office. Data were collected from study participants after explaining the purpose and procedure of the study and assuring the confidentiality of their results then obtaining informed written consent. The laboratory diagnosis of study participants was done without cost. Both the negative and positive results were given back to the doctor for appropriate management. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank Hawassa University College of Medicine and Health Sciences and School of Medical Laboratory Science for sponsoring the research and Hawassa University Comprehensive Specialized Hospital for unreserved material and reagent supply that made the study possible. We extend our gratitude to all data collectors for their cooperation and efforts during the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was obtained.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Getachew K, Tamirat A, Adane M. A retrospective study on prevalence and antimicrobial susceptibility patterns of bacterial isolates from urinary tract infections in Tikur Anbessa Specialized Teaching Hospital Addis Ababa, Ethiopia, 2011. Ethiop J Health Dev. 2013;27(2):111–117.
2. Gajdács M, Ábrók M, Lázár A, Burián K. Comparative epidemiology and resistance trends of common urinary pathogens in a tertiary-care hospital: a 10-Year Surveillance Study. Medicina. 2019;55:356. doi:10.3390/medicina55070356
3. Scott K, George AS, Ved RR. Taking stock of 10 years of published research on the ASHA programme: examining India’s national community health worker programme from a health systems perspective. Health Res Policy Syst. 2019;17(1):29. doi:10.1186/s12961-019-0427-0
4. Alanazi MQ, Alqahtani FY, Aleanizy FS. An evaluation of E. coli in urinary tract infection in emergency department at KAMC in Riyadh, Saudi Arabia: retrospective study. Ann Clin Microbiol Antimicrob. 2018;17(1):3. doi:10.1186/s12941-018-0255-z
5. Sewify M, Nair S, Warsame S, et al. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. J Diabetes Res. 2016;2016:6573215. doi:10.1155/2016/6573215
6. Gajdács M, Urbán E. Comparative epidemiology and resistance trends of proteae in urinary tract infections of inpatients and outpatients: a 10-Year Retrospective Study. Antibiotics. 2019;8:91. doi:10.3390/antibiotics8030091
7. Gajdács M, Burián K, Terhes G. Resistance levels and epidemiology of non-fermenting gram-negative bacteria in urinary tract infections of inpatients and outpatients (RENFUTI): a 10-Year Epidemiological Snapshot. Antibiotics. 2019;8:143. doi:10.3390/antibiotics8030143
8. Vasudevan R. Urinary tract infection an overview of the infection and the associated risk factors. J Microbiol Exp. 2014;1(2).
9. Tiruneh M, Yifru S, Gizachew M, et al. Changing trends in prevalence and antibiotics resistance of uropathogens in patients attending the Gondar University Hospital, Northwest Ethiopia. Int J Bacteriol. 2014;1–7.
10. Gajdács M. The importance of reporting clinical and epidemiological data in urology: local experiences and insights from the international literature. Medicina. 2020;56(11):581. doi:10.3390/medicina56110581
11. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. National Center for Health Statistics. Vital Health Stat 13. 2011;169:1–38.
12. Michno M, Sydor A, Walaszek M, Sulowicz W. Microbiology and drug resistance of pathogens in patients hospitalized at the Nephrology Department in the South of Poland. Pol J Microbiol. 2018;67(4):517–524. doi:10.21307/pjm-2018-061
13. Toner L, Papa N, Aliyu SH, Dev H, Lawrentschuk N, Al-Hayek S. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in hospital urinary tract infections: incidence and antibiotic susceptibility profile over 9 years. World J Urol. 2016;34(7):1031–1037. doi:10.1007/s00345-015-1718-x
14. Kabew G, Abebe T, Miheret A. A retrospective study on prevalence and antimicrobial susceptibility patterns of bacterial isolates from urinary tract infections in Tikur Anbessa Specialized Teaching Hospital Addis Ababa, Ethiopia. Ethiop J Health Dev. 2011;27(2):112–117.
15. Gezmu T, Regassa B, Manilal A. Prevalence, diversity and antimicrobial resistance of bacteria isolated from the UTI Patients of Arba Minch Province, Southern Ethiopia. Transl Biomed. 2016;7(3):81. doi:10.21767/2172-0479.100081
16. Hailay A, Zereabruk K, Mebrahtom G, Aberhe W, Bahrey D. Magnitude and its associated factors of urinary tract infection among adult patients attending Tigray Region Hospitals, Northern Ethiopia. Int J Microbiol. 2019;2020.
17. Duffa Y, Kitila K, Gebretsadik D, Bitew A. Prevalence and antimicrobial susceptibility of bacterial uropathogens isolated from pediatric patients at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. Int J Microbiol. 2018;2018:8492309.
18. Gebremariam G, Legese H, Woldu Y, Araya T, Hagos K, Wasihun A. Bacteriological profile, risk factors and antimicrobial susceptibility patterns of symptomatic urinary tract infection among students of Mekelle University, northern Ethiopia. BMC Infect Dis. 2019;19:950. doi:10.1186/s12879-019-4610-2
19. Belete Y, Asrat D, Woldeamanuel Y, Yihenew G, Gize A. Bacterial profile and antibiotic susceptibility pattern of urinary tract infection among children attending Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia. Infect Drug Resist. 2019;12:3575–3583. doi:10.2147/IDR.S217574
20. Kiros T, Asrat D, Ayenew Z, Tsige E. Bacterial urinary tract infection among adult renal transplant recipients at St. Paul’s hospital millennium medical college, Addis Ababa, Ethiopia. BMC Nephrol. 2019;20(1):289. doi:10.1186/s12882-019-1485-9
21. Gajdács M, Ábrók M, Lázár A, Burián K. Increasing relevance of Gram-positive cocci in urinary tract infections: a 10-year analysis of their prevalence and resistance trends. Sci Rep. 2020;10(1):17658. doi:10.1038/s41598-020-74834-y
22. Gajdács M. The concept of an ideal antibiotic: implications for drug design. Molecules. 2019;24(5):892. doi:10.3390/molecules24050892
23. Kebamo S, Dabsu R, Deressa A, Gebire M. Urinary Tract infection: bacterial etiologies, drug resistance profile and associated risk factors among diabetic patients attending NRH. Am J Curr Microbiol. 2017;5(1):19–32.
24. Abdulahi II, Gebre-Selassie S. Common bacterial pathogens and their antimicrobial susceptibility patterns in patients with symptomatic urinary tract infections at HiwotFana and Jugal Hospitals, Harar City, Eastern Ethiopia. Galore Int J Health Sci Res. 2018;3(2).
25. Beyene G, Tsegaye W. Bacterial uropathogens in urinary tract infection and antibiotic susceptibility pattern in Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21(2):141–146. doi:10.4314/ejhs.v21i2.69055
26. Hryniewicza K, Szczypab K, Sulikowskab A, Jankowskia K, Betlejewskab K, Hryniewiczb W. Antibiotic susceptibility of bacterial strains isolated from urinary tract infections in Poland. J Antimicrob Chemother. 2001;47:773–780. doi:10.1093/jac/47.6.773
27. Chandrasekhar D, Dollychan A, Roy BM, Cholamughath S, Parambil JC. Prevalence and antibiotic utilization pattern of uropathogens causing community-acquired urinary tract infection in Kerala, India. J Basic Clin Physiol Pharmacol. 2018;29(6):671–677. doi:10.1515/jbcpp-2018-0015
28. Commission FDroEPC. Summary and statistical report of the 2007 population and housing census results; 2015.
29. Woldemariam HK, Geleta DA, Tulu KD, et al. Common uropathogens and their antibiotic susceptibility pattern among diabetic patients. BMC Infect Dis. 2019;19(1):43. doi:10.1186/s12879-018-3669-5
30. Cheesbrough M. District Laboratory Practice in Tropical Countries.
31. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4_ts):493–496. doi:10.1093/ajcp/45.4_ts.493
32. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2019.
33. Bitew A, Molalign T, Chanie M. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect Dis. 2017;17(1):654. doi:10.1186/s12879-017-2743-8
34. Alo MN, Saidu AY, Ugah UI, Alhassan M. Prevalence and antbiogram of bacterial isolates causing urinary tract infections at Federal Teaching Hospital Abakaliki I (FETHA I). Br Microbiol Res J. 2015;8(2):403–417. doi:10.9734/BMRJ/2015/16696
35. Otajevwo FD. Urinary tract infection among symptomatic outpatients visiting a Tertiary Hospital Based in Midwestern Nigeria. Glob J Health Sci. 2013;5(2). doi:10.5539/gjhs.v5n2p187
36. Obiofu E, Ige O, Iroro O. Antimicrobial susceptibility pattern of urinary isolates from outpatients suspected for urinary tract infection. GSC Biol Pharm Sci. 2018;05(03):001–011.
37. Abate D, Kabew G, Urgesa F, Meaza D. Bacterial etiologies, antimicrobial susceptibility patterns and associated risk factors of urinary tract infection among diabetic patients attending diabetic clinics in Harar, Eastern Ethiopia. East Afr J Health Biomed Sci. 2017;1(2):11–20.
38. Getu Y, Ali I, Lema T, Belay H, Yeshetela B. Bacteriuria and antimicrobial susceptibility pattern among HIV patients attending ALERT Center, Addis Ababa, Ethiopia. Am J Health Res. 2017;5(3):76–82. doi:10.11648/j.ajhr.20170503.14
39. Fenta G, Legese M, Weldearegay G. Bacteriuria and their antibiotic susceptibility patterns among people living with HIV attending Tikur Anbessa Specialized and Zewditu Memorial Hospital ART Clinics, Addis Ababa, Ethiopia. J Bacteriol Parasitol. 2016;7(5). doi:10.4172/2155-9597.1000292
40. Willet W, Radovic V. Urinary tract pathogens and Antibiotic sensitivity patterns in Dar es Salaam. East Afr Med J. 1976;53:685–692.
41. Seifu WD, Gebissa AD. Prevalence and antibiotic susceptibility of Uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect Dis. 2018;18(1):30. doi:10.1186/s12879-017-2911-x
42. Marami D, Balakrishnan S, Seyoum B. Prevalence, antimicrobial susceptibility pattern of bacterial isolates, and associated factors of urinary tract infections among HIV-Positive Patients at Hiwot Fana Specialized University Hospital, Eastern Ethiopia. Can J Infect Dis Med Microbiol. 2019;2019:6780354. doi:10.1155/2019/6780354
43. Ogundeji A. Prevalence of urinary tract infection (UTI) and antimicrobial susceptibility pattern among patients attending National Hospital Abuja, Fct - Nigeria. South Am J Public Health. 2015;3(1).
44. Nader M, Tasnim T, Neetu F, Ethika Hossain E, Mosharraf A. Prevalence and antibiogram of UTI in a Tertiary Care Hospital (Chattagram Maa-O-Shishu Hospital); 2018.
45. Gutema T, Weldegebreal F, Marami D, Teklemariam Z. Prevalence, antimicrobial susceptibility pattern, and associated factors of urinary tract infections among adult diabetic patients at Metu Karl Heinz Referral Hospital, Southwest Ethiopia. Int J Microbiol. 2018;2018:7591259. doi:10.1155/2018/7591259
46. Kumar Maji S, Maity C, Kumar Halder S, Paul T, Kumar Kundu P, Chandra Mondal K. Studies on drug sensitivity and bacterial prevalence of UTI in Tribal Population of Paschim Medinipur, West Bengal, India. Jundishapur J Microbiol. 2012;6(1):42–46. doi:10.5812/jjm.4756
47. Nigussie D, Amsalu A. Prevalence of uropathogen and their antibiotic resistance pattern among diabetic patients. Turk J Urol. 2017;43(1):85–92. doi:10.5152/tud.2016.86155
48. Pardeshi P. Prevalence of urinary tract infections and current scenario of antibiotic susceptibility pattern of bacteria causing UTI. Indian J Microbiol Res. 2018;5(3):334–338.
49. Tektook N, Al-Lehibi KI, Al-Husseinei R. Prevalence some pathogenic bacteria causing UTI in diabetic patients in/specialized center for endocrinology and diabetes of Baghdad City-Iraq. Med J Babylon. 2017;14:260–266.
50. Gajdács M, Bátori Z, Ábrók M, Lázár A, Burián K. Characterization of resistance in gram-negative urinary isolates using existing and novel indicators of clinical relevance: a 10-year data analysis. Life. 2020;10(2):16. doi:10.3390/life10020016
51. Kabugo D, Kizito S, Ashok DD, et al. Factors associated with community-acquired urinary tract infections among adults attending assessment centre, Mulago Hospital Uganda. Afr Health Sci. 2016;16(4):1131–1142. doi:10.4314/ahs.v16i4.31
52. Taye S, Getachew M, Desalegn Z, Biratu A, Mubashir K. Bacterial profile, antibiotic susceptibility pattern and associated factors among pregnant women with urinary tract infection in Goba and Sinana Woredas, Bale Zone, Southeast Ethiopia. BMC Res Notes. 2018;11(1):799. doi:10.1186/s13104-018-3910-8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.