Back to Journals » Drug Design, Development and Therapy » Volume 14
Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory Properties (Mini Review)
Authors Jenab A, Roghanian R, Emtiazi G
Received 9 May 2020
Accepted for publication 26 August 2020
Published 18 September 2020 Volume 2020:14 Pages 3787—3801
DOI https://doi.org/10.2147/DDDT.S261283
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Tin Wui Wong
Anahita Jenab, Rasoul Roghanian, Giti Emtiazi
Biological Science and Technology, Department of Cellular and Microbiology, University of Isfahan, Hezar Jerib, Isfahan, Iran
Correspondence: Anahita Jenab Tel/ Fax +983137932455
Email [email protected]
Abstract: Inflammation is part of the body’s complex biological response to harmful stimuli such as damaged cells, pathogens, or irritants. It is a protective response involving blood cells, immune cells, and molecular mediators. The inflammation not only can eliminate the primary cause of cell injury but also clears out necrotic cells, tissue damaged from the original insults and inflammatory process. Furthermore, it can initiate tissue repair. Pro-inflammatory cytokines are produced predominantly by activated macrophages and are involved in the up-regulation of inflammatory reactions. They are involved in further regulating inflammatory reactions. There is ample evidence that some pro-inflammatory cytokines, such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α), are involved in the pathological pain process. Some of the natural compounds promote cytokines production and inhibit inflammatory responses. The natural compounds which are produced from microorganisms such as omega-3 fatty acid, cyclic peptide, antimicrobial peptide, oligosaccharides, and polysaccharides can reduce inflammation and could be easily incorporated into the diet without any adverse effects. For example, SCFA (short-chain fatty acids), peptide bacteriocin, and polycyclic peptide bacteriocin (nisin) could be used in the treatment of atherosclerosis, orthopedic postoperative infections, and mycobacterium tuberculosis infection, respectively. Also, fatty acids (saturated and unsaturated fatty acids) can be introduced as anti-inflammatory drugs. This review article summarizes bacterial natural compounds with modulating effects on cytokines that are surveyed which may have potential anti-inflammatory drug-like activity.
Keywords: bacterial natural materials, secondary metabolite, drug-like activity, anti-inflammatory activity, cytokine
Introduction
In nature, there are unlimited sources of pharmaceutical agents, and the origin of several modern medicines are found in natural products. The natural products include chemical compounds either as standardized extracts or as pure compounds produced by living organisms with biological effects on other organisms. Due to the availability of incomparable chemical diversity, natural product has interestingly enormous opportunities for new drugs.1 Natural materials are found in multicellular origins such as plants, animals, marine sources, sponges, snails, and unicellular origins like bacteria and yeasts with pharmaceutical properties. For example, Borojó fruit with antihypertensive, diuretic, antitumor, healing, anti-inflammatory, aphrodisiac, and immunological effects,2 Austroeupatorium inulaefolium (H.B.K.) essential oil with antimicrobial activity,3 and the lavender essential oil with anti-inflammatory, antimicrobial and antioxidant activity were examined.4
In addition to temperature, the body maintains many factors of homeostasis. For example, the concentration of different ions in the blood must be constant, along with pH and glucose concentration. Maintaining homeostasis at any level is important for maintaining overall body function. The immune system is one of the most dynamic in the body. The mechanisms that keep immune homeostasis must be engaged in the continuous turnover of cell proliferation and apoptosis in many lineages in different locations. The mechanisms must also deal with challenges eg responses to a variety of pathogens, cancer or drug, and reset the status to the normal situations. The cell types involved in homeostasis in the immune system are immune cells such as B cell, dendritic cells, Natural killer cells (NK cells), and epithelial cells.5
Anti-inflammatory cytokines are a variety of immune regulatory molecules with the response to pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, INF-γ, and TNF-α. Anti-inflammatory cytokines include antagonist IL-1 receptor, IL-4, IL-6, IL10, IL-11, and IL-13.6
Nitric oxide (NO) radicals and COX-2 are produced during inflammation. NO is produced by oxygen nitric oxide synthase (iNOS) from oxygen and L-arginine; this enzyme is regulated during the inflammatory process. Like iNOS, COX-2 is regulated precisely in response to infectious agents, atherosclerosis, and many malignancies. The up-regulation of iNOS and COX-2 during inflammation is controlled by the pro-inflammatory transcription factor NF-κB.7 Some of the members of the toll‐like receptor (TLR) family, especially TLR4, are now known as the main receptors for LPS. On the other hand, IκB is a suppressive protein that binds to the NFκB transcription factor in a normal condition. So, the degradation of IκB stimulated by LPS leads to the activation of this special transcription factor for COX‐2 mRNA induction (Figure 1).8
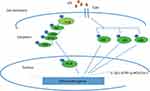 |
Figure 1 Schematic representation of the mechanisms in LPS-induced inflammation associated with iNOS and COX2. |
Unicellular organisms like bacteria and yeasts include probiotics from the gastrointestinal tract (GIT) that could be useful for inflammatory bowel disease and treatment of infectious diarrhea.2 Common commensal microbes such as Lactobacilli, Bifidobacteria, Bacteroides fragilis, E.coli, Bacillus sp, etc can produce the natural compounds with anti-inflammatory and immunomodulatory properties.
Culturable microbial varieties in the Hypersaline Lake belong to the various genera like Halorubrum, Haloarcula, Salinibacter, Salicola, Rhodovibrio, Proteobacteria, Firmicutes, and Actinobacteria. They could be useful in biotechnological applications, such as treatment of saline wastewater and the production of salt-tolerant enzymes, β-carotene, compatible solutes, biofuels, and bioplastics.9 Bacteria associated with marine algae produce natural compounds such as genus Alteromona. The genus Alteromona is found only in Laurencia Pacifica, generally isolated in the seawater and produced high molecular weight polysaccharide compounds used in the industries and medicines.10 Also, several marine bacteria could be introduced as producers of secondary metabolites. Chorommobacteria marinum produces secondary metabolites with antibacterial activity against Escherichia coli (E.coli), Pseudomonas aureginosa (P.aureginosa), Staphylococcus aureus (S.aureus) and are useful for the treatment of pneumonitis, osteitis, arthritis, endocarditis, and localized abscesses. Also, Flavobacterium uliginosum (F. uliginosum) with anti-cancerous activity against sarcoma cells could be used in the treatment of viral tumors.10 The cationic peptides with bacterial origin can prevent the secretion of pro-inflammatory cytokines (eg, TNF-α, IL- 1) by the host and are useful in acne vulgaris therapy.11
Natural microbial compounds have several advantages. The most important properties of the natural microbial compounds are their microbial origin, as their specific microbial producers; their unique chemical structures and their interaction with the environment, as various biological activities.12,13 Natural compounds such as omega-3 fatty acid, cyclic peptide, antimicrobial peptide, acetyl derivatives, oligosaccharides, and polysaccharides can reduce inflammatory responses.
This review concentrates on the natural bacterial material with a modulating effect on cytokines and their application in anti-inflammatory drug-like activity (Tables 1–3) (Figure 2–3). The advantages of using bacteria as anti-inflammatory inhibitors are their faster and more natural growth than other microorganisms in large-scale. Additionally, bacterial cell mass could be used without enzyme purification.14
 |
Table 1 Effects of Bacterial Bio-Active Materials Containing Peptide on Cytokine Production and Disease Therapy |
 |
Table 2 Effects of Bacterial Bio-Active Materials Containing Fatty Acids on Cytokine Production and Disease Therapy |
 |
Table 3 Effects of Other Bacterial Bio-Active Materials on Cytokine Production and Disease Therapy |
 |
Figure 2 Summary of the bacterial bio-active compounds, active components, their producing bacteria, and potential mechanism of their actions (anti-inflammatory). |
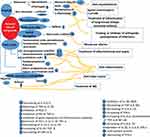 |
Figure 3 Summary of the bacterial bio-active compounds, active components, the potential mechanism of their actions, and treatment of different diseases. |
Bacterial Natural Compounds with Anti-Inflammatory Activities
Biosurfactants
Biosurfactants are natural products derived from bacteria, yeasts, or fungi. They are amphiphilic compounds, including a polar section (soluble in water) and a non-polar section (insoluble in water). The amphiphilic structure of biosurfactant is useful to reduce surface tension. This feature is used in various industries such as petroleum, petrochemical, pharmaceutical, cosmetics, medicine, agriculture, textiles, food industries, and many others.15 Microorganisms use different types of organic compounds as a source of carbon and energy for their growth. When the source of carbon is an insoluble substrate such as hydrocarbons (CxHy), microorganisms facilitate their diffusion into the cell by producing different types of substances, the biosurfactants. They decrease surface tension in bacteria. A biosurfactant may have one of the following structures: glycolipids, a polysaccharide–lipid complex, phospholipid, mycolic acid, lipoprotein, or lipopeptide. Production of biosurfactant can be induced by hydrocarbons or other water-insoluble substrates, the culture conditions, such as temperature, pH, agitation, the concentration of P, Fe, N, Mg, and Mn ions in the medium and dilution rate in continuous culture.16 Microbial bio-surfactants have more advantages than chemical surfactants. They are higher foaming capability, less toxic, environmentally compatible, higher biodegradability, specific activity at extreme pH, temperatures, high selectivity. Also, it can be obtained from renewable food sources.15 Bacteria such as Bacillus, Pseudomonas, Rhodococcus, Arthrobacter, Mycobacterium, etc. can make biosurfactants. Bio-surfactant produced from Bacillus mojavensis showed antibacterial activities. So, it could be used as a natural product with potential medicinal usages. It needs more studies about the mechanisms of the treatment related to this bio-surfactant.17 Some lipopeptides like N-palmitoyl-S-(2,3-bis(palmitoyloxy)-(2RS)-propyl)-(R)-cysteinyl-a and alanyl-glycine (Pam3Cys-Ala-Gly), are a synthetic analog of the N-terminal part of bacterial lipoprotein and capable of activating macrophages and B cells in vitro. After injecting the lipopeptide to mice, low levels of IL-6 and no levels of TNF-α were detected in the serum.16 In contrast, bacterial LPS induced pro-inflammatory cytokines such as IL-6, IL-11, and TNF-α, indicating the lack of immune cell response to lipopeptide While, bacterial LPS induces very high amounts of IL-6, IL-1, and TNF-α.18 Labeo rohita fingerlings were injected intraperitoneally with purified biosurfactant from Bacillus licheniformis VS16 at various concentrations. It was shown that bio-surfactant produced by Bacillus licheniformis VS16 cause to down-regulates of pro-inflammatory cytokines TNF-α and IL-1β. But the expression of anti-inflammatory cytokines IL-10 and TGF-β were up-regulated.15 Bio-surfactant can inhibit biofilm formation, and it removes cadmium (Cd) from tested vegetables such as radish, ginger, potato, and carrot so that it could be useful for food industries.15
Surfactin is a bacterial cyclic lipopeptide produced by B. subtilis. Because of its multifunctional interactions with biological systems, it has several physiological and biochemical activities. The anti-inflammatory mechanism of surfactin in lipopolysaccharide (LPS)-stimulated macrophages showed that surfactin prevents the formation of inflammatory agents like IL-1β and iNOS; also, it decreases TNF-α and nitric oxide levels in response to septic shock.19,20 TLR4 is the central receptor for LPS; thus, the TLR4 signal transduction pathway is the major pathway that mediates LPS-induced inflammation. The results show that surfactin down-regulated the LPS-induced TLR4 protein expression of macrophages. It was also shown that surfactin has an anti-inflammatory property by reducing the activation of nuclear factor-κB (NF-κB), which is involved in the nuclear factor-κB (NF-κB) cell signaling pathways.20,21 The anti-inflammatory activity of surfactin was demonstrated in the interaction of LPS with macrophage cells. The proposed mechanism of the anti-inflammatory activity includes interaction with cytosolic phospholipase A2 (PLA2), inhibition of lipoteichoic acid (LTA)-induced NF-κB, activation of signal transducer and activator of transcription-1 (STAT-1), modulation of the TLR4 and the nuclear factor-κB (NF-κB) cell signaling pathways, and increased phosphorylation of STAT-3.21 There are few reports of clinical trials. Significant efforts are needed to develop and use these materials as therapeutic drugs.21
Other cyclic lipopeptides like fengycin, and iturin lipopeptides, which are produced by B. subtilis have a variety of capabilities in biological activities, containing interactions with biofilms, anti-fungal, anti-tumor, anti-virus, anti-platelet, and anti-inflammatory properties.21
Polysaccharide
Polysaccharides are long chains of carbohydrate molecules, specifically polymeric carbohydrates that are composed of monosaccharide units joined together by glycosidic bonds. Polysaccharides contain more than ten monosaccharide units, whereas oligosaccharides contain three to ten monosaccharide units. They are classified according to their biological functions into intracellular storage polysaccharides (glycogen), capsular polysaccharides that are close to the cell surface, and extracellular bacterial polysaccharides. Extracellular bacterial polysaccharide such as xanthan, alginate, cellulose, and levan is produced by Xanthomonas campestris, Pseudomonas and Azotobacter, Acetobacter xylinum, Leuconostoc mesenteroides respectively with different applications.22 Also, exopolysaccharide (EPS) producing lactic acid bacteria strains have a variety of health benefits for their hosts such as anti-inflammatory, antioxidant, antitumor, and stress-tolerant effects.23
2-1-Kefiran is a polysaccharide (Branched glucogalactan) produced by lactic acid bacteria of Kefir. Due to the antimicrobial activity of kefiran against isolated Pseudomonas, Rhizoctonia, and S. aureus, kefiran nanofiber was introduced as a biocontrol agent for food packaging and food preservation.24 Also, Jenab et al examined the amount of the entrapped platelets in kefiran and the released platelets from kefiran by coulter counter. Results showed that, in the beginning, platelets are decreased, and then the platelets are released from kefiran. So, Kefiran may have the potential to be used for surface bleeding. The presence of nisin in kefiran with lantibiotics properties is an advantage of kefiran. It induces both CD4+ and CD8+ T-lymphocytes populations.25 Interleukin-4 (IL-4) and interleukin-5 (IL-5) were decreased to the normal levels after administration of kefiran in BALB/c mice stimulated with ovalbumin.26 So, Kefiran could be used to treat pulmonary inflammation in a murine model.26 Kefiran is not only an excellent promising of allergic bronchial asthma therapy and food packaging but also is an accurate candidate as a drug for the treatment of inflammatory, bacterial infection, tumor cells, surface bleeding. On the other hand, kefiran extraction is easily done at a low cost by ethanol.24,25 Also, Jenab et al confirmed that no significant changes were measured for the level of IL-6 in PAN/kefiran 5% nanofiber treated PBMC cell cultures compared to the control (p ≥ 0.05), while PAN nanofiber revealed the enhancement level of IL-6. So, the kefiran-PAN nanofiber may be promising for the neural stem cell culture, especially for repairing the injured spinal cord. So, kefiran is safer and user-friendly to humans and animals with no side effects, which may have potential anti-inflammatory drug-like activity.27
2-2-Exopolysaccharide (EPS) from the probiotic spore-forming bacterium Bacillus subtilis Protects Mice from acute colitis induced by the enteric pathogen Citrobacter rodentium. It can broadly inhibit T cell activation and thus control T cell-mediated immune responses in various inflammatory diseases.28
2-3-Polysaccharide capsules in the intestine. The capsular polysaccharide of Bacteroides cellulosilyticus and Bifidobacterium bifidum induces IL-10. Due to that 2,4,6-trinitrobenzene sulfonic acid (TNBS) induces chemical colitis, this polysaccharide can attenuate TNBS-induced colitis.29 Also, Capsular polysaccharide of Bacteroides fragilis induces IL-10. It protects against TNBS-induced colitis and Helicobacter hepaticus-induced colitis. Bifidobacterium longum decreases IFN-γ, IL-12, TNF-α, IL-17, and IL-6 production and protects against the T cell transfer model of colitis.28 EPS from Streptococcus thermophilous in the intestine decreases IL-6, IFN-γ, and TNF-α production. EPS from Pediococcus parvulus, the commensal bacterium in the intestine, decreases TNF-α and IL-8 production. EPS from Faecalibacterium prausnitzii the commensal bacterium in the intestine, decreases IL-12 and IFN- γ and increases IL-10 secretion through TLR-2 signaling by Lactobacillus plantarum attenuates DSS-colitis. Bifidobacterium longum decreases.28
2-4- Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 was examined in the rat model. It decreases expression levels of pro-inflammatory mediators IL-1β, TNF-α,/and iNOS, and increases the level of anti-inflammatory cytokines such as IL-10 and IL-6, while neutrophil infiltration was not changed. The EPS CG11 was examined as antihyperalgesic and anti-edematous agents. Intraperitoneal administration of EPS CG11 was used in a model of inflammatory rats induced by carrageenan injection in the hind paw. EPS CG11 decreased the hind paw swelling and pain sensations (mechanical hyperalgesia) in a dose-dependent manner.
It was measured by the plethysmometer and von Frey anesthesiometer, respectively. So, this bacterial EPS has the antihyperalgesic effect as the novel property of bacterial EPSs.30
2-5- The EPS derived from Bacillus licheniformis BioE-BL11 and Leuconostoc mesenteroides BioE-LMD18, isolated from Korean fermented kimchi inhibited secretion of the pro-inflammatory cytokine IL-6 in lipopolysaccharide-stimulated RAW264.7 mouse macrophage. Also, it enhanced the secretion of the anti-inflammatory cytokine IL-10 in a dose-dependent manner.31
2-6- The mucous variant of Lactobacillus rhamnosus RW-9595M induced low or no TNF-α and IL-6 and decreased the inflammatory cytokine. Also, the EPS of Lactobacillus rhamnosus RW-9595M increased the IL-10 produced by macrophages. Whereas, conditioned media were produced by macrophages treated with parental Lactobacillus rhamnosus induced higher levels of TNF-α, IL-6, and IL-12 but inhibited IL-10 production. So, the EPS from Lact. rhamnosus RW-9595M may be useful as a new immunosuppressive product in dairy food.32
Acetyl Derivatives
Indol-3 acetic acid (IAA) is a derivative of indole, containing a carboxymethyl residue. It is the most important and the most abundant auxin hormone that stimulates the growth of plants.33,34 It is produced by Rhizobium strains, symbiotic, Paenibacillus non-symbiotic bacteria and Pseudomonas fluorescens.35 IAA with antioxidant effect shows anti-inflammatory activity against croton oil- and arachidonic acid-induced mouse ear edema.36 The indole-3-acetic acid produced by Burkholderia heleia plays a role as a phenylacetic acid antagonist to inhibit tropolone biosynthesis in Burkholderia plantarii.37 Burkholderia heleia PAK1-2 is a potential biocontrol agent that can suppress B. plantarii virulence.
Fatty Acids
Fatty acids have long and linear aliphatic chains. They are classified according to the link between the carbon including saturated fatty acids and unsaturated fatty acids and the other based on the length of the chain including SCFA (short-chain fatty acids), MCFA (medium-chain fatty acids), LCFA (long-chain fatty acids) and VLCFA (very-long-chain fatty acids). Due to the limited sources of ω3 and ω6 fatty acids from animal and plant sources, attention has been focused on microbial production like marine bacteria (Shewanella sp. strain SCRC-2738).38 The amounts and relative ratio of acetate, glycerol, carbohydrate, lipid, and nitrogenous substances available in the medium; oxygen supply; pH; and the age of the culture influence the fatty acid content of the bacteria. It should be mentioned that the extraction of bacterial fatty acids is difficult. Prevention of NO and TNF-α production in LPS stimulated macrophages were related to the higher level of Saturated Fatty Acids (SFAs) and Poly Unsaturated Fatty Acids (PUFA), and a lower concentration of Mono Unsaturated Fatty Acids (MUFAs). The composition of the fatty acids can influence anti-inflammatory activities.39
Unsaturated Fatty Acids (ω3 and ω6)
Fatty acid chains have at least one double bond. Unsaturated Fatty acids (ω3 and ω6) are precursors of lipid intermediates. Marine bacteria like Vibrio cyclitrophicus were identified as EPA producer.38 Each strain of Cellulophaga and Pibocella could produce EPA and DHA, respectively. While Polaribacter could produce only DHA or only EPA.40 Shewanella marinintestina contains a significant amount of EPA. Their functions in inflammatory regulation are very important. ω6 fatty acids like arachidonic acid increase inflammation, while ω3 Fatty acids like eicosapentaenoic acid and docosahexaenoic play a role as anti-inflammatory agents. Production of inflammatory cytokines like TNF-α and IL-1β was inhibited. ω3 fatty acids inhibit the formation of ω6 fatty acids-derived pro-inflammatory eicosanoids (eg, PGE2 and LTB4).41 Also, the bacterium Moritella marina produces high levels of DHA. The membranes of Vibrio marinus strain MP-1 contain substantial amounts of DHA.42 Also, arachidonic acid-producing bacteria from marine have been isolated, including; Psychroflexus torquis, Psychroflexus pacifica, Krokinobacter eikastus, and Krokinobacter diaphorus Aureispira marina (a gliding bacterium). They produce the most significant amounts of arachidonic acid (about 40%) that show anti-inflammatory activities.43
Saturated Fatty Acids
Saturated fatty acids are long-chain carboxylic acids, including 12 and 14 carbon atoms like stearic acid without double bonds. They play critical roles in immune responses. It forms from linoleic acid produced by Clostridium proteoclasticum with anti-inflammatory activity.44 Fatty acid analyses revealed the presence of the heneicosanoic acids, pentadecanoic, hexadecanoic, and heptadecanoic acid as primary ester-bound fatty acids in LPS from Chlamydophila psittaci 6BC.45 These fatty acids can influence the anti-inflammatory activity through the inhibition of NO and TNF-α.39
Short-Chain Fatty Acids
Short-chain fatty acids (SCFAs) are fatty acids, including 1–6 carbon atoms. Studies in both animals and humans showed that SCFA reduced cholesterol levels, and they play a significant role in colon health. SCFAs, mainly acetate, propionate, and butyrate, can inhibit from MAPK (mitogen-activated protein kinase) signaling pathway and activation of NF-κB (nuclear factor-κ light-chain) enhancer of activated B cells via activation fatty acid (FFA) receptors type 2, 3 (FFA2 and FFA3 receptors) and GPR109A (G protein-coupled receptor 109A) or inhibition of HDACs (histone deacetylases) (Figure 4). It inhibits gene expression, including inflammatory cytokines, chemokines, and adhesion molecules, which play essential roles in the improvement of atherosclerosis and sepsis.46 The main butyrate producers are Clostridium IV group (Faecalibacterium prausnitzii), and Clostridium XIVa group (Eubacterium rectale, E. halii, Roseburia). Also, the main propionate producers are Bacteroides, and the main acetate producer are Clostridium XIVa group (Blautia hydrogeotrophyca).47
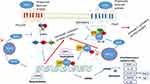 |
Figure 4 The downstream effect of short-chain fatty acids (SCFA) occurs with the regulation of MAPK signaling pathway and activation of NF-κB through two pathways: 1- activation of receptor FFA2, FFA3 receptors 2- inhibition of HDAC (histone deacetylase).46 |
The overexpression of several isoforms of HDACs has been identified in different types of cancerous cells, inflammatory diseases, and neurological diseases. A role for HDAC inhibitors is the suppression of IFN-γ, IL-10, IL-1β, and TNF-α.48 Valeric acid (pentanoic acid) and caproic acid (hexanoic acid) were identified as histone deacetylase enzymes (HDAC) inhibitors, produced with high concentration by Ruminococcaceae bacterium CPB648 and Megasphaera massiliensis MRx0029.49
Peptides
Antimicrobial peptides (AMP) are groups of various antimicrobial compounds. They are of interest due to the importance of pathogenic resistance to conventional antibiotics.50 These compounds include two groups of bacteriocins and antibacterial peptides as based on their biosynthesis mechanism. Bacteriocins are synthesized compounds by ribosomes that are produced in some bacteria and against the others. While antibiotic peptides are not synthesized by ribosomes. They are produced by compression reactions and complex stages using large non-ribosome peptide syntheses (NRPS) enzyme.51,52 Bacteriocins produced by lactobacilli are based on the building and biochemical specifications.51 Bacteriocins are extracellularly released peptides, which are produced by Gram-positive (+) and Gram-negative (-) bacterial species. Gram-negative bacteriocins are typically classified by size. Microcins are less than 20 kDa in size, colicin-like bacteriocins are 20 to 90 kDa in size and tailocins or so-called high molecular weight bacteriocins. Also, bacteriocins are divided into four groups based on their structure.
- 1-Lanthia antibiotics: Active and small peptides against bacteria, containing unusual amino acids of lantinum.
- 2-Small peptides (< 10 kDa), resist heating without lanthionine, and active against membranes.
- Proteins are unstable against heat and large (>10kDa).
- Complex bacteriocins containing lipid or carbohydrate moieties.51
Some antimicrobial peptides are useful as bio-control, such as bacteriocins produced by Bacillus pumilus ZED17, and DFAR strains. These bacteriocins have the antifungal effect against Rhizoctonia solani (agent of the fungal diseases in plants) and inhibitory activity for seed germination.53 Peptide antibiotics such as Actinomycin, Gramisidine, Surfactin are useful in the industry and medical drugs.54,55 Some bacteriocins not only used for antimicrobial activity but also have the potential for usage in high blood pressure treatment like ancovenin. Its mechanism of action in antibacterial activity is that they target the bacterial cell membranes, deplete the transmembrane potential and/or the pH gradient and form membrane pores, resulting in membrane disruption and cellular leakage.56 To be applied as drug agents, they have some obstacles, such as their toxicities and high cost of construction.52 Today, there is a high potential for the usage of antimicrobial peptides, and it needs more studies to be done in this field.
Several bacteriocins have been produced by lactic acid bacteria. Nine lactic acid bacteria with anti-listeria activity were identified and used in food additives.57
LAB is used in anti-mycobacterial (Mycobacterium tuberculosis) therapy. Nisin is the bacteriocin produced by LAB. It increases the bactericidal activity of mononuclear phagocytes. This activity occurs through increasing autophagy-inducing cytokine-like IFN-γ levels and reducing IL-4 and IL-13 that is followed to down-regulate the lung Th2 response, which is known to restrict autophagy. The treatment with probiotics can modulate the immune responses in the lung, which increases the regulatory T cell response in the treatment of PBMCs and macrophages with combined M. tuberculosis and LAB.58,59
Microcins are bacteriocins produced by E.coli in the ribosomal pathway. Microcins with the Bactericide effect have smart mechanisms to cross from the outer and inner membrane of gram-negative bacteria. Microcins use trojan horse strategies and destroy their competitors. It could be used for novel, efficient antibiotics. Pro-inflammatory cytokines levels were determined in intestinal porcine cells line (IPEC-J2 cells) after treatment with biogenic Microcin j25 (MccJ25) and challenge with (enterotoxigenic Escherichia coli K88) ETEC K88. MccJ25 increases the level of IL-6 and IL-10 anti-inflammatory cytokines and modulates the level of tumor necrosis factor-α through inhibition of MAPK and NFκB activation. MccJ25 can be used to protect against ETEC K88, which induce intestinal damage.60,61
Bacteriocin produced by Enterococcus faecium has anti‐listerial activity in the sterile milk that might be useful as a natural preservative.62 On the other hand, E. faecium can produce the neurochemical dopamine from the dietary substrate. Both dopamine and E. faecium can modulate the immune system and increase the production of anti-inflammatory IL-4 and are useful in the treatment of inflammatory diseases. E. faecium probiotics are good enough in IBD therapy. After the per oral administration of E. faecium strain NCIMB 11181 in broiler chickens, the serum levels of IL-4, IL-10, IL-1β, IL-2, IL-6, and IFN-γ are changed.62 Bacteriocins produced by Lactobacillus rhamnosus with antibacterial effect showed significant inhibitory effects on S.aureus biofilm formation. Also, it decreases the level of C Reactive Protein (CRP) and IL-6 in the serum following surgery and infectious diseases. So, bacteriocins could be used in the prevention of postoperative orthopedic infections.63
Lipopeptides, such as amphomycins, polymyxins, teicoplanins, and bacitracin are well-known for immunomodulatory activity. For example, daptomycin was shown to be an immunomodulator. It suppresses the cytokine expression after host immune response stimulation by methicillin-resistant S. aureus. Also, it is a membrane permeabilizing lipopeptide.64
The synthetic bacterial lipopeptide Pam3Cys-Ala-Gly, which is capable of activating macrophages and B cells in vitro, was injected into the mice. After injection, a little increase of IL-6 was detected in the serum without affecting the level of TNF-α.18
Cell-penetrating peptides (CPPs) can pass the cellular membrane. This process can be done alone or in association with bio-active cargo. YopM can down-regulate the transcription of TNF-α, and interleukins 12, 15, and 18 (pro-inflammatory cytokines) without affection on anti-inflammatory cytokines in infected mice with Yersinia pestis (Y.pestis). YopM cell uptake, mediated by the N-terminal -helical domain, is essential to counteract the pro-inflammatory response induced by LPS. It was analyzed in HL60-derived macrophages with either recombinant YopM or the short YopM87-C derivatives, followed by stimulation with LPS (1 g/mL). So, just YopM (without infection with Yersinia) could be suggested as a tool for protein delivery.65,66
Lactobacillus delbrueckii and Streptococcus thermophilus are lactic acid bacteria (LAB), which can inhibit localized inflammatory diseases. Beside conventional therapy, they could be used as an additional treatment. Their usage causes down-regulation of the inflammatory cytokines such as IL-17 and IL-12.67
Other Bacterial Natural Compounds
Secondary Metabolites
Bacillus sp isolated from Neogene permafrost has secondary metabolites, which increases the secretion of pro-inflammatory cytokines (TNF-α, IL-1β, IL-8, IL-2, and IFNγ) and anti-inflammatory cytokines (IL-4 and IL-10) by human peripheral blood mononuclear cells (PBMC). The activity of these metabolites depends on the incubation temperature. Cold isolated Bacillus metabolites at −5°C increase the secretion of Th1-dependent cytokines such as IFN γ. Warm isolated Bacillus metabolites at 37°C increase the secretion of Th2-dependent cytokines such as IL-4. So, metabolites of Bacillus spare materials for evolution and progressing immunomodulating drugs.68
Equol
Isolated new intestinal bacteria, CS1, CS2, and CS3, showed that belonging to the genus Pediococcus and Lactobacillus can produce equol or its related intermediates.69
Pretreatment of PBMC with equol reduces the production of interferon-gamma) IFN- γ).70
Butanol
Bifidobacterium adolescentis (B. adolescentis), which is one of the LAB strains, produces the butanol extract. The butanol extract significantly causes to boost the production of NO and TNF-α, which are cytotoxic for tumor cells.71,72 It was shown that the butanol extract of B.adolescentis SPM0212 dose-dependently prevented the growth of Caco-2, HT-29, and SW480 cells by 70%, 30%, and 40%, respectively, at 200 μg/mL. It was shown that the LAB by-product has anti-tumor immune effects.71 Therefore, this extract has anti-proliferative activity against human colon cancer.73
Polyketide
Camporidine A, polyketide alkaloid, from a gut bacterium of carpenter ant Camponotus kiusiuensis (Streptomyces sp. STA1) displayed an anti-inflammatory activity by suppressing nitric oxide production induced by lipopolysaccharide after treatment of mouse macrophages with these compounds in the iNOS assay.74
Conclusion
In this review, the bacterial natural compounds with modulating effect on cytokines are surveyed, which may have an anti-inflammatory drug like activity. The primary mechanism of the impact of natural products is through modulating cytokines. Cytokines can down-regulate and, or up-regulate different genes. It can affect their associated transcription factors to reduce the signs and symptoms of diseases or disease therapy. The main bacterial natural components are fatty acids, peptide, antimicrobial peptide, secondary metabolite, polysaccharide, and oligosaccharide. The effects of these natural products are mediated mainly by cytokines like TNF-α, IL-1β, IL-6, which are the main pro-inflammatory cytokines and IL-10 that are anti-inflammatory cytokine. Some of these bacterial natural compounds have been used as anti-inflammatory drugs such as biosurfactant of B. licheniformis and Fatty acids (saturated and unsaturated fatty acids). Other bacterial natural compounds could be used as treating drugs such as SCFA (butyrate, propionate, and acetate) in the treatment of atherosclerosis and sepsis, cyclic lipopeptide (surfactin) in treating of septic shock, and peptide bacteriocin in treating and inhibiting of orthopedic postoperative infections. Also, bacteriocin (nisin) is a polycyclic peptide with anti-mycobacterium tuberculosis activity and microcin with anti enterotoxigenic E.coli k88 properties. Among all, kefiran as a polysaccharide natural compound is cheap and user-friendly with easy extraction. Kefiran could be promising as a drug for the treatment of inflammatory especially in repairing injured spinal cord by using kefiran-PAN nanofiber. Because it is confirmed that no significant changes were measured for the level of IL-6 in PAN/kefiran nanofiber treated cells compared to the control (p ≥ 0.05). Also, It is confirmed that morphological changes (differentiation) of PC12 cells were shown in PAN-kefiran 10% nanofiber.27 On the other hand, new unknown bacteria are discovered taxonomically. The new metabolites produced by these new unknown bacteria could be useful in various sections.11,75 They may have significant influences on the treatment of different diseases. This theory requires further studies to be done.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Colegate SM, Molyneux RJ, editors. Bio-active natural products: detection, isolation, and structural determination.
2. Chaves-López C, Usai D, Donadu MG, et al. Potential of Borojoa patinoi Cuatrecasas water extract to inhibit nosocomial antibiotic-resistant bacteria and cancer cell proliferation in vitro. Food Funct. 2018;9(5):2725–2734. doi:10.1039/C7FO01542A
3. Bua A, Usai D, Donadu MG, et al. Antimicrobial activity of Austroeupatorium inulaefolium (HBK) against intracellular and extracellular organisms. Nat Prod Res. 2018;32(23):2869–2871. doi:10.1080/14786419.2017.1385014
4. Donadu MG, Usai D, Mazzarello V, et al. Change in Caco-2 cells following treatment with various lavender essential oils. Nat Prod Res. 2017;31(18):2203–2206. doi:10.1080/14786419.2017.1280489
5. Huntington ND, Gray DH. Immune homeostasis in health and disease. Immunol Cell Biol. 2018;96(5):451–452. doi:10.1111/imcb.12043
6. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat Inflamm. 2014;2014.
7. Moita E, Gil-Izquierdo A, Sousa C, et al. Integrated analysis of COX-2 and iNOS derived inflammatory mediators in LPS-stimulated RAW macrophages pre-exposed to Echium plantagineum L. bee pollen extract. PLoS One. 2013;8(3):e59131. doi:10.1371/journal.pone.0059131
8. Murakami A, Ohigashi H. Targeting NOX, INOS, and COX‐2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121(11):2357–2363. doi:10.1002/ijc.23161
9. Naghoni A, Emtiazi G, Amoozegar MA, et al. Microbial diversity in the hypersaline Lake Meyghan, Iran. Sci Rep. 2017;7(1):11522. doi:10.1038/s41598-017-11585-3
10. Soria-Mercado IE, Villarreal-Gómez LJ, Rivas GG, et al. Bioactive compounds from bacteria associated to marine algae. Biotechnology-Molecular Studies and Novel Applications for Improved Quality of Human Life. Croatia. 2012;25–44.
11. Marta Guarna M, Coulson R, Rubinchik E. Anti-inflammatory activity of cationic peptides: application to the treatment of acne vulgaris. FEMS Microbiol Lett. 2006;257(1):1–6. doi:10.1111/j.1574-6968.2006.00156.x
12. Berdy J. Bio-active microbial metabolites. J Antibiot (Tokyo). 2005;58(1):1–26. doi:10.1038/ja.2005.1
13. Marinelli F, Genilloud O, Fedorenko V, et al. Specialized bioactive microbial metabolites: from gene to product. Biomed Res Int. 2015;2015.
14. Kaur K, Dattajirao V, Shrivastava V, et al. Isolation and characterization of chitosan-producing bacteria from beaches of Chennai, India. Enzyme Res. 2012;2012:1–6. doi:10.1155/2012/421683
15. Giri SS, Sen SS, Jun JW, Sukumaran V, Park SC. Role of Bacillus licheniformis VS16-derived biosurfactant in mediating immune responses in Carp Rohu and its application to the food industry. Front Microbiol. 2017;8:514. doi:10.3389/fmicb.2017.00514
16. Karanth NG, Deo PG, Veenanadig NK. Microbial production of biosurfactants and their importance. Curr Sci. 1999;10:116–126.
17. Fanaei M, Emtiazi G. Microbial assisted (Bacillus mojavensis) production of bio-surfactant lipopeptide with potential pharmaceutical applications and its characterization by MALDI-TOF-MS analysis. J Mol Liq. 2018;268:707–714. doi:10.1016/j.molliq.2018.07.103
18. Hauschildt S, Beuscher HU, Jung G, et al. Intraperitoneal injection of synthetic bacterial lipopeptides does not cause a rise in circulating inflammatory cytokines. FEMS Immunol Med Microbiol. 1994;8(1):77–82. doi:10.1111/j.1574-695X.1994.tb00428.x
19. Seydlová G, Svobodová J. Review of surfactin chemical properties and the potential biomedical applications. Cent Eur J Med. 2008;3(2):123–133.
20. Zhang Y, Liu C, Dong B, et al. Anti-inflammatory activity and mechanism of surfactin in lipopolysaccharide-activated macrophages. Inflammation. 2015;38(2):756–764. doi:10.1007/s10753-014-9986-y
21. Zhao H, Shao D, Jiang C, et al. Biological activity of lipopeptides from. Bacillus Appl Microbiol Biotechnol. 2017;101(15):5951–5960. doi:10.1007/s00253-017-8396-0
22. Schmid J, Sieber V, Rehm B. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol. 2015;26(6):496.
23. Jin H, Jeong Y, Yoo SH, et al. Isolation and characterization of high exopolysaccharide-producing Weissella confusa VP30 from young children’s feces. Microb Cell Fact. 2019;18(1):110. doi:10.1186/s12934-019-1158-1
24. Jenab A, Roghanian R, Emtiazi G, et al. Manufacturing and structural analysis of antimicrobial kefiran/polyethylene oxide nanofibers for food packaging. Iran Polym J. 2017;26(1):31–39. doi:10.1007/s13726-016-0496-7
25. Jenab A, Roghanian R, Emtiazi G. Encapsulation of platelet in kefiran polymer and detection of bioavailability of immobilized platelet in probiotic kefiran as a new drug for surface bleeding. J Med Microbiol. 2015;4(3–4):45–55.
26. Kwon OK, Ahn KS, Lee MY, et al. Inhibitory effect of kefiran on ovalbumin-induced lung inflammation in a murine model of asthma. Arch Pharm Res. 2008;31(12):1590–1596. doi:10.1007/s12272-001-2156-4
27. Jenab A, Roghanian R, Ghorbani N, et al. The Efficacy of Electrospun PAN/Kefiran Nanofiber and Kefir in Mammalian Cell Culture: Promotion of PC12 Cell Growth, Anti-MCF7 Breast Cancer Cells Activities, and Cytokine Production of PBMC. Int J Nanomed. 2020;15:717. doi:10.2147/IJN.S232264
28. Hsieh SA, Allen PM. Immunomodulatory Roles of Polysaccharide Capsules in the Intestine. Front Immunol. 2020;11.
29. Antoniou E, Margonis GA, Angelou A, et al. The TNBS-induced colitis animal model: An overview. Ann Med Surg. 2016;11:9–15. doi:10.1016/j.amsu.2016.07.019
30. Dinić M, Pecikoza U, Djokić J, et al. Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats. Front Pharmacol. 2018;17(9):1. doi:10.3389/fphar.2018.00001
31. Kook SY, Lee Y, Jeong EC, et al. Immunomodulatory effects of exopolysaccharides produced by Bacillus licheniformis and Leuconostoc mesenteroides isolated from Korean kimchi. J. Funct. Foods. 2019;54:211–219. doi:10.1016/j.jff.2019.01.003
32. Bleau C, Monges A, Rashidan K, et al. Intermediate chains of exopolysaccharides from Lactobacillus rhamnosus RW‐9595M increase IL‐10 production by macrophages. J Appl Microbiol. 2010;108(2):666–675. doi:10.1111/j.1365-2672.2009.04450.x
33. Zaghian S, Shokri D, Emtiazi G. Co-production of a UV-stable bacteriocin-like inhibitory substance (BLIS) and indole-3-acetic acid hormone (IAA) and their optimization by Taguchi design in. Bacillus pumilus Ann Microbiol. 2012;62(3):1189–1197. doi:10.1007/s13213-011-0359-6
34. Kim D, Kim H, Kim K, et al. The protective effect of indole-3-acetic acid (IAA) on H2O2-damaged human dental pulp stem cells is mediated by the AKT pathway and involves increased expression of the transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) and its downstream target heme oxygenase 1 (HO-1). Oxid Med Cell Longev. 2017;2017.
35. Shokri D, Emtiazi G. Indole-3-acetic acid (IAA) production in symbiotic and non-symbiotic nitrogen-fixing bacteria and its optimization by Taguchi design. Curr Microbiol. 2010;61:217–225. doi:10.1007/s00284-010-9600-y
36. Jones LH, Abdalla DS, Freitas JC. Effects of indole-3-acetic acid on croton oil-and arachidonic acid-induced mouse ear edema. Inflamm Res. 1995;44(9):372–375. doi:10.1007/BF01797863
37. Wang M, Tachibana S, Murai Y, et al. Indole-3-acetic acid produced by Burkholderia heleia acts as a phenylacetic acid antagonist to disrupt tropolone biosynthesis in Burkholderia plantarii. Sci Rep. 2016;6(1):22596. doi:10.1038/srep22596
38. Elrazak AA, Ward AC, Glassey J. Polyunsaturated fatty acid production by marine bacteria. Bioprocess Biosyst Eng. 2013;36(11):1641–1652. doi:10.1007/s00449-013-0936-0
39. Ahmad TB, Rudd D, Kotiw M, et al. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Mar Drugs. 2019;17(3):155. doi:10.3390/md17030155
40. Bianchi AC, Olazábal L, Torre A, et al. Antarctic microorganisms as a source of the omega-3 polyunsaturated fatty acids. World. J Microbiol Biotechnol. 2014;30(6):1869–1878. doi:10.1007/s11274-014-1607-2
41. Kang JX, Weylandt KH. Modulation of inflammatory cytokines by omega-3 fatty acids. Lipids Health Dis. 2008;133–143.
42. Abedi E, Sahari MA. Long‐chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr. 2014;2(5):443–463. doi:10.1002/fsn3.121
43. Hosoya S, Arunpairojana V, Suwannachart C, et al. Aureispira marina gen. nov., sp. nov., a gliding, arachidonic acid-containing bacterium isolated from the southern coastline of Thailand. Int J Syst Evol Microbiol. 2006;56(12):2931–2935. doi:10.1099/ijs.0.64504-0
44. Wallace RJ, Chaudhary LC, McKain N, et al. Clostridium proteoclasticum: a ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol Lett. 2006;265(2):195–201. doi:10.1111/j.1574-6968.2006.00487.x
45. Rund S, Lindner B, Brade H, et al. Structural analysis of the lipopolysaccharide from Chlamydophila psittaci strain 6BC. Eur J Biochem. 2000;267(18):5717–5726. doi:10.1046/j.1432-1327.2000.01635.x
46. Li M, van esch BC, Wagenaar GT, et al. Pro-and anti-inflammatory effects of short-chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–59. doi:10.1016/j.ejphar.2018.05.003
47. Mikelsaar M, Sepp E, Štšepetova J, et al. Biodiversity of intestinal lactic acid bacteria in the healthy population. Advan Microbiol Infect Dis Public Health. 2016;4:1–64.
48. Zhu X, Zhou Y, Wang Y, et al. Production of high-concentration n-caproic acid from lactate through fermentation using a newly isolated Ruminococcaceae bacterium CPB6. Biotechnol Biofuels. 2017;10(1):102. doi:10.1186/s13068-017-0788-y
49. Yuille S, Reichardt N, Panda S, et al. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS One. 2018;13(7):e0201073. doi:10.1371/journal.pone.0201073
50. Lewies A, Du Plessis LH, Wentzel JF. Antimicrobial Peptides: the Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob Proteins. 2018;18:1–2.
51. Lee HJ, Kim HY. Lantibiotics, class I bacteriocins from the genus. Bacillus J Microbiol Biotechnol. 2011;21(3):229–235. doi:10.4014/jmb.1010.10017
52. Seo MD, Won HS, Kim JH, et al. Antimicrobial peptides for therapeutic applications: a review. Molecules. 2012;17:12276–12286. doi:10.3390/molecules171012276
53. Dehghanifar S, Keyhanfar M, Emtiazi G. Production and partial purification of thermostable bacteriocins from Bacillus pumilus ZED17 and DFAR8 strains with antifungal activity. Mol Biol Res Commun. 2019;8(1):41–49. doi:10.22099/mbrc.2019.31563.1367
54. Avendaño C, Menéndez JC. Anticancer drugs acting via radical species, photosensitizers, and photodynamic therapy of cancer. Medicinal Chemistry of Anticancer Drugs.
55. David JM, Rajasekaran AK. Gramicidin A: A New Mission for an old antibiotic. J Kidney Cancer VHL. 2015;2(1):15–24. doi:10.15586/jkcvhl.2015.21
56. Todorov SD. Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action: produção, organização genética e modo de ação. Braz J Microbiol. 2009;40(2):209–221. doi:10.1590/S1517-83822009000200001
57. Mirhosseini M, Nahvi I, Emtiazi G, et al. Characterisation of anti-Listeria monocytogenes bacteriocins from Enterococcus faecium strains isolated from dairy products. Int J Dairy Technol. 2010;63:55–61. doi:10.1111/j.1471-0307.2009.00543.x
58. Sivaraj A, Sundar R, Manikkam R, et al. Potential applications of lactic acid bacteria and bacteriocins in anti-mycobacterial therapy. Asian Pac J Trop Dis. 2018;11(8):453–459. doi:10.4103/1995-7645.240080
59. Fernández A, Rodríguez JM, Bongaerts RJ, et al. Nisin-controlled extracellular production of interleukin-2 in Lactococcus lactis strains, without the requirement for a signal peptide sequence. Appl Environ Microbiol. 2007;73(23):7781–7784. doi:10.1128/AEM.01247-07
60. Yu HT, Ding XL, Li N, et al. Dietary supplemented antimicrobial peptide microcin J25 improves the growth performance, apparent total tract digestibility, fecal microbiota, and intestinal barrier function of weaned pigs. J. Animal Sci. 2017;95(11):5064–5076. doi:10.2527/jas2017.1494
61. Yu H, Ding X, Shang L, et al. Protective ability of biogenic antimicrobial peptide microcin J25 against Enterotoxigenic Escherichia Coli-induced intestinal epithelial dysfunction and inflammatory responses IPEC-J2 cells. Front Cell Infect Microbiol. 2018;8.
62. Villageliú D, Lyte M. Dopamine production in Enterococcus faecium: A microbial endocrinology-based mechanism for the selection of probiotics based on neurochemical-producing potential. PLoS One. 2018;13(11):e0207038. doi:10.1371/journal.pone.0207038
63. Zhou B, Zhang D. Antibacterial effects of bacteriocins isolated from Lactobacillus rhamnosus (ATCC 53103) in a rabbit model of knee implant infection. Exp Ther Med. 2018;15(3):2985–2989. doi:10.3892/etm.2018.5790
64. Torres MD, de la Fuente-nunez C. Reprogramming biological peptides to combat infectious diseases. ChemComm. 2019;55(100):15020–15032.
65. Rüter C, Buss C, Scharnert J, et al. A newly identified bacterial cell-penetrating peptide that reduces the transcription of pro-inflammatory cytokines. J Cell Sci. 2010;123(13):2190–2198. doi:10.1242/jcs.063016
66. Höfling S, Grabowski B, Norkowski S, et al. Current activities of the Yersinia effector protein YopM. Int J Med Microbiol. 2015;305(3):424–432. doi:10.1016/j.ijmm.2015.03.009
67. Hur SJ, Kang SH, Jung HS, et al. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 2012;32(11):801–816. doi:10.1016/j.nutres.2012.09.013
68. Kalenova LF, Kolyvanova SS, Bazhin AS, et al. Effects of Secondary Metabolites of Permafrost Bacillus sp. on Cytokine Synthesis by Human Peripheral Blood Mononuclear Cells. Exp Biol Med. 2017;163(2):235–238. doi:10.1007/s10517-017-3774-2
69. Kwon JE, Lim J, Kim I, et al. Isolation and identification of new bacterial strains producing equol from Pueraria lobata extract fermentation. PLoS One. 2018;13(2):e0192490. doi:10.1371/journal.pone.0192490
70. Mace TA, Ware MB, King SA, et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci Rep. 2019;9(1):5068. doi:10.1038/s41598-019-41687-z
71. Lee DK, Jang S, Kim MJ, et al. Anti-proliferative effects of Bifidobacterium adolescentis SPM0212 extract on human colon cancer cell lines. BMC Cancer. 2008;8(1):310. doi:10.1186/1471-2407-8-310
72. Bahmani S, Azarpira N, Moazamian E. Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk J Gastroenterol. 2019;30(9):835. doi:10.5152/tjg.2019.18451
73. Zhong L, Zhang X, Covasa M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J Gastroenterol. 2014;20(24):7878–7886. doi:10.3748/wjg.v20.i24.7878
74. Hong SH, Ban YH, Byun WS, et al. Camporidines A and B: Antimetastatic and Anti-inflammatory Polyketide Alkaloids from a Gut Bacterium of Camponotus kiusiuensis. J Nat Prod. 2019;82(4):903–910. doi:10.1021/acs.jnatprod.8b01000
75. Naghoni A, Emtiazi G, Amoozegar MA, et al. REP-PCR analysis to study prokaryotic biodiversity from Lake Meyghan. Int Lett Nat Sci. 2017;61:69–84.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
