Back to Journals » Infection and Drug Resistance » Volume 14
Bacterial Meningitis Among Adult Patients at University of Gondar Comprehensive Specialized Referral Hospital
Authors Tigabu A , Jember A, Nega T, Wubishet G, Misganaw H, Goshu T, Negash M
Received 10 December 2020
Accepted for publication 28 January 2021
Published 15 February 2021 Volume 2021:14 Pages 565—574
DOI https://doi.org/10.2147/IDR.S296792
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Abiye Tigabu,1 Abiyot Jember,2 Temesgen Nega,2 Getachew Wubishet,2 Hana Misganaw,2 Tigist Goshu,2 Markos Negash3
1Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, University of Gondar, Gondar, Ethiopia; 2School of Biomedical and Laboratory Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Immunology and Molecular Biology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Abiye Tigabu
Department of Medical Microbiology, University of Gondar, Postal Address: 196, Gondar, Ethiopia
Tel +251-918-192721
Email [email protected]
Background: Bacterial meningitis is a bacterial infection that causes inflammation of the membranes that surround the brain and spinal cord. The most frequent causes of bacterial meningitis are Neisseria meningitidis, Streptococcus pneumoniae, Listeria monocytogenes, and Haemophilus influenzae. This study aimed to determine bacterial meningitis and their antibiotic susceptibility patterns among adult patients.
Methods: A retrospective cross-sectional study was conducted on records of 3,683 patients to determine bacterial meningitis and their antibiotic susceptibility patterns from 2011 to 2020. Cerebrospinal fluid samples were collected, inoculated on blood and chocolate agar plates, and then incubated at 37°c for 24 hours. Bacterial identification performed using morphological characters, Gram stain, and biochemical tests. And then antimicrobial susceptibility tests were done using modified Kirby–Bauer disk diffusion technique. Records of 3,683 culture results were collected and reviewed using a checklist from the registration book. Finally, data was entered, cleared, and checked using Epi-info version 7 and exported to SPSS version 20 for analysis.
Results: Of the 3,683 patients, the overall prevalence of culture-positive bacterial meningitis was 1.28% (47/3683). Of them, bacterial meningitis in males was 1.61% (33/2052). Streptococcus pneumoniae (32%, 15/47) was the commonest isolate followed by Staphylococcus aureus, (12.80%, 6/47), Escherichia coli, (12.80%, 6/47), and Neisseria meningitidis, (10.60%, 5/47). Out of 47 culture-positive isolates, 15 of them were MDR isolates. Ceftriaxone, chloramphenicol, ciprofloxacin, vancomycin, clindamycin, and erythromycin were the most effective antibiotics whereas penicillin, tetracycline, and cotrimoxazole were the least effective antibiotics for isolates. Gender (P = 0.047, AOR = 0.528, CI = 0.282– 0.99) is significantly associated with bacterial meningitis.
Conclusion: The prevalence of bacterial meningitis among adult patients was 1.28%. Males are at high risk for bacterial meningitis compared to females. Therefore, infection preventive measures are required with a particular focus on adult patients. Further research is needed to explore the epidemiology and risk factors of bacterial meningitis.
Keywords: bacterial meningitis, adult patients, antimicrobial susceptibility pattern, Gondar
Background
Microbial meningitis remains a common infectious disease worldwide, caused by bacterial, viral, fungal, or protozoan agents that causes inflammation of membranes that surrounds the brain and spinal cord. Bacteria and viruses are the most common causes of meningitis. But bacterial meningitis (BM) is usually severe and common.1 The most common etiologic agents of BM are S. aureus, E. coli, H. influenzae, N. meningitidis, S. pneumoniae, and L. monocytogenes.2 The classic symptoms of bacterial meningitis are fever, neck stiffness, altered mental status, and headache.3 But the classical signs of bacterial meningitis are not always present in adults, and we cannot rule out based on the classical signs and symptoms alone.4,5 Bacterial meningitis is a severe infectious disease of the membranes lining the brain resulting in high mortality and morbidity.6 Accurate and timely identification of the etiological agents is vital to initiate public health measures and appropriate management.7
The incidence of bacterial meningitis is between 3 and 5 per 100,000 people per year, and more than 2,000 deaths are reported annually in USA.8 The incidence of bacterial meningitis is a significant burden in adults with a mortality rate of up to 30% and it requires prompt recognition and treatment.9,10 S. pneumoniae, H. influenzae, and N. meningitidis have been responsible for 118.400, 83.000, and 75.000 deaths, respectively. N. meningitidis was accountable for the majority of BM epidemics in the meningitis belt of Sub-Saharan Africa.6,11 S. pneumoniae becomes a leading cause of meningitis among adults.12 Adult bacterial meningitis is caused by P. aeruginosa, which usually found in the hospital area and patients with a post neurosurgical state.13 The introduction of vaccines for S. pneumoniae and N. meningitidis has reduced the burden of BM in adults.14
Microbiological laboratory examination of cerebrospinal fluid (CSF) is the most definitive investigation for bacterial meningitis and guides possible choice of antibiotics and duration of therapy like third-generation cephalosporin is the initial antibiotics of choice in the absence of penicillin allergy and bacterial resistance. Amoxicillin is used if L. monocytogenes are suspected in adults.15 Besides, mass vaccinations available for vaccine-preventable pathogens, bacterial meningitis is a major cause of public health problem, mortality and morbidity in tropical and subtropical countries including Ethiopia.16 The rapid emergence and spread of antimicrobial-resistant bacteria have raised considerable public health concern in both developed and developing countries. Ethiopia is one of the developing countries with bacterial profile and antibiotic susceptibility pattern was not well studied among meningitis suspected adult patients. This cross-sectional retrospective study could give significant information about the prevalence and antimicrobial susceptibility pattern of bacterial meningitis and also to indicate the prevention and control measures. Therefore, this study aimed to determine the prevalence and antimicrobial susceptibility patterns of bacterial meningitis among adult patients at the University of Gondar comprehensive specialized referral hospital.
Methods
Study Area
The study was conducted at the University of Gondar comprehensive specialized referral hospital, which serves more than five million people in Gondar town and the surrounding area. The town has 8 health centers, 21 private clinics, and one referral hospital which has more than 500 beds that provides health services such as surgery, internal medicine, pathology, TB/HIV, dermatology, antenatal care, delivery, postnatal care, laboratory, pharmacy, maternal and neonatal care, and other services for the population of Gondar town and surrounding areas.
Study Design, Period and Data Collection
A retrospective cross-sectional study was conducted to determine the prevalence and antimicrobial susceptibility patterns of bacterial meningitis among adult patients at the University of Gondar comprehensive specialized hospital from 2011 to 2020. Data records of 3,683 patients were collected and reviewed using a checklist from the registration book at medical bacteriology unit. Information concerning laboratory test results, age, sex, and hospitalization status of patient’s collected from 2011 to 2020 in the registration book using a data collection format.
Laboratory Inoculation and Identification
Cerebrospinal fluid samples were collected by the physician between the 4th and 5th lumbar vertebrae and each CSF sample was inoculated onto blood and chocolate agar plates, and incubated aerobically with 5% CO2 at 37°C for 24 hours. Samples that were culture positive on blood and chocolate agar plates, and the isolates obtained were identified using standard microbiological methods including colony morphology, Gram’s stain reaction, and standardized biochemical tests such as indole production, lactose fermentation, hydrolysis of urea, citrate utilization, lysine decarboxylation, oxidase test, motility test, mannitol fermentation, catalase, and coagulase tests. A suspension of a pure colony from each confirmed culture isolate was performed by using 0.85% sterile normal saline for antimicrobial susceptibility testing and adjusted at 0.5 MacFarland standard. Using a sterile cotton tip applicator stick, the suspension was distributed evenly on Muller-Hinton agar (5% sheep blood supplemented for fastidious bacterial isolates), and a modified Kirby–Bauer disk diffusion technique implemented for antibiotic susceptibility pattern using different antibiotics.
Data Management and Statistical Analysis
The quality of data was assured using a structured data collection format, asking laboratory staff how data registered including abbreviations in the laboratory and cross-checking by members of the data collector. Data entered into EPI-Info version-7 to check data completeness and clearance then transferred to SPSS version-20 for analysis. Frequency distribution, percentages, and summary statistics were used to describe the study population and antimicrobial results. Logistic regression was computed to assess statistical association for age, sex and relevant variables, and the significance of statistical association was assured using p-value <0.05 at 95% confidence interval (CI).
Results
Socio-Demographic Characteristics of Study Participants
In this study, a total of 3,683 adult patients greater than 18 years of age were included at the University of Gondar teaching hospital during the study period. Out of these, 55.70% (2052/3683) were males. The mean age of the study participants was 36.12 years with an SD of ±14.41 with an age range of 18–97 years. Approximately, 33% (1199/3683) of the study participants belonged to 18–27 years of age. The majority, 90% (3313/3683) of patients were admitted in hospital and 32.27% (1069/3313) of them belong 18–27 years of age. In this study, cerebrospinal fluid samples collected by lumbar puncture, and the most frequently observed patient’s age was 30 followed by 40. Most of the study participants were involved in 2018 and 2014, 13.90% (512/3683), and 11.50% (425/3683), respectively. The age and sex distribution of patients involved in this study are presented (Table 1).
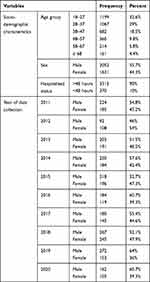 |
Table 1 Frequency of Study Participants by Age Groups, Gender and Year at University of Gondar Comprehensive Specialized Hospital, Gondar, Northwest Ethiopia, 2020 |
Prevalence of Culture-Positive Bacterial Meningitis and Risk Factors
A total of 3,683 cerebrospinal fluid samples were analyzed; the prevalence of culture-positive bacterial meningitis among adult patients greater than 18 years was 1.28% (47/3683), and 12 different types of bacterial isolates were found. Of these, S. pneumoniae (31.90%, 15/47) was the commonest isolated bacteria followed by S. aureus, (12.80%, 6/47), E. coli, (12.80%, 6/47), and N. meningitidis, (10.60%, 5/47) (Figure 1). The highest prevalence of culture-positive bacterial meningitis was observed among 18–27 (12, 0.33%), and 28–37 (18, 0.49%) years of study participants. Culture-positive bacterial meningitis was high in less than 50 years’ old patients 1.17% (43/3683) compared to above 50 years, 0.11% (4/3683). The prevalence of culture-positive bacterial meningitis among male patients was 1.61% (33/2052) and 1.16% (43/3683) among hospitalized patients, which is higher than their non-hospitalized patients, 0.12% (4/3683). Highest frequency of culture-positive bacterial isolates was found in 2019, 0.27% (10/3683) and the most frequently isolated bacteria were found at age of 32 and 40, 5 (0.14%) and 4 (0.11%), respectively. Male participants were 0.53 times at risk for bacterial meningitis (P = 0.047; AOR = 0.528; CI = 0.282–0.99) compared to female participants. However, age (P = 0.871; AOR =1.017; CI = 0.827–1.25) and places of patient visits (P = 0.705; AOR = 0.819; CI = 0.292–2.30) were not significantly associated with bacterial meningitis (Table 2).
 |
Figure 1 Frequency and percentage of bacterial isolates at University of Gondar Comprehensive Specialized Hospital, Gondar, Northwest Ethiopia, 2020. |
Trends of Bacterial Meningitis
Over the ten years study periods, the prevalence of culture-positive bacterial meningitis was higher in 2019 (0.27%, 10), 2015 (0.19%, 7), and 2011 (0.19%, 7), while lower in 2018 (0.05%, 2), and 2020 (0.05%, 2) (Figure 2).
 |
Figure 2 Trends of bacterial meningitis by years at University of Gondar Comprehensive Specialized Hospital, Gondar, Northwest Ethiopia, 2020. |
Antimicrobial Susceptibility Patterns of Bacterial Isolates
Bacterial antimicrobial susceptibility tests done for bacterial isolates, and ampicillin (100%), ceftriaxone (100), chloramphenicol (77.80%), ciprofloxacin (83.30%), penicillin (100%), vancomycin (87.50%), and erythromycin (80%) are effective antimicrobial agent for S. pneumoniae isolates. However, four S. pneumoniae isolates were resistant to cotrimoxazole (66.80%). Cefoxitin (100%), clindamycin (100%), and erythromycin (66.80%) are the most effective drug against S. aureus isolates (Table 3). On the other hand, ciprofloxacin (60%), gentamicin (66.80%) effective for E. coli isolates. But five E. coli isolates were resistant to ampicillin (100%). Amoxicillin (100%) and erythromycin (100%) are effective to treat N. meningitidis, and ceftazidime (100%) is an effective drug for K. pneumoniae isolates (Table 4).
 |
Table 3 Antimicrobial Susceptibility Profile of Gram-Positive Bacterial Isolates at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from January to August, 2020 |
 |
Table 4 Antimicrobial Susceptibility Profile of Gram-Negative Bacterial Isolates at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from January to August, 2020 |
Multidrug-Resistant Isolates
Multidrug-resistant (MDR) isolates refer to an isolate that is resistant to at least one antibiotic in three or more drug classes. In this study, the prevalence of MDR isolates was 31.90% (15/47). Out of 47 culture-positive isolates, 15 of them were MDR isolates. Among gram positives, MDR was observed in S. aureus, CoNS, S. pneumoniae, and S. viridians. While among gram negative, MDR was observed in N. meningitidis, E. coli, K. pneumoniae, K. ozanae and NLFGR (Table 5).
 |
Table 5 Multidrug-Resistant Patterns for Gram-Positive and Gram-Negative Bacteria at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, from January to August, 2020 |
Discussion
Bacterial meningitis, a life-threatening worldwide disease, has to be reviewed periodically because of specific microorganisms responsible for infection vary with time, geography, and patient age. It has become a disease of adults with a significant mortality rate that ranges from 20% to 30%. We performed this study to summarize prevalence figures obtained from CSF samples, with an exclusive focus on adults. The overall prevalence of culture-positive bacterial meningitis among adult patients in this study is 1.28%, which is higher than a study carried in Indonesia (0.68%).17 However, it is lower than reported in Ethiopia (6.90%),18 Malawi (5.25%),19 South African (10.70%),20 Kenya (11.20%),21 Netherlands (13%),22 Yemen (52.70%),23 and Qatar (53.60%),24 which differs noticeably among studies, might be due to differences in characteristics and geographical distribution of the study population, sample size, diagnostic techniques, and differences in infection control policies. We noticed a significant prevalence of bacterial meningitis among adult patients, which indicates adults remain the population where the disease meningitis prevalent and strict prevention strategies are required.
The present study showed that S. pneumoniae, S. aureus, E. coli, and N. meningitidis were the predominant pathogens that caused bacterial meningitis. S. pneumoniae was the predominant pathogen, and it remains an important cause of bacterial meningitis, which is in agreement with the results of a previous study by Amaya-Villar et al,25 Van de Beek et al,26 Wall Eet al,27 Mook-Kanamori et al,28 Adriani et al,29 Weisfelt et al,30 and Mirecka A.31 However, other studies reported that N. meningitidis,18 M. tuberculosis,20 coagulase-negative staphylococci,24 and L. monocytogenes32 were the leading cause of bacterial meningitis. Moreover, S. aureus and E. coli were the second frequent etiological agent, which is comparable with a study conducted by Mook-Kanamori et al and Mirecka A.28,31 E. coli and other Gram-negative bacteria are not common etiology of BM in patients below 50 years old. However, in this study, they are causes of bacterial meningitis below 50 years old adults. We noticed a significant increase in the prevalence of BM among adult patients (18–50 years). The possible reason might be there may be predisposing, immunocompromising factors or presence of cerebral shunts among these age groups.
The trends of bacterial meningitis among adult patients tend to decrease in 2013 (0.08%), 2014 (0.08%), 2016 (0.08%), 2017 (0.08%), 2018 (0.05%), and 2020 (0.05%). However, the prevalence of bacterial meningitis significantly increased in 2011 (0. 22%), 2012 (0.16%), 2015 (0.19%), and 2019 (0.27%). The highest prevalence of bacterial meningitis was observed in 2019 (0.27%), and the lowest prevalence of bacterial meningitis was observed in 2018 (0.05%) and 2020 (0.05%). The prevalence of bacterial meningitis was not constantly decreased or increased in this study. However, differs noticeably between years, which might be due to the difference in the management of the disease, the prevention and infection control policies from year to year.
In this study, ampicillin (100%), ceftriaxone (100%), chloramphenicol (77.80%), ciprofloxacin (83.30%), penicillin G (100%), vancomycin (87.50%), and erythromycin (80%) are the most effective antimicrobial agent for S. pneumoniae isolates. This finding was in agreement with studies conducted by Purwanto et al and by Gudina et al.17,33 However, a study by Assegu Fenta D et al.18 and Khan et al.24 reported that S. pneumoniae was resistant to ceftriaxone and penicillin. Cefoxitin (100%) and clindamycin (100%) are the most effective drugs for S. aureus isolates while erythromycin (66.80%) and amoxicillin (66.80%) are functional against S. aureus isolates. However, a study conducted by Assegu Fenta et al.18 reported that S. aureus isolates were found to be (100%) resistant to amoxicillin. On the other hand, ciprofloxacin (60%) is effective against E. coli isolates but resistant for ampicillin (100%) which is comparable with a study conducted by Gordon et al.19 Furthermore, amoxicillin (100%), and erythromycin (100%) are effective for N. meningitidis isolates, but resistant for penicillin (100%). However, Mirecka et al.31 reported that penicillin was effective for N. meningitidis isolates. The differences in the susceptibility pattern of the isolates might be due to the differences in the management of antibiotics and diagnostic techniques employed.
Even if there are several factors considered as risk factors for bacterial meningitis, and we found gender is significantly associated with bacterial meningitis in adult patients. The proportion of bacterial meningitis among male patients was higher than females. Male patients were 0.53 times more at risk of acquiring the disease meningitis as compared with female patients. This higher prevalence in males might be due to males more exposed to smoking, alcohol drinking and debilitating disease which makes them more vulnerable to bacterial infection. In this study, age is not significantly associated with meningitis (p > 0.05). However, a study by Abdulrab et al,23 Amaya-Villar et al.25 and Van de Beek et al.26 reported that age was independently associated with bacterial meningitis. Hospital-acquired bacterial meningitis among hospitalized patients were higher than non-hospitalized patients. But hospitalization is not significantly associated with bacterial meningitis (p > 0.05).
Conclusions and Recommendations
In conclusion, the prevalence of bacterial meningitis among adult patients was 1.28%. Males are at high risk for bacterial meningitis compared to females. Therefore, infection preventive measures are required with a particular focus on adult patients. Further research is needed to explore the epidemiology and risk factors of meningitis.
Abbreviations
BM, bacterial meningitis; CSF, cerebrospinal fluid; CI, confidence interval; OR, odds ratio.
Data Sharing Statement
All data generated or analyzed during this study were included in this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.
Ethical Approval and Consent to Participate
Ethical approval was obtained from the University of Gondar ethical review committee. A legal permission and support letter were obtained from College of Medicine and Health Sciences hospital director office. Patient’s informed consent was not required due to the secondary data, and this study was conducted in accordance with the Declaration of Helsinki. The objectives of the study were explained to the heads of the hospital director and health care providers who worked at medical microbiology department and clarification was given before starting data collection from the registrations and client cards. To ensure confidentiality of information from participant’s records, no personal identifiers were recorded in the client information extraction pre-designed form, and data secured from participant records were not available to anyone except for the main investigator.
Acknowledgments
We would like to thank all participants of this research, University of Gondar Hospital, the staff of Medical Microbiology, School of Biomedical and Laboratory Science, College of Medicine and Health Sciences for their contribution to the maturation and the success of this research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Rashid LR Clinical manifestations of bacterial meningitis; 2018.
2. Domingo P, Pomar V, Benito N, Coll P. The changing pattern of bacterial meningitis in adult patients at a large tertiary university hospital in Barcelona, Spain (1982–2010). J Infect. 2013;66(2):147–154. doi:10.1016/j.jinf.2012.10.030
3. Lin AL, Safdieh JE. The evaluation and management of bacterial meningitis: current practice and emerging developments. Neurologist. 2010;16(3):143–151. doi:10.1097/NRL.0b013e3181d14185
4. van de Beek D, Brouwer MC, de Gans J, Verstegen MJ, Spanjaard L, Pajkrt D. Richtlijn bacterial meningitis. Clin Neurol Neurosurg. 2013.
5. Van de Beek D, Cabellos C, Dzupova O, et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect. 2016;22:S37–62. doi:10.1016/j.cmi.2016.01.007
6. Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17. doi:10.1186/1478-7954-11-17
7. Greenhow TL, Hung -Y-Y, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595–599. doi:10.1097/INF.0000000000000225
8. Nur HE, Jamaiah I, Rohela M, Nissapatorn V Bacterial meningitis: a five-year (2001–2005) retrospective study at University Malaya medical center (UMMC), Kuala Lumpur, Malaysia; 2008.
9. McGill F, Heyderman RS, Panagiotou S, Tunkel AR, Solomon T. Acute bacterial meningitis in adults. Lancet. 2016;388(10063):3036–3047. doi:10.1016/S0140-6736(16)30654-7
10. Lukšić I, Mulić R, Falconer R, Orban M, Sidhu S, Rudan I. Estimating global and regional morbidity from acute bacterial meningitis in children: assessment of the evidence. Croat Med J. 2013;54(6):510–518. doi:10.3325/cmj.2013.54.510
11. Mohammed I, Iliyasu G, Habib AG. Emergence and control of epidemic meningococcal meningitis in sub-Saharan Africa. Pathog Glob Health. 2017;111(1):1–6. doi:10.1080/20477724.2016.1274068
12. Veltman JA, Bristow CC, Klausner JD. Meningitis in HIV‐positive patients in sub-Saharan Africa: a review. J Int AIDS Soc. 2014;17(1):19184. doi:10.7448/IAS.17.1.19184
13. Pai S, Bedford L, Ruramayi R, et al. Pseudomonas aeruginosa meningitis/ventriculitis in a UK tertiary referral hospital. QJM. 2016;109(2):85–89. doi:10.1093/qjmed/hcv094
14. Van Ettekoven CN, van de Beek D, Brouwer MC. Update on community-acquired bacterial meningitis: guidance and challenges. Clin Microbiol Infect. 2017;23(9):601–606. doi:10.1016/j.cmi.2017.04.019
15. Chaudhuri A, Martin P, Kennedy P, et al. EFNS guideline on the management of community‐acquired bacterial meningitis: report of an EFNS Task Force on acute bacterial meningitis in older children and adults. Eur J Neurol. 2008;15(7):649–659. doi:10.1111/j.1468-1331.2008.02193.x
16. Rashid H, Khandaker G, Booy R. Vaccination and herd immunity: what more do we know? Curr Opin Infect Dis. 2012;25(3):243–249. doi:10.1097/QCO.0b013e328352f727
17. Purwanto DS, Loho T, Tafroji W, et al. Isolation and identification of Streptococcus pneumoniae serotype 6B from a patient with bacterial meningitis infection in Jakarta, Indonesia. Access Microbiol. 2020;acmi000123.
18. Assegu Fenta D, Lemma K, Tadele H, Tilahun BT, Derese B. Antimicrobial sensitivity profile and bacterial isolates among suspected pyogenic meningitis patients attending at Hawassa University Hospital: cross-sectional study. BMC Microbiol. 2020;20(1):1–10. doi:10.1186/s12866-019-1672-7
19. Gordon SB, Walsh AL, Chaponda M, et al. Bacterial meningitis in Malawian adults: pneumococcal disease is common, severe, and seasonal. Clin Infect Dis. 2000;31(1):53–57. doi:10.1086/313910
20. Britz E, Perovic O, Von Mollendorf C, et al. The epidemiology of meningitis among adults in a South African province with a high HIV prevalence, 2009–2012. PLoS One. 2016;11(9):e0163036. doi:10.1371/journal.pone.0163036
21. Gituro CN, Nyerere A, Ngayo MO, Maina E, Githuku J, Boru W. Etiology of bacterial meningitis: a cross-sectional study among patients admitted in a semi-urban hospital in Nairobi, Kenya. Pan Afr Med J. 2017;28(Suppl1). doi:10.11604/pamj.supp.2017.28.1.9383
22. Van Veen KE, Brouwer MC, Van Der Ende A, Van De Beek D. Bacterial meningitis in diabetes patients: a population-based prospective study. Sci Rep. 2016;6(1):1–7. doi:10.1038/srep36996
23. Abdulrab A, Algobaty F, Salem AK, Mohammed YA. Acute bacterial meningitis in adults: a hospital-based study in Yemen. Jpn J Infect Dis. 2010;63(2):128–131.
24. Khan FY, Abu-Khattab M, Almaslamani EA, et al. Acute bacterial meningitis in Qatar: a hospital-based study from 2009 to 2013. Biomed Res Int. 2017;2017:1–8. doi:10.1155/2017/2975610
25. Amaya-Villar R, García-Cabrera E, Sulleiro-Igual E, et al. Three-year multicenter surveillance of community-acquired Listeria monocytogenes meningitis in adults. BMC Infect Dis. 2010;10(1):324. doi:10.1186/1471-2334-10-324
26. Van de Beek D, De Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351(18):1849–1859. doi:10.1056/NEJMoa040845
27. Wall EC, Everett DB, Mukaka M, et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale-up and Haemophilus influenzae type b vaccination, 2000–2012. Clin Infect Dis. 2014;58(10):e137–1345. doi:10.1093/cid/ciu057
28. Mook-Kanamori BB, Fritz D, Brouwer MC, Van Der Ende A, Van De Beek D. Intracerebral hemorrhages in adults with community associated bacterial meningitis in adults: should we reconsider anticoagulant therapy? PLoS One. 2012;7(9):e45271. doi:10.1371/journal.pone.0045271
29. Adriani KS, Brouwer MC, Baas F, Zwinderman AH, Van der Ende A, Van de Beek D. Genetic variation in the β2-adrenocepter gene is associated with susceptibility to bacterial meningitis in adults. PLoS One. 2012;7(5):e37618. doi:10.1371/journal.pone.0037618
30. Weisfelt M, De Gans J, Van Der Ende A, Van De Beek D. Community-acquired bacterial meningitis in alcoholic patients. PLoS One. 2010;5(2):e9102. doi:10.1371/journal.pone.0009102
31. Mirecka A. Etiological agents of bacterial meningitis in adults and antibiotic susceptibility of Streptococcus pneumoniae isolated from 2009–2016 from patients of regional specialist hospital of Dr Wł. Biegański in Lódź. Przegl Epidemiol. 2018;72(3):313–324. doi:10.32394/pe.72.3.8
32. van Veen KE, Brouwer MC, Van Der Ende A, Van De Beek D. Bacterial meningitis in solid organ transplant recipients: a population‐based prospective study. Transpl Infect Dis. 2016;18(5):674–680. doi:10.1111/tid.12570
33. Gudina EK, Tesfaye M, Wieser A, Pfister HW, Klein M. Outcome of patients with acute bacterial meningitis in a teaching hospital in Ethiopia: a prospective study. PLoS One. 2018;13(7):e0200067. doi:10.1371/journal.pone.0200067
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

