Back to Journals » Infection and Drug Resistance » Volume 15
Bacterial Keratitis Following Small Incision Lenticule Extraction
Authors Li J, Ren SW, Dai LJ, Zhang B, Gu YW, Pang CJ, Wang Y
Received 21 March 2022
Accepted for publication 3 August 2022
Published 17 August 2022 Volume 2022:15 Pages 4585—4593
DOI https://doi.org/10.2147/IDR.S367328
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Jin Li,1 Sheng-Wei Ren,1 Li-Juan Dai,1 Bo Zhang,1 Yu-Wei Gu,1 Chen-Jiu Pang,1 Yan Wang2
1Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, People’s Hospital of Zhengzhou University, Henan University People’s Hospital, Zhengzhou, People’s Republic of China; 2Clinical College of Ophthalmology Tianjin Medical University, and The Tianjin Eye Hospital Tianjin Eye Institute, Tianjin Key Laboratory of Ophthalmology and Visual Science, Tianjin, People’s Republic of China
Correspondence: Chen-Jiu Pang, Henan Provincial People’s Hospital, Henan Eye Hospital, Henan Eye Institute, People’s Hospital of Zhengzhou University, Henan University People’s Hospital, Zhengzhou, People’s Republic of China, Tel +8613939018599, Email [email protected]; [email protected] Yan Wang, Clinical College of Ophthalmology Tianjin Medical University, and The Tianjin Eye Hospital Tianjin Eye Institute, Tianjin Key Laboratory of Ophthalmology and Visual Science, Tianjin, People’s Republic of China, Tel +8613602089393, Email [email protected]
Purpose: To describe the development of bacterial keratitis after small incision lenticule extraction in 5 patients and to explore its appropriate therapies.
Methods: We retrospectively summarized the clinical treatments of five patients with postoperative bacterial infection after small incision lenticule extraction, who were referred to our hospital from 2019 to 2021.
Results: Five male patients had undergone bilateral SMILE in the local hospital due to myopia aged from 18 to 26 years. The onset of keratitis during 1– 3 days postoperatively and four of them were severe infection (2 bilateral, 2 unilateral). In five cases, 1 patient (1 eye) who was infected mild keratitis after SMILE was treated with only topical antibiotics; the others who respond poorly to topical antibiotics require surgical treatment, which 1 patient (1 eye) infected necrotic mass of the corneal cap was scraped and irrigated with antibiotic, and 3 patients (5 eyes) were treated by converting the cap to flap, curetting the necrotic tissue and irrigating with the antibiotic solution. In all patients, the duration from onset to resolution was 1– 5 weeks. The final uncorrected visual acuity was above 20/32.
Conclusion: Owing to the upward popularity of refractive surgery, the incidence of keratitis after SMILE should not be ignored. Early diagnosis and timely treatment of post-SMILE keratitis are essential. For severe keratitis that fails to respond to topical antibiotics, the corneal cap should be opened as a flap.
Keywords: small incision lenticule extraction, bacterial keratitis, refractive surgery, postoperative infection, therapy
Introduction
Small incision lenticule extraction (SMILE) has been applied clinically for nearly 11 years in corneal refractive surgery since it was first reported in 2011, and is becoming increasingly widespread due to its safety, accuracy, fast recovery, biomechanical advantage over LASIK.1–3 SMILE corrects myopic errors by removing an intrastromal lenticule through a small arcuate incision without creating a flap, leaving a pocket in the corneal stroma.2 The complications of small incision lens extraction have been reported in the past, such as epithelial erosions, diffuse lamellar keratitis, corneal ectasia, infection.4–8 Among them, infectious keratitis after SMILE is a rare but dreaded complication. It can be sight threatening if not managed properly. At present, limited evidence is available on the optimal management of infectious keratitis in patients who have undergone SMILE.6–8 Herein, we report a series of cases of post-SMILE bacterial keratitis in 5 patients (7 eyes), including 2 patients with severe bilateral keratitis, and all patients were successfully treated (Table 1). To date, this is the largest case series of its kind to be reported. We hope our findings will help guide the management of bacterial keratitis after SMILE.
 |
Table 1 Summary of Cases of Infectious Keratitis After SMILE |
Case Reports
Case 1
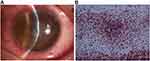
A 19-year-old man visited our hospital with a 3-day history of decreased vision, pain, redness, and photophobia in the right eye. Five days ago, he had undergone bilateral SMILE for the correction of myopia (right eye, −3.75 diopters; left eye, −4.0 diopters) in the local hospital. After the surgery, he had been prescribed topical levofloxacin 0.5% and topical fluorometholone 0.1% to be instilled 6 times per day.9 However, due to pain, redness of the right eye, and a history of drug allergies, the patient chose to discontinue the antibiotics since postoperative day 2, and his symptoms worsened. An examination at our hospital revealed the uncorrected visual acuity (UCVA) of 20/50 in the right eye and 20/25 in the left eye. Slit-lamp examination revealed 3 dense, round, white infiltrates at the central cap-stromal bed interface along with a cellular reaction (Figure 1A). The anterior chamber was deep with no cells or flares. Oct-optic (cassia) of the eye revealed that the central cornea was cloudy up to the cap-stromal bed interface (Figure 1B). The left eye was normal. No fungi or amoebae were found on confocal laser microscopy of the right eye. Bacterial keratitis of the right eye was suspected. The patient was treated empirically with moxifloxacin 0.5% and cefazolin 0.5% eye drops instilled every 30 min, along with gatifloxacin ointment at bedtime. Two days later, topical antibiotics were tapered in frequency every 1 hour. After 1 week, the clinical symptoms improved, and the infiltrates decreased in size and density. Topical antibiotics were changed to every 2 hours, and fluorometholone 0.1% was added 3 times daily. After 1 month, the infiltrates disappeared, and the uncorrected visual acuity (UCVA) of the right eye was 20/20.
Case 2
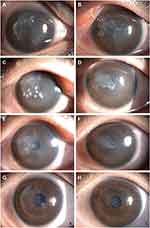
A 23-year-old man underwent SMILE for the correction of refractive errors of −4.00/-0.50× 40° in his right eye and −4.00 diopters in his left eye. After the surgery, prophylactic antibiotics were administered at a frequency of 4 times per day. At 1 day after the operation, the UCVA was 20/20 in both eyes. On postoperative day 3, the patient developed pain and redness in his left eye, and his UCVA was 20/50, and then he was referred to our hospital for treatment. Slit-lamp examination revealed a paracentral, white, cross-shaped infiltrate at the corneal cap–stromal bed interface (Figure 2A). There was no improvement after 2 days of empirical topical instillations of antibiotics, and the corneal cap underwent melting. After obtaining consent from the patient, we enlarged the original incision with the corneal microscissor along the nasal edge of the corneal cap to less than 2 quadrants near the lesion area. The infiltrate was fully scraped and used for smear and culture examinations. The smear revealed Gram-positive cocci, so the cap and stromal bed were soaked with Ceftriaxone (2 mg/mL) solution. However, culture tests returned negative results, possibly due to the application of topical antibiotics. We continued to treat the patient with levofloxacin 0.5% and tobramycin 0.3% alternated every 30 min for three days. After then, the patient received topical antibiotics every 1 hour. After 2 weeks, the infiltration regressed, the levofloxacin 0.5% and tobramycin 0.3% were reductive to every 3 hours, and fluorometholone 0.1% was added to the treatment (4 times a day). One month after the surgical intervention, the patient’s UCVA was 20/20, and only a trace of a residual peripheral scar was present (Figure 2B).
Case 3
A 26-year-old man was referred to our hospital after undergoing bilateral, sequential SMILE for the correction of myopia (−6.25 diopters in both eyes). The SMILE procedure was completed uneventfully, and the postoperative UCVA was 20/20 in both eyes. Two days after the procedure, the patient developed blurry vision; his pain intensified, and his UCVA was 20/200 in the left eye. A diagnosis of infectious keratitis was made by the treating doctor, who then prescribed topical levofloxacin 0.5% every 30 min. However, this treatment failed to resolve the corneal infection, and the patient was referred to our hospital 3 days after SMILE. At this time, his VA had reduced to hand motions. We detected a 1.5×1.5mm2 melt in the central anterior corneal cap accompanied by diffuse dense infiltration at the corneal cap–stromal bed interface, and multiple keratic precipitates in the left eye (Figure 3A). The infected necrotic mass was scraped, and the scrapings were immediately used for smear and culture examinations. Pocket irrigation of the lesion was performed with amikacin (10mg/mL) ophthalmic solution. The smear examination showed Gram-positive cocci (Figure 3B), and a diagnosis of bacterial keratitis after SMILE in the left eye was made. The patient was managed with hourly topical levofloxacin 0.5% and cefazolin 0.5% eye drops as well as intramuscular for Cefamezin injection 2.0 g every 8 hours and intravenous for ascorbic acid injection 1.0 g every 24 hours. After 3 days, symptomatic improvement occurred; the UCVA was 20/100 in the left eye, and the infiltrate decreased in both size and density. A culture grew Staphylococcus epidermidis, and it sensitive to levofloxacin and cefazolin, which were subsequently continued. By day 6 of the treatment, the epithelial defect healed, and the UCVA improved to 20/50, so the topical antibiotics were tapered down to 4 times per day, After 1 month of topical antibiotics therapy, the keratitis resolved completely, and fluorometholone 0.1% eyedrops 4 times a day were initiated. At 3 months, the UCVA improved to 20/25, the original infiltrate had left only a thin scar and the topical drugs were stopped.
Case 4
An 18-year-old man with myopia (right eye, −3.25 diopters; left eye, −3.75 diopters) uneventfully underwent SMILE at a local hospital. He was routinely administered tobramycin 0.3%, fluorometholone 0.1%, and lubricant eye drops after the surgery. However, he returned to the hospital on the second day after the surgery due to pain, redness, tearing, and photophobia in both eyes. His visual acuity had decreased from 20/25 on postoperative day 1 to counting fingers at 10 cm in both eyes on postoperative day 2. Infectious keratitis affecting both corneas was suspected. Intensive topical treatment (ie, once every 30 minutes) with levofloxacin 0.5% and tobramycin 0.3% failed to elicit an improvement after 2 days, and the patient was referred to our hospital for further management on postoperative day 4.
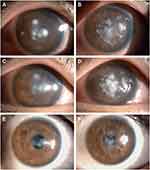
An examination at our hospital showed that the patient’s VA was hand motions along with ocular pain, redness, purulent discharge in both eyes. Slit-lamp examination revealed several dense, white multiple infiltrates of differing sizes at the interface between the corneal cap and stromal bed, along with corneal edema and immune rings on the corneal endothelium (Figure 4A and B). After obtaining consent from the patient and his family, we immediately scraped the necrotic tissues near the original small incisions in both eyes with sterile cellulose sponges and irrigated the corneal cap–stromal bed interface with amikacin solution (10 mg/mL) in the operating room. After half an hour, smear examination showed Gram-positive cocci, and a diagnosis of bilateral keratitis after SMILE was made. The patient was administered the intensive therapy with topical levofloxacin 0.5% and tobramycin 0.3% every 20 minutes, intramuscular for Cefamezin injection 2.0g every 8 hours, and oral levofloxacin (0.5g, once daily). On the next day after this operation, the infiltrates near the incision areas had decreased, but the infiltrates in the central part of the cornea had increased and those in the right eye had become more obvious (Figure 4C and D). Thus, irrigation of only the corneal pocket through the SMILE incision was insufficient. On the same day, with the consent of the patient, the original incision was enlarged with the two separate corneal microscissors along the edge of the corneal cap to about half of the pocket under topical anesthesia in both eyes, and the corneal cap was opened to fully scrape the necrotic tissue, which was subjected to smear and culture examination respectively. Then, the lesion was thoroughly rinsed with ceftriaxone (2 mg/mL) ophthalmic solution to limit the spread of the infection. The smear tests indicated Gram-positive cocci. The systemic antibiotic therapy was continued as before, and the frequency of topical antibiotic administration was changed to once every 30 minutes. On day 7 of the corneal infection, the patient reported a considerable decrease in pain and photophobia (Figure 4E and F), so the systemic antibiotics were discontinued. The culture test showed that no bacteria and fungi were present 1 week later, and the patient was asymptomatic at this time. At 1 month, the infiltrate eventually consolidated and it appeared that the infection had been controlled. We then tapered the topical antibiotics to once every 4 h, and initiated treatment with fluorometholone 0.1% 3 times a day on the following week. The UCVA improved to 20/50 in the right eye and 20/32 in the left eye within 1 month. At 1 year after the last procedure, the corneal showed residual stromal scarring (Figure 4G and H), and the UCVA improved to 20/32 and 20/25 in the right and left eyes, respectively.
Case 5
Bilateral SMILE was performed for an 18-year-old man with refractive errors of −3.25 −1.75 × 5° and −4.25 −1.50 × 175° diopters in the right and left eye, respectively. The procedure was completed uneventfully at a local hospital, and the patient was administered topical levofloxacin 0.5% and fluorometholone 0.1% once every 6 h after surgery. The UCVA was 20/20 in both eyes on the day after SMILE. On postoperative day 1, however, the patient developed pain and decreased vision. Slit-lamp examination revealed 3 small, mild paraxial corneal infiltrates in the corneal pocket of the right eye, and multiple, round, white stromal infiltrates at the corneal cap–stromal bed interface in the left eye. The frequency of topical antibiotic administration was increased to once every 30 minutes. In addition, the corneal cap–stromal bed interface in both eyes was irrigated with vancomycin (10 mg/mL) on 2 separate occasions. However, the symptoms continued to worsen, and the infection progressed in both eyes. The culture of the washing fluid mixed with physiological saline revealed Streptococcus pneumoniae infection in the left eye, and bacterial keratitis was confirmed on postoperative day 4. Meanwhile, the patient was referred to our hospital.
At the time of presentation in our hospital, the VA was light perception in both eyes, and the patient was markedly photophobic. Slit-lamp examination revealed round, white infiltrates involving the central corneal cap and the underlying stromal bed that had increased in severity since their onset in the right eye (Figure 5A). Multiple white infiltrates in the mid-peripheral area and diffuse cell infiltration at the corneal cap–stromal bed interface were observed in the left eye (Figure 5B). The patient was administered intensive treatment with topical levofloxacin 0.5%and tobramycin 0.3% every 20 minutes, intramuscular for Cefamezin injection 2.0g every 8 hours, and oral levofloxacin (0.5g, once daily). At 5 days after SMILE, the infectious infiltrates were still progressing. Based on our experience in Case 4, we immediately enlarged the original incision with the corneal microscissors along the edge of the corneal cap to more than half of the pocket in both eyes after obtaining consent from the patient. The bilateral corneal caps were opened to fully scrape the infiltrates and then thoroughly rinsed with ceftriaxone (2 mg/mL) ophthalmic solution to limit the spread of the infection. Two days later, the right eye improved (Figure 5C). Unfortunately, the infiltrates in the left eye were not under control (Figure 5D). Therefore, irrigation of the corneal cap–stromal bed interface and scrape the infectious mass was repeated for the left eye. After 1 week, the patient was asymptomatic, and the infiltrates gradually consolidated and diminished. Topical fluorometholone 0.1% was applied in both eyes 3 times daily from 1 month onwards. The topical antibiotics and steroid were gradually tapered according to the clinical condition. At 6 months, the UCVA improved to 20/25 and 20/32 in the right and left eyes, respectively. Slit-lamp examination showed residual stromal scarring (Figure 5E and F).
Discussion
Despite the rapid advances in laser corneal refractive surgery technologies, corneal infection after refractive surgery remains a serious sight-threatening complication. The lack of epithelial protection renders the cornea more susceptible to infection after surface ablation than after a lamellar procedure. The incidence of corneal infection after surface ablation is relatively high, at 0.2%.10 In contrast, lamellar procedures such as LASIK, in which an epithelial barrier develops early after the operation and provides a certain protective effect, are associated with a postoperative infection rate of 0.03%.11 SMILE is relatively a new surgical method, in which femtosecond laser technology is used to create an intrastromal lenticule that is extracted through a small peripheral corneal incision to alter the shape of the cornea.2 Although both are stromal surgeries, the surgical incision in SMILE is smaller, and the corneal lamellar pocket is relatively closed; thus, the rate of postoperative corneal infection should theoretically be lower. To our knowledge, there is limited evidence in managing bacterial keratitis after SMILE.6–8 One case of bilateral keratitis caused by Streptococcus pneumoniae following SMILE was treated by irrigation with povidone-iodine solution and topical corticosteroids, resulting in a favorable outcome.6 Chan et al7 reported the successful use of PACK-CXL in treatment of unilateral keratitis caused by Staphylococcus after SMILE. Liu et al8 described the case of severe bilateral non-tuberculous mycobacterial keratitis after SMILE and its successful management with topical and oral antibiotics. In China, as the number of SMILE procedures increases, sporadic post-SMILE corneal infections are being reported.12
In view of the relatively closed lamellar pocket, it enables rapid pathogen growth, and the penetration of topical therapy is limited. Moreover, the drainage of the irrigation fluid may be impeded. Therefore, this study demonstrated that the importance of the prompt and aggressive management of vision-threatening bacterial keratitis after SMILE. In our study, we reported 5 cases of bacterial keratitis after SMILE, including 2 cases of simultaneous infection of both eyes. We summarize our experience as follows: For patients with mild, small, limited lesions, topical antibiotics can first be applied. If the infected area shrinks, the drug treatment should be continued. If the infection cannot be controlled and the lesion expands, the corneal cap should be opened and changed to a flap timely; the necrotic tissue should be thoroughly scraped off and sent for smear and culture tests to identify the pathogenic microorganisms, and irrigation or soaking of the stromal bed and flap itself with an antibiotic solution should be done. If the cap has involved, the necrotic tissue should be scraped, and the corneal pocket should be thoroughly irrigated with the antibiotic solution through the necrotic cap. For patients with multiple severe corneal interface infections, converting the cap to flap should be attempted without delay, as well as rinsing with the proper antibiotic solution and thoroughly scraping the lesion, and administering combined treatment with systemic and local antibiotics. Although corneal scarring may be worse, we believe that the benefit of changing cap to flap and eradicating the infection far outweighs the scarring of the cornea. It is worth mentioning that after infection control, the appropriate and reasonable application of corticosteroid eye drops is required to reduce corneal scar formation and restore vision to the maximum extent.
The rational use of antibiotics before and after surgery is also critical. In our patients, symptom onset occurred early, on postoperative days 1–3. All the culture results in our report showed bacteria that are commonly found in the conjunctival sac.13,14 It should be noted that topical antibiotics must be used before surgery to prevent infection. Although the preoperative ocular examination in referring hospital did not report clinical signs of periocular pathology, such as blepharitis or dacryocystitis, the prophylactic therapy during perioperative period is essential to prevent this type of infection, and should include the cleaning of the eyelashes, lid margins, and conjunctiva as well as an appropriate frequency and duration of antibiotic eye drop administration.
Conclusions
In summary, bacterial keratitis following SMILE is a rare but serious vision-threatening complication. We emphasize the different treatment options depending on the severity of the infection after SMILE. For severe infections, the corneal pocket should be opened into the flap and thoroughly rinsed with antibiotics at an early stage.
Abbreviations
SMILE, small incision lenticule extraction; UCVA, uncorrected visual acuity; VA, visual acuity; LASIK, laser-assisted in situ keratomileusis; PACK- CXL, photo-activated chromophore for keratitis corneal cross-linking; R, right; L, left.
Data Sharing Statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of Henan Eye Hospital & Henan Eye Institute. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from individual participants.
Consent for Publication
All data published here are under the consent for publication. Written informed consent was obtained from all individual participants included in the study.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Wang Y, Ma J, Zhang J, et al. Incidence and management of intraoperative complications during small-incision lenticule extraction in 3004 cases. J Cataract Refract Surg. 2017;43:796–802. doi:10.1016/j.jcrs.2017.03.039
2. Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol. 2011;95:335–339. doi:10.1136/bjo.2009.174284
3. Guo H, Hosseini-Moghaddam SM, Hodge W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: a systematic review and meta-analysis. BMC Ophthalmol. 2019;19(1):167. doi:10.1186/s12886-019-1165-3
4. Wang Y, Ma J, Zhang J, et al. Postoperative corneal complications in small incision lenticule extraction: long-term study. J Refract Surg. 2019;35(3):146–152. doi:10.3928/1081597X-20190118-02
5. Wang Y, Cui C, Li Z, et al. Corneal ectasia 6.5 months after small-incision lenticule extraction.J. Cataract Refract Surg. 2015;41:1100–1106. doi:10.1016/j.jcrs.2015.04.001
6. Chehaibou I, Sandali O, Ameline B, et al. Bilateral infectious keratitis after small-incision lenticule extraction. J Cataract Refract Surg. 2016;42:626–630. doi:10.1016/j.jcrs.2016.03.024
7. Chan TC, Chow VW, Jhanji V. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for bacterial keratitis after small incision lenticule extraction (SMILE). J Refract Surg. 2017;33:278–280. doi:10.3928/1081597X-20170126-01
8. Liu HY, Chu HS, Chen WL, et al. Bilateral non-tuberculous mycobacterial keratitis after small incision lenticule extraction. J Refract Surg. 2018;34:633–636. doi:10.3928/1081597X-20180827-01
9. Li Y, Wang Y. Chinese expert consensus on perioperative medication in laser corneal refractive surgeries (2019). Chin Med Sci J. 2020;35(1):1–12. doi:10.24920/003552
10. de Rojas V, Llovet F, Martínez M, et al. Infectious keratitis in 18,651 laser surface ablation procedures. J Cataract Refract Surg. 2011;37:1822–1831. doi:10.1016/j.jcrs.2011.04.037
11. Llovet F, de Rojas V, Interlandi E, et al. Infectious keratitis in 204586 LASIK procedures. Ophthalmology. 2010;117:
12. Yang S, Li Y, Jiang Y. A case of streptococcus infection after small incision lenticule extraction. Zhonghua Yan Ke Za Zhi. 2018;54:464–465.
13. Pang CJ, Wang LY, Sun ST, et al. The investigation of microbial culturing of microkeratome blades and sponges used in laser in situ keratomileusis. Zhonghua Yan Ke Za Zhi. 2012;48(5):394–397.
14. Fan C, Yang B, Huang Y. Efficacy of 0.5% levofloxacin and 5.0% povidone-iodine eyedrops in reducing conjunctival bacterial flora: metagenomic analysis. J Ophthalmol. 2020;2020:1780498. doi:10.1155/2020/1780498
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.