Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 10 » Issue 1
Bacterial flora in the sputum and comorbidity in patients with acute exacerbations of COPD
Authors Boixeda R , Almagro P , Díez-Manglano J , Cabrera F, Recio J, Martin-Garrido I, Soriano JB
Received 16 May 2015
Accepted for publication 6 July 2015
Published 1 December 2015 Volume 2015:10(1) Pages 2581—2591
DOI https://doi.org/10.2147/COPD.S88702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Ramon Boixeda,1 Pere Almagro,2,3 Jesús Díez-Manglano,4 Francisco Javier Cabrera,5 Jesús Recio,6 Isabel Martin-Garrido,7 Joan B Soriano8
On behalf of the COPD and Pluripathological Patients Groups of the Spanish Internal Medicine Society
1Internal Medicine Department, Hospital de Mataró – CSDM, Universitat Autònoma de Barcelona, Mataró, Barcelona, Spain; 2Internal Medicine Department, Hospital Mútua de Terrassa, Terrassa, 3Universitat de Barcelona, Barcelona, Spain; 4Internal Medicine Department, Hospital Royo Villanova, Zaragoza, Zaragoza, Spain; 5Internal Medicine Department, Hospital General Universitario Gregorio Marañón, Universidad Complutense, Madrid, Spain; 6Internal Medicine Department, Hospital Vall d’Hebrón, Barcelona, Barcelona, Spain; 7Internal Medicine Department, Hospital Quirón San Camilo, Madrid, Madrid, Spain; 8Instituto de Investigación Hospital Universitario de la Princesa (IISP), Universidad Autónoma de Madrid, Cátedra UAM-Lindel, Madrid, Spain
Objective: To determine in patients admitted with an acute exacerbation of chronic obstructive pulmonary disease (AE-COPD) the association between the isolation of potential pathogens in a conventional sputum culture and comorbidities.
Patients and methods: The ESMI study is a multicenter observational study. Patients with AE-COPD admitted to the Internal Medicine departments of 70 hospitals were included. The clinical characteristics, treatments, and comorbidities were gathered. The results of conventional sputum cultures were recorded.
Results: A total of 536 patients were included, of which 161 produced valid sputum and a potentially pathogenic microorganism was isolated from 88 subjects (16.4%). The isolation of Pseudomonas aeruginosa (30.7%) was associated with a greater severity of the lung disease (previous admissions [P= 0.026], dyspnea scale [P=0.047], post-broncodilator forced expiratory volume in 1 second (FEV1) [P=0.005], and the BODEx index [P=0.009]); also with higher prevalence of cor pulmonale (P=0.017), heart failure (P=0.048), and cerebrovascular disease (P=0.026). Streptococcus pneumoniae (26.1%) was associated with more comorbidity according to number of diseases (P=0.018); notably, peripheral artery disease (P=0.033), hypertension (P=0.029), dyslipidemia (P=0.039), osteoporosis (P=0.0001), and depression (P=0.005).
Conclusion: Patients with AE-COPD and P. aeruginosa present higher severity of COPD, while those with S. pneumoniae present greater comorbidity. The potentially pathogenic microorganism obtained in the sputum culture depends on the associated comorbidities.
Keywords: chronic obstructive pulmonary disease, comorbidities, hospitalization, sputum culture, etiology of exacerbations
Introduction
Acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) have a great impact on health status,1 disease progression,2 and prognosis.3
Up to 50%–70% of AE-COPD can be attributed to respiratory infections by viruses or bacteria, even more in the most severe patients.4 Finding a purulent sputum suggests, but does not prove, a bacterial etiology, since 25%–50% of COPD patients are colonized by potentially pathogenic microorganisms (PPM).5 Bacterial colonization has been associated with the frequency and severity of COPD exacerbations.6
Most guidelines recommend the use of antibiotics whenever two or more of the Anthonisen’s criteria,7 ie, increased dyspnea, increased sputum volume, and increased sputum purulence, are met. Nevertheless, sputum purulence seems to be the main factor associated with infection.8,9
The choice of antimicrobial agent depends on the suspected microorganism, based on the clinical circumstances,10 the severity of the COPD,11 and the presence of comorbidities (diabetes mellitus, liver cirrhosis, chronic kidney failure, or heart disease), infections, or previous antibiotherapy.
Even though the presence of comorbidities is associated with a higher risk of therapeutic failure and modifies the choice of antibiotic according to recommendations of scientific societies,12,13 such indications are mainly based on expert opinion, not on prospective studies. Hence, studies are needed to prove whether the presence of comorbidities bears any relation to the microorganism causing AE-COPD and might help to choose an antibiotic treatment empirically. This way both therapeutic failure and occurrence of resistance could be reduced.
Our primary aim was to assess the comorbidities of patients admitted to Internal Medicine services and its association to bacteriological isolation through conventional sputum culture, seeking to obtain clinical features that might help a microbiological diagnosis.
Materials and methods
The ESMI (Spanish acronym for COPD in Internal Medicine Services) is an epidemiological multicenter research study, cross-sectional in the first stage and longitudinal later on. The inclusion period lasted a year (October 2009 to October 2010) and its methodology has been previously described elsewhere.14 In brief, the ten first consecutive patients attended to in each of 70 participating hospitals for AE-COPD were studied, whether they required hospitalization or not. The main aim was to describe the comorbidities and their relation to mortality and hospital readmissions within the first 3 months after discharge.
For this research, only patients admitted with a confirmed AE-COPD were included, since guidelines recommend carrying out a sputum culture in this population, that usually presents with a severe or very severe exacerbation along with therapeutic failure.15,16 In conclusion, this study was a multicenter, cross-sectional study conducted to identify isolated bacteria in the sputum of exacerbated COPD patients and to relate them to the comorbidities. The COPD diagnosis required spirometric confirmation (post-bronchodilator FEV1/forced vital capacity (FVC) <0.7). Patients admitted for causes other than AE-COPD and those who could not undergo the spirometry or did not meet the spirometric criteria were excluded.
All patients included were assessed during admission, and their clinical and functional data were gathered through a specifically designed questionnaire. The Charlson index17 was used to asses comorbidities, without age adjustments and including COPD, as well as a number of other diseases not included in this index that we considered especially relevant, such as history of myocardial infarction, arterial hypertension, venous thromboembolic disease, arrhythmia, anemia, dyslipidemia, or osteoporosis.14
Other data gathered included body mass index, the modified Medical Research Council (mMRC) dyspnea scale,18 the usual treatment prior to admission, basal gasometry at admission, and C-reactive protein (CRP). The functional status was also assessed, using Katz index19 at baseline. The BODEx index20 (replaces exercise capacity with exacerbations) score was calculated.
Finally, conventional sputum samples were taken during the admission according to the usual clinical practice. The sputum was considered valid following the criteria of Murray and Washington.21
In order to compare clinical characteristics in relation to the sputum results, patients were divided according to the microorganism isolated in their culture, comparing them with the rest of the patients with a valid culture but different microorganism results.
Eventually, this resulted in the division of patients into three groups, and based on whether FEV1 is above or under 50%,10,22 we grouped the patients depending on the isolation of Pseudomonas aeruginosa or Enterobacteriaceae (group 1); Streptococcus pneumoniae, Haemophilus influenzae or Moraxella catarrhalis (group 2); or non-potential pathogen microorganisms (nPPM) (group 3).
Statistical analysis
Qualitative variables are expressed as absolute frequency and percentages (%), and quantitative variables as mean and standard deviation in case of normal distribution, and as median otherwise. For the bivariate analysis, we used the chi-square test or Fisher’s exact test whenever it was required. To study the differences between averages, we used Student’s t-test or Mann–Whitney U-test whenever appropriate. The analysis was carried out with the SPSS 15.0 statistical package, and every analysis was based on the bilateral hypothesis with a statistical significance level under P<0.05.
The study was approved by the Clinical Trials Committee of the Hospital Mútua de Terrassa that acted as a coordinating center. All patients accepted taking part freely and signed an informed consent document.
Results
Out of a total of 679 identified patients, 606 were included in the ESMI study. Of these, 70 (11.5%) were discharged directly from Emergency Department, not requiring hospitalization in the ward, and were hence excluded. Compared with patients who did require hospitalization, those discharged from Emergency Department were younger and had better pulmonary function and fewer comorbidities (P<0.001). Furthermore, it was possible to obtain a conventional sputum sample from a smaller percentage of this group (26.1% vs 47.8%, P<0.05).
A sputum sample was obtained from 256 (47.8%) out of 536 patients. The most frequent reasons for not obtaining it from the remaining 280 (52.2%) patients were that they could not expectorate (101 patients, 18.8%) and that they were not asked to during their hospitalization (172 patients, 32.1%). In the case of seven other patients, the cause was not registered. Figure 1 shows the flowchart of participants.
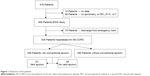  | Figure 1 Flowchart of the patients. |
Characteristics of patients
The clinical and demographic characteristics of patients are presented in Table 1. The mean age was 73.2 (9.5) years (range 41–94). In all, 486 (90.8%) were men, with an average post-bronchodilator % predicted FEV1 of 48.3 (15.7), and their average Charlson index score was 3.14 (2.0). Compared with the participants who did not produce sputum, the COPD patients who produced a sputum sample had a significantly higher smoking exposure, suffered more hospitalizations for AE-COPD in the previous year, and required long-term oxygen therapy (P<0.05).
Table 2 presents the characteristics of exacerbations. Prior to admission, systemic corticosteroids or antibiotics had been administered to 21.9% and 31.7% of subjects, respectively. Compared with the participants who did not produce sputum, the COPD patients who produced a sputum sample more frequently experienced an increase of expectoration, change of color in the sputum, and fever. Furthermore, their hospital stays were longer (P<0.05). The sputum was valid for culture in 161 patients. Compared with those who did not provide a sputum, they had shown symptoms prior to admission for more days (6.4 vs 5.2; P=0.038), they had higher CRP values (mg/L) in Emergency Department (63.2 vs 35; P=0.03) and their hospital stay was longer (10.9 vs 9.1 days; P=0.04).
Bacteriologic isolation
Out of 161 patients who provided a sputum sample that was valid for culture, saprophytic flora (nPPM) was isolated in 73 (44.8%). The culture was positive for PPM in 88 of all 536 AE-COPD cases who required hospitalization (16.4%). The most frequently isolated microorganism was P. aeruginosa in 27 (30.7%), followed by S. pneumoniae in 23 (26.1%), Enterobacteriaceae in 18 (20.4%), H. influenzae in 14 cases (15.9%), and finally M. catharrhalis in 6 (6.8%) (Figure 2).
Bacterial flora in the sputum and comorbidity
Table 3 presents the various comorbidities according to isolation in sputum cultures. In Figure 3, we show the different characteristics of patients according to the microbiological diagnostic of the sputum culture.
Patients with P. aeruginosa isolation in their sputum (27) more frequently had cor pulmonale (P=0.01), heart failure (P=0.04), and cerebrovascular disease (P=0.02). Also, these patients have a more severe (mean %FEV1 41.1 vs 49.1; P=0.005), higher BODEx index scores (6 vs 4.7; P=0.008), greater probability of having required admissions for AE-COPD during the previous year (92.6% vs 71.6%; P=0.02), greater dyspnea according to the mMRC scale (2.7 vs 2.3; P=0.047), a higher number of exacerbations (according to stratification used in BODE; P=0.01), greater purulence of sputum at admission (P=0.02), and they more frequently used inhaled corticosteroids (100% vs 88%, P=0.04). The number of hospitalizations during the previous year (2.3 vs 1.7; P=0.07) and of days of hospital stay (14.5 vs 10.2; P=0.05) were higher, but not significantly.
Patients with S. pneumoniae isolation had more comorbidities (5.9 vs 2.7; P=0.002), and greater probability of having a Charlson index above three, though without statistical significance (52.2% vs 32.6%; P=0.07). The comorbidities more frequently associated with S. pneumoniae isolation were hypertension (P=0.04), dyslipidemia (P=0.03), peripheral vascular disease (P=0.03), osteoporosis (P=0.000), and depression (P=0.005). According to the characteristics of the present exacerbation, these patients had fewer previous days with symptoms (4.7 vs 6.7; P=0.01), and according to the Anthonisen criteria greater increase of sputum (P=0.02) and of purulence (P=0.04). As for the previous treatment, they presented more use of β-lactams prior to admission (P=0.02) and statins (P=0.002). According to the treatment during the exacerbation, there was greater use of diuretics (P=0.02). There is a tendency to a greater smoking history measured in packs/year (48.5 vs 59.5; P=0.06) and a longer span of time between exacerbation and COPD diagnosis, in years (12.2 vs 9.1; P=0.04).
Patients with Enterobacteriaceae isolation were younger (66.6 vs 74 years; P=0.04), had lesser functional impairment according to Katz index (5.6 vs 5.2; P=0.04), and more frequently presented with mild liver disease (P=0.01). Also notable, they showed less fever (P=0.02) and comorbidities (arterial hypertension [P=0.022], dyslipidemia [P=0.007], and peripheral vascular disease [P=0.046]). Comparing the treatment of this group of patients, we found the less use of statins (P=0.001) and a greater need of continuous positive airway pressure (P=0.01). Finally, no differences appear as for AE-COPD severity factors.
Comparing patients with H. influenzae isolation in the sputum with the non-H. influenzae group, their differential characteristics are a greater presence of peptic ulcer (P=0.04) and atrial fibrillation (P=0.01). Comparing treatments, they have lesser need of home oxygen therapy (P=0.03). They also required fewer days of hospital stay over the previous 12 months (P=0.04) and in the current AE-COPD admission (P=0.02).
No differences are clear when comparing patients with M. catharralis isolation in the sputum with the remainder.
Finally, if we compare patients with nPPM isolation in the sputum (73 patients) with the rest of the group, their differential characteristics are fewer admissions over the previous year (P=0.01), lesser presence of sputum increase (P=0.008) and sputum purulence (P=0.02), lesser presence of cor pulmonale (P=0.02), less depression (P=0.03), less need of continuous positive airway pressure (P=0.003), shorter hospital stays (P=0.02), lower CRP values (P=0.04), and fewer associated comorbidities (P=0.046). As for severity of the COPD, they had a lower FVC in milliliter (P=0.03).
We analyzed the characteristics of patients according to the results of the sputum culture, by grouping these results according to the isolation of P. aeruginosa or Enterobacteriaceae (group 1); S. pneumoniae, H. influenzae, or M. catharralis (group 2); or nPPM (group 3). The results are presented in Table 4. COPD patients in group 1 had significantly more admission in the previous year, increased sputum, and more heart failure; while those in group 2 had more osteoporosis (P<0.05).
Discussion
Our study shows that COPD patients hospitalized for an AE-COPD with P. aeruginosa isolation present greater severity of their disease, with worse levels of respiratory parameters as measured with the predicted %FEV1 after bronchodilatation, greater dyspnea by the mMRC scale, higher scores in the BODEx index, and more hospitalizations over the previous year. The isolation of S. pneumoniae, in turn, is associated with more associated comorbidities, assessed by the number of comorbidities or with the Charlson index. Finally, patients with isolation of enterobacteria are younger, have less functional impairment, and more use of non-invasive mechanical ventilation similar to continuous positive airway pressure. Thus, we have noted that the diseases associated to COPD differ depending on the microorganism isolated in the sputum.
These data are relevant for clinical practice, as they help decide the empirical antibiotic treatment for each kind of patients in those AE-COPD that require hospitalization. Presently, according to clinical practice guidelines,15,16 it is advised to determine the antibiotic treatment for AE-COPD depending on the patient’s comorbidities, since they affect the risk of therapeutic failure. According to our results, this comorbidity varies depending on the etiology of the bacterial exacerbation.
Only a recent study has described the identification of bacterial etiology in relation to the clinical characteristics of patients. Miravitlles et al10 identified factors independently associated with bacterial growth, such as current smoking and H. influenzae; longer periods between exacerbations and S. pneumoniae; or decrease in FEV1 and P. aeruginosa.
Traditionally, bacterial identification has been defined depending on the severity of the COPD, since S. pneumoniae, H. influenzae, and M. catharralis are isolated in patients with predicted FEV1 >50%, and Enterobacteriaceae and P. aeruginosa in those with FEV1 <50%, as verified by Miravitlles et al10 and Eller et al.22
Other authors have also identified a greater growth of P. aeruginosa in patients with lower levels of FEV1,23,24 and other factors of greater severity of COPD.25,26 García-Vidal et al27 defined the BODEx index, the number of hospital admissions in the previous year, treatment with corticoids, and prior isolation of P. aeruginosa as risk factors.
COPD is a heterogeneous disease that requires categorizing patients in subgroups in order to optimize their management in clinical practice, identifying traits of the disease with clinical significance. Likewise, there are exacerbation subtypes depending on the number of previous days with symptoms,28 or by etiology. There have been attempts to typify different exacerbation subtypes through biomarkers. Bafadhel et al29 described three types of exacerbation: bacterial, viral, and eosinophilic. Among the bacterial causes, comorbidity might orient us toward a certain etiological agent.
On bacterial etiology the conventional study of sputum plays an important role. It is the simplest and most accessible method for diagnosing the etiology of bacterial infections in AE-COPD. Nevertheless, its value is debatable in some cases due to bacterial colonization in certain patients,30 and to the increasing importance of other, non-bacterial etiologies such as respiratory viruses, that are isolated in more than 50% of cases, frequently associated with bacteria.31 Most guidelines recommend conducting a sputum culture in case of severe exacerbation, the presence of risk factors for multiresistant bacteria, or if there is therapeutic failure.16,17,32,33
Most studies have focused on the risk factors for growth in the sputum culture for P. aeruginosa, while obtaining similar results to ours about other microorganisms (although with better results in obtaining valid sputum samples from patients admitted with AE-COPD, as they induced sputum). For instance, a study similar to ours27 included 188 patients, obtaining quality sputum samples from 119 subjects (63%). Of the quality cultures, 55% were for PPM and the remaining 45% for nPPM, identical to our results.
One of the factors predicting growth of PPM in cultures is the purulence of the sputum, but it must be observed by clinicians, since it is less reliable when reported by the patients themselves.34 This has led researchers to look for other predictors of purulence in the sputum, such as FEV1 <35% and body mass index <22.35 Also, Larsen et al23 identified the number of neutrophils in peripheral blood as a predictor of growth in the sputum. Roche et al24 associated it to the presence of bronchiectasis, chronic home oxygen therapy, and lower levels of FEV1%. Recently, Bafadhel et al36 had shown that patients with persistently positive sputum samples have particularly severe neutrophilic airway inflammation and poor clinical outcomes, and the H. influenzae was the most commonly isolated pathogen.
Thus, for instance, the presence of Anthonisen’s criteria (type I) in patients with decreased lung function has been described as indicative of antibiotic treatment.37 Nevertheless, recent studies point out that purulence in the sputum remains the most important factor.9
Finally, we observed that the most frequently isolated microorganism was P. aeruginosa (30.7%), followed by S. pneumoniae (26.1%), Enterobacteriaceae (20.4%), H. influenzae (15.9%), and M. catharralis (6.8%). We did not encountered any patient with Staphylococcus aureus. Other authors in Spain have also identified a similar rate.25,27
Our study has several limitations. First, the reduced number of women, similar to other studies conducted in Spain, probably due to its late entry to smoking in Spain. Second, most of the patients included in the study had been hospitalized in Internal Medicine Services, and probably presented more associated pathologies than those admitted to Pneumology departments. In Spain, 40%–50% of COPD exacerbations are attended to in Internal Medicine Services, which usually care for older patients with more comorbidities;38 however, the data about mortality and readmissions shown in our study are similar to those obtained by another paper, studying 1,200 patients admitted to a variety of services and hospitals.39 Also, the difficulty of identifying the etiology of exacerbation through the sputum has been pointed out before. In addition, a significant percentage of the patients were already being treated with antibiotics prior to their hospitalization, which hampers studying their case through conventional sputum samples. A final limitation is that computed tomography was not undertaken as part of the study protocol, so we are not in a position to determine whether the presence of bronchiectasis was an important cofactor in these subjects.
In summary, the etiology of bacterial infections in AE-COPD can be implied through characteristics of patients such as their associated comorbidities. P. aeruginosa, for instance, is associated with a greater severity of COPD itself; S. pneumoniae, with a disease not as severe but with a longer time of evolution, more comorbidities, and more vascular risk factors; and Enterobacteriaceae are associated with younger patients in need of continuous positive airway pressure.
Acknowledgments
The authors would like to thank all the investigators of the ESMI Study, Working Group on COPD of the Spanish Society of Internal Medicine that took part in the study (Supplementary material).
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. | ||
Anzueto A. Impact of exacerbation on COPD. Eur Respir Rev. 2010;19:113–118. | ||
Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. | ||
De Serres G, Lampron N, La Forge J, et al. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J Clin Virol. 2009;46:129–133. | ||
Rosell A, Monsó E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:891–897. | ||
Patel IS, Seemungal TAR, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonization and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–764. | ||
Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. | ||
Miravitlles M, Moragas A, Hernández S, Bayona C, Llor C. Is it possible to identify exacerbations of mild to moderate COPD that do not require antibiotic treatment? Chest. 2013;144(5):1571–1577. | ||
Soler N, Esperatti M, Ewig S, Huerta A, Agustí C, Torres A. Sputum purulence-guided antibiotic use in hospitalised patients with exacerbations of COPD. Eur Respir J. 2012;40:1344–1353. | ||
Miravitlles M, Espinosa C, Fernández-Laso E, et al. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40–46. | ||
Monsó E, Rosell A, Bonet G, et al. Lower airway colonization in chronic bronchitis: risk factors. Eur Respir J. 1997;10(Suppl 25):147S. | ||
Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. | ||
Miravitlles M, Monsó E, Mensa J, et al. Tratamiento antimicrobiano de la agudización de la EPOC. Arch Bronconeumol. 2008;44:100–108. | ||
Almagro P, Cabrera FJ, Diez J, et al. Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD. The ESMI study. Chest. 2012;142:1126–1133. | ||
Guía Española del EPOC (Gesepoc). Arch Bronconeumol. 2012;48(Suppl 1):2–58. | ||
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Available from: http://www.goldcopd.org/. Accessed January 2, 2015. | ||
Charlson ME, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. | ||
Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. | ||
Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged: the index of ADL; a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. | ||
Soler-Cataluña JJ, Martínez-García MA, Sanchez LS, Tordera MP, Sánchez PR. Sever exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2008;103(5):682–689. | ||
Murray PR, Washington JA. Microscopic and bacteriologic analysis of expectorated sputum. Mayo Clin Proc. 1975;50(6):339–344. | ||
Eller J, Ede A, Schaberg T, Niederman MS, Mauch H, Lode H. Infective exacerbations of chronic bronchitis: relation between bacteriologic etiology and lung function. Chest. 1998;113:1542–1548. | ||
Larsen MV, Janner JH, Nielsen SD, Friis-Moller A, Ringbaek T, Lange P. Bacteriology in acute exacerbation of chronic obstructive pulmonary disease in patients admitted to hospital. Scand J Infect Dis. 2009;41:26–32. | ||
Roche N, Kouassi B, Rabbat A, Mounedji A, Lorut C, Huchon G. Yield of sputum microbiological examination in patients hospitalized for exacerbations of chronic obstructive pulmonary disease with purulent sputum. Respiration. 2007;74:19–25. | ||
Almagro P, Salvadó M, Garcia-Vidal C, et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84:36–43. | ||
Nowetaka K, Frankowska M, Grzelewska-Rzymowska I. Exacerbations of COPD and the role of sputum bacteriological examination. Pneumonol Alergol Pol. 2006;74:396–402. | ||
García-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J. 2009;34:1072–1078. | ||
Aaron SD, Donaldson GC, Whitmore GA, Hurst JR, Ramsay T, Wedzicha JA. Time course and pattern of COPD exacerbation onset. Thorax. 2012;67:238–243. | ||
Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. | ||
Bari MR, Hiron MM, Zaman SM, Rahman MM, Ganguly KC. Microbes responsible for acute exacerbation of COPD. Mymensingh Med J. 2010;19:576–585. | ||
Sethi S. Molecular diagnosis of respiratory tract infection in acute exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2011;52(S4):S290–S295. | ||
Balter M. Canadian guidelines. Can Respir J. 2003;10:248–258. | ||
Woodhead M. Guidelines for the management of adult lower respiratory tract infections. Eur Respir J. 2005;1138–1180. | ||
Daniels JM, de Graaf CS, Vlaspolder F, Snijders D, Jansen HM, Boersma WG. Sputum color reported by patients is not a reliable marker of the presence of bacteria in acute exacerbations of COPD. Clin Microbiol Infect. 2010;16:583–588. | ||
Tsimogianni AM, Papiris SA, Kanavaki S, et al. Predictors of positive sputum cultures in exacerbation of COPD. Respirology. 2009;14:1114–1120. | ||
Bafadhel M, Haldar K, Barker B, et al. Airway bacteria measured by quantitative polymerase chain reaction and culture in patients with stable COPD: relationship with neutrophilic airway inflammation, exacerbation frequency, and lung function. Int J COPD. 2015;10:1075–1083. | ||
Stolz D, Tamm M. Discriminate use of antibiotics for exacerbation of COPD. Curr Opin Pulm Med. 2009;15:126–132. | ||
San Román Terán C, Guijarro Merino R, Gómez Huelgas R, Montero Ribas L. Epidemiología hospitalaria de la EPOC en España. Rev Clin Esp. 2007;207(Suppl 1):3–7. | ||
Pozo-Rodríguez F, Alvarez CJ, Castro-Acosta A, et al; AUDIPOC Spain Group. Clinical audit of patients admitted to hospital in Spain due to exacerbation of COPD (AUDIPOC study): method and organisation. Arch Bronconeumol. 2010;46:349–357. |
Supplementary material
Investigators of the ESMI Study, Working Group on COPD of the Spanish Society of Internal Medicine
Elena Güell Farré (Hospital Sant Joan Despi Moises Broggi), Enrique Calderón Sandubete (Hospital universitario Virgen del Rocío), Jordi Guerrero González (Hospital general Mateu Orfila), Rosa Garcia Contreras (Hospital Universitario Virgen del Rocio), Adriana Gómez Gigirey (Complexo Arquitecto Marcide de Ferrol), Carlos Dueñas Gutiérrez (Hospital General Yagüe), Elena Zubillaga Azpiroz (Hospital Donostia), Carmen Romero Pérez (Hospital Severo Ochoa de Leganés), Fátima del Molino Sanz (Hospital General de Catalunya), Jeronimo Nieto López-Guerrero (Hospital de Cantoblanco), Anna Arjol Boga (Fundacion privada - Hospital de Mollet), Jose Miguel Hernandez Rey (Hospital Punta Europa), María Melero Bascones (Hospital General de Albacete), Alberto Muela Molinero (Hospital Monte San Isidro Complejo Asistencial de León), Fernando Javier Sánchez Lora (Hospital de Antequera), Jesús Castiella Herrero (Fundación Hospital Calahorra), Juan Carlos Martín Escudero (Hospital Universitario Rio Hortega), Maria Cruz Almendros Rivas (Hospital de Palamós), Natalia Costa Ferrer (Hospital Can Misses de Ibiza), Olga Torres Bonafonte (Hospital de Sant Pau), Belen Alonso (Hospital Juan Negrín, Gran Canaria), Pablo Espejo Salamanca (Hospital de Manacor), Gabriel Zubillaga Garmendia (Hospital Donostia), José Antonio Díaz Peromingo (Hospital da Barbanza), Montserrat Pérez Pinar (Hospital General Villarobledo), Pilar Román Sánchez (Hospital General de Requena), Vicente Giner Galvañ (Hospital Verge dels Lliris), José Portillo Sánchez (Hospital General de Ciudad Real), Mario Fernández Ruíz (Hospital Universitario 12 de Octubre), Jose Manuel Murcia Zaragoza (Hospital Vega Baja de Orihuela).
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






