Back to Journals » Infection and Drug Resistance » Volume 15
Bacterial Contamination Level of Indoor Air and Surface of Equipment in the Operation Room in Dil-Chora Referral Hospital, Dire Dawa, Eastern Ethiopia
Received 15 July 2022
Accepted for publication 20 August 2022
Published 31 August 2022 Volume 2022:15 Pages 5085—5097
DOI https://doi.org/10.2147/IDR.S380774
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Robel Mekonnen Yimer,1 Mesfin Kebede Alemu2
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Dire Dawa University, Dire Dawa, Ethiopia; 2Department of Public Health, College of Medicine and Health Sciences, Dire Dawa University, Dire Dawa, Ethiopia
Correspondence: Robel Mekonnen Yimer, P.O.Box 1362, 3000 Dire Dawa University, Dire Dawa, Ethiopia, Tel +251 913 278 009, Fax +251 25 112 79 71, Email [email protected]; [email protected]
Purpose: This study investigated the bacterial contamination level of the indoor air and surface of the operation room, surgical, and gynecology wards of Dilchora Referral hospitals between January and August 2020.
Methods: A laboratory based cross-sectional study was carried out on the OR and wards of Dilchora referral hospital in Eastern Ethiopia. A passive air sampling method was used to collect 128 indoor air samples; the bacterial load was enumerated and the result was expressed as colony forming units (CFU/m3). Additional qualitative analysis was carried out to identify particular bacterial species that were isolated from the indoor air and swabs taken from the surface of the equipment using conventional techniques. All laboratory data were entered and analyzed using MS Excel 2007 and SPSS version 20.
Results: The mean bacterial counts of 94.63 CFU/dm/hr in major OR during active time as well as 509.75 and 509.38 CFU/dm/hr in male and female clothing rooms during the afternoon were unacceptable (> 450 CFU/dm2). Similarly, 43.75% of the bacterial counts found in the afternoon samples fell short of Fisher’s criterion. The difference between the bacterial counts recorded in the morning and afternoon was significant (p=0.000). A total of 54 (42.2%) indoor air samples and 28 (93.3%) cotton swabs were positive for bacterial growth, with S. aureus (51.04%) and Bacillus sp (55%) being the dominant bacteria isolated from indoor air and the surface of equipment, respectively.
Conclusion: The bacterial load of investigated wards is considerably “high” to “very high”, which implies a significant risk of hospital acquired infections. Therefore, devising effective control strategies targeted on surface cleansing and sterilizing of the air environment and practicing periodic microbial surveillance of the hospital environment is a paramant.
Keywords: bacterial contamination, operation room, Dilchora referral hospital
Introduction
The operation room (OR) is one of the most important medical divisions in the hospital where surgeries are carried out.1 The OR demands a relatively sterile setting with the fewest airborne microbes.2 Nevertheless, the OR is considered to be a complex setting that carries a significant risk of infection for surgical team and patients due to prolonged exposure to multiple dangers, including chemical, physical, and biological elements.3 The amount of contamination level in the hospital environment is what essentially determines the prevalence of surgical site infection (SSI), the key clinical indicator of quality patient care, and infection prevention.4,5 Prior estimates indicated that airborne transmission was responsible for 10–24% of SSIs.6
Because enclosed areas can confine aerosols and allow them to build up to infectious levels, the indoor air environment has the potential to put patients at greater risk than the outdoor air environment.7 Multiple reservoirs, including ventilation systems, unfiltered air and antiseptic solutions, drainage of wounds, patient transportation and collection bags, the surgical team, the volume of indoor traffic, theater gowns, footwear, gloves, and hands, the use of improperly sterilized equipment, contaminated environments, and grossly contaminated surfaces, have all been identified as sources of indoor air contamination.8
A wide range of bacterial pathogens is associated with contamination of the air, surface, and equipment of OR and SWs, including staphylococci aureus (S. aureus), coagulase-negative Staphylococci (CoNS), E. coli, Proteus spp, Pseudomonas aeruginosa (P. aeruginosa), Bacillus spp, Enterococcus spp, and Enterobacter spp.8,9 These pathogens have the potential of prolonged survival on the surface of inanimate items/equipment and air, capable of initiating infections, acquiring new resistant genes, and disseminating within the units and hospital environment. Few studies carried out in Ethiopia showed a higher level of bacterial contamination from OR and SW indoor air and came to the conclusion that bacterial contamination is a major problem in these units and was significantly over the established acceptable levels.10,11 A high degree of microbial contamination highlights the necessity for ongoing surveillance targeted at preventing hospital-acquired infections (HAIs).12
The prevalence of SSIs had continued to rise as a result of microbial contamination in the OR and surgical wards (SWs).8,13 The clinical impact on the patient and the surgical team is significant, with a consequent increase in morbidity and mortality rates,8,14 duration of hospital stays, and overall expenses among patients admitted for SSI.15 A relevant increase in hospital stay, extra medical costs, and mortality following HAI due to multi-drug resistant organisms (MDROs) was also reported by previous studies.16–18 Moreover, a study by Guglielmo et al indicated a significant increase in the duration of hospitalization of 34.0 days for P. aeruginosa and 32.6 days for K. pneumoniae, while for Gram-positive bacteria, the most significant extension was found for MRSA (18.0 days), with an overall direct cost attributable to alert organisms’ infections of 1,409,000 € (11,549 € per patient).15
Measuring microbial air quality is a fundamental step for risk management.19 It allows us to confirm the presence of biological agents, identify critical situations, and validate the preventive measures adopted. To date, in Dire Dawa Ethiopia, no previous study regarding the microbial air contamination level of hospital environments, lack of epidemiological data about aerosole bacterial profile, and also the effectiveness of IPC measures in current use has never been evaluated.
This current study was conducted in terms of 1) providing up-to-date epidemiological data on frequently isolated bacterial species of air contaminants; 2) providing reliable and evidence-based findings on the risk of airborne infection in order to implement targeted preventive and control measures; and 3) providing valuable information used as an index for environmental cleanliness, a measure of their bearing on human health, and as a source HAI.
Materials and Methods
Study Area
Dil-Chora Referral Hospital (DCRH), which is found in Dire Dawa Administration served as the study setting. Dire Dawa is situated 550 kilometers from the capital, Addis Abeba. Its latitude and longitude are 9°36ʹN 41°52ʹE. According to the Central Statistical Agency of Ethiopia’s (CSA) population prediction for 2019/2020, there are 506,609 people living in the administration, of whom 313,000 (63%) people live in the city and the remaining 180,000 (37%) are rural residents.20
DCRH is the only referral hospital in the city with 300 beds and provides various types of medical services. Five major operations – both elective and emergency – are performed daily in the general surgical department. As a referral hospital, DCRH has no demarcated catchment area and it gives referral services to all Eastern Ethiopian populations.
Study Design and Period
A laboratory-based cross-sectional study was carried out between January and August 2020 in the OR and SWs of Dilchora Referral Hospital.
Selection of Operating Rooms and Wards for the Study
DCRH was selected purposively since it was the only convenient hospital for the current study; the other hospitals were acting as COVID-19 treatment centers. DCRH has two ORs: major OR (OR-1) and Gyn OR (OR-2), each comprising of one male clothing room and one female clothing room (MCR-1 and FCR-1 and MCR-2 and FCR-2) and one sterilization/material store room (STR/MSR-1 and STR/MSR-2). Accordingly, both OR-1 and OR-2, FCR-1, MCR-2, and STR/MSR-1 were selected randomly. Similarly, out of the eight male and female surgical wards (SWs) and five maternity wards (MWs), one ward was selected from each (MSW-3, FSW-1, and MW-3) at random by lottery method. Finally, a total of eight sampling sites (OR-1, OR-2, FCR-1, MCR-2, STR/MSR-1, MSW-3, FSW-1, and MW-3) were selected for the study.
Laboratory Methods of Sample Collection and Analysis
Collection of Cotton Swab Samples
A total of 30 cotton swabs were taken from the surface of articles and equipment within the OR and SWs based on swab sampling procedures, as described previously.21 A cotton swab that has been wet with sterile normal saline rolled several times on the surface of selected equipment 1 cm by 1 cm·area/cm2/surfaces, and then labeled properly before transporting to the microbiology laboratory for processing.
Collection of Indoor Air Samples
Indoor air samples from eight different locations were collected in eight rounds. In each round, the indoor air in FCR-1, MCR-2, STR/MSR-1, MSW-3, FSW-1, and MW-3 were collected twice a day, during the morning (8:00–10:00 am) and in the afternoon (7:00–9:00 pm). Whereas, air sample in OR-1 and OR-2 collected during active time (surgical procedure) and passive (at rest) time. Totally, 128 indoor air samples collected by using a passive air sampling technique that was standardized by the Index of Microbial Air Contamination (IMA), which is the number of CFU counted on a 9 cm in diameter Petri dish (63.585 cm2 areas) with 2% nutrient agar that was left open to the air according to the 1/1/1 scheme (for 1 hour, 1 meter above the floor and about 1 meter away from walls and major obstacles).22
Bacterial Enumeration and Identification
After air exposure, the nutrient agar plates were covered and labeled properly, and then transported to the microbiology laboratory for processing. The swabs alongside the plates were inoculated onto 5% Sheep blood agar (Oxoid, UK) and MacConkey agar (Oxoid, UK) and incubated at 37°C for 24 hours. After overnight incubation, the number of discrete macroscopic colonies on nutrient agar plates were counted manually, and the number of airborne bacteria were expressed both as CFU/plate/time which in turn transformed in CFU/m3 by using the following Omelyansky’s formula: [N=5a*104 (bt)−1]; where N=CFU/m3; a is the number of colonies per petridish; b is the petridish square centimeter diameter; and t is the exposure time in minute.23
Further presumptive bacterial isolates were identified by microscopic examination of Gram-stained smears, colonial morphology and hemolysis pattern, and biochemical tests. Consequently, those suspected colonies were sub-cultured on an appropriate culture media based on their Gram-reaction. Gram-positive Staphylococci were cultured onto mannitol salt agar (MSA) (Oxoid, UK), while Streptococci and Bacilli onto 5% Sheep blood agar (Oxoid, UK). Gram-negative rods were cultured onto MacConkey agar (Oxoid, UK). In the end, basic bacteriological procedures such as catalase, coagulase, Mannitol and lactose fermentation, KIA, motility-urease-indole test, oxidase and citrate test, nitrate reduction test, and bacitracin susceptibility test were used to identify the specific types of bacterial species.24
Quality Control of Laboratory Procedures
The principal investigator gave two days training given for six sample collectors and two laboratory technologists about the aim of the study, the various methods and procedures of sample collection techniques and the role of aseptic techniques during sample collection and processing. All samples were checked for proper and correct labeling and transported safely. Two supervisors were assigned to perform the supervision of sample collection procedures on daily basis.
During laboratory analysis, standard operating procedures had followed, calibrated equipment used for measuring reagents and all other materials were checked for proper functionality. Culture media prepared and sterilized following the manufacturer’s instruction. After that, 5% of the batch was incubated at 35–37°C overnight to check the sterility of the culture media and watch for bacterial growth. Finally, the media that had evidence of bacterial development had been discarded. The American Type Culture Collection (ATCC) strains including S. aureus ATCC25923 and E. coli ATCC25922 strains were used as quality control organisms to observe the entire bacteriological process.
Statistical Analysis
All laboratory data were entered and analyzed on Microsoft Office Excel 2007 and Statistical Package for Social Sciences (SPSS) version 20. Descriptive statistics such as mean and standard deviation, frequency, and percentage were used to present the findings and interpreted according to scientifically determined baseline values adopted by the European Cooperation for Accreditation of Laboratories.22 An effect size of mean bacterial air counts among the investigated wards was tested using a one-way ANOVA test.
Ethical Consideration
Ethical approval for the study was obtained from the Institutional Review Board, College of Medicine and Health Sciences, Dire Dawa University (Approval number: DPH/23/0042). Indoor air and cotton swab samples were collected after securing permission letter from Dire Dawa Regional Health Bureau and Dilchora Referral Hospital.
Result
Bacterial Concentration of Indoor Air
A total of 128 indoor air samples were collected in eight rounds, two times a day each round. As displayed in Table 1, the Major OR had the highest bacterial load of 124 CFU/dm2/hr (1,625 CFU/m3) during active/surgery time, and the Gyn OR had the least, 3 CFU/dm2/hr (39 CFU/m3) taken at rest. When it comes to mean bacterial counts of both ORs, it was found that the major OR had 94.63 CFU/dm2/hr (1,104.12 CFU/m3) during operation time while it was 7.75 CFU/dm2/hr (101.75 CFU/m3) during at rest/passive time. On the contrary, mean bacterial counts of 73.63 CFU/dm2/hr (965 CFU/m3) and 7.37 CFU/dm2/hr (96.75 CFU/m3) were recorded in Gyn OR during operation time and at rest, respectively. When these differences among the means during active and rest time were tested, it was statistically significant (p<0.000).
 |
Table 1 Bacterial Concentration of Indoor Air Taken from Major and Gyne ORs and Wards Expressed as CFU/dm2/Hr and CFU/m3, in Dilchora Referral Hospital, Dire Dawa, Ethiopia |
Regarding the intermediate and non-critical zone of OR, a higher bacterial load of 647 CFU/dm2/hr. and a lower count of 246 CFU/dm2/hr were recorded at MCR-2 and STR/MSR-1, respectively. Similarly, mean bacterial concentration of 509.75 CFU/dm2/hr (6680.5 CFU/m3) in MCR-2 and 509.38 CFU/dm2/hr (6675.75 CFU/m3) in FCR-1 during afternoon time were unacceptable (>450 CFU/dm2). The difference between the means of bacterial counts taken in the morning and afternoon was statistically significant (p<0.000).
Considering the indoor air bacterial load of surgical and maternity wards, it was found that MSW-3 during the afternoon and MW-3 in the morning showed the highest, 648 CFU/dm2/hr. and lowest, 86 CFU/dm2/hr. respectively. Similarly, higher mean bacterial counts of 485.88 CFU/dm2/hr (6,367.62 CFU/m3) in MSW-3 and lower 137.25 CFU/dm2/hr (1,798.75 CFU/m3) in MW-3 were recorded. The difference among the means of the investigated wards was found to be statistically significant (p<0.000).
Table 2 compares the proportion of bacterial concentration in indoor air against the standard acceptable limit set by Fisher. Generally, 33/80 (41.25%) bacterial loads of sampled indoor air were found to be unacceptable while 42/80 (52.5%) were acceptable. More specifically, around 43.75% of bacterial load sampled in Major OR, 56.25% of MCR-2, and FCR-1 were beyond tolerable limits (unacceptable range) compared to the standard. However, only 25% of bacterial counts in Gyn OR and STR/MSR-1 fell under unacceptable limits (Table 2).
 |
Table 2 Percentage of Indoor Air of Different Sites Against the Standard, in Dilchora Referral Hospital, Dire Dawa, Ethiopia |
Table 3 displays the variation in sampling time of indoor air to the standard set by IMA. Accordingly, 28 (43.75%) of bacterial counts recorded in the afternoon samples were unacceptable compared to the standard set by Fisher. On the contrary, only 11 (17.2%) samples taken in the morning time were beyond the tolerable limit. The difference in sampling time during the morning and in the afternoon was statistically significant, p-value=0.000.
 |
Table 3 Time Variation of Indoor Air Samples Against the Standard, in Dilchora Referral Hospital, Dire Dawa, Ethiopia |
One-way ANOVA was tested to compare the mean bacterial concentrations in three separate wards (MSW-3, FSW-1, and MW-3) and three intermediate zones of the OR (FCR-1, MCR-2, and STR/MSR-1), as shown in Tables 4 and 5. Accordingly, the test revealed that the investigated wards had a significant mean bacterial concentration difference (p=0.001). Moreover, the estimated effect size was considerably high (0.549).
 |
Table 4 ANOVA for Mean Difference of Bacterial Load and Magnitude of the Effect Size Among Investigated Wards and Clothing Rooms, in Dilchora Referral Hospital, Dire Dawa, Ethiopia |
 |
Table 5 ANOVA for Mean Difference of Sampling Time Variations in Bacterial Load Among Investigated Wards and Rooms, in Dilchora Referral Hospital |
Profile of Bacterial Species Isolated from Indoor Air and Surfaces
A total of 158 samples (128 indoor air and 30 cotton swab samples) were collected and analysed. Out of 128 indoor air samples, 54 (42.2%) showed bacterial growth with a total yield of 96 isolates. Similarly, of 30 cotton swab samples taken from the surface of articles and equipment, 28 (93.3%) were positive for bacterial growth yielding 36 isolates in total (Figures 1 and 2).
 |
Figure 1 Proportion of bacteria isolated from Indoor air of operation theater and surgical wards in Dilchora Referral Hospital, Ethiopia. |
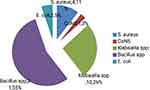 |
Figure 2 Bacterial species Isolated from Surface articles and equipment of OR in Dilchora Referral Hospital, Ethiopia. |
The majority (96; 71.6%) of the isolated bacteria recovered from the air, and the remaining 38 (28.4%) recovered from the surfaces of articles and equipment. Gram-positive isolates were predominating at 115 (85.28%) while gram negatives were 19 (14.18%). Moreover, mixed growth was reported on 20/82 (24.4%) samples.
Distribution of Bacterial Species Isolated from Indoor Air
Regarding the distribution of bacteria isolated from various wards (Figure 1), the MSW-3 had the highest percentage of bacteria isolates (19.8%), followed by the MCR-2 (18.75%) and the gyn OR had the lowest percentage (3.12%). S. aureus (51.04%) was the dominant isolates followed by coagulase-negative staphylococci (CoNS) at 19.79%, and Bacillus sp was least isolated at 4.2%. More specifically, S. aureus was isolated in all sites, while Streptococcus sp was isolated from all sites except in Gyn OR.
Distribution of Bacterial Species Isolated from Surface Samples
Figure 2 depicts the proportion of bacteria isolated from the surface of articles and equipment within the OR. A total of five bacterial genera were isolated, Bacillus sp being the major bacterial isolates (55%), followed by Klebsiella sp (26%). Whereas E. coli and CoNS were isolated at 5% and 3%, respectively. Moreover, the distribution of the isolates from different surfaces includes door and/or locker handlers (5 bacterial spp), linen and patient cloth (7), chairs (4), light switch (3), operation coaches (4), trolleys (5), and sink (8).
Discussion
The physical, chemical, and biological characteristics of the indoor environment have an impact on the general population’s health and wellbeing. However, the interior environment’s quality might potentially put human occupiers in danger and is difficult to define or control.25 Estimating the quantity and types of airborne microorganisms is important because hospital indoor air contains a variety of microbial populations. These populations can be used as an indicator of the cleanliness of the environment, as well as an indicator of how they relate to human health and as a source of HAIs.26,27
Our study revealed that the Major OR had the highest bacterial load of 124 CFU/dm2/hr (1,625 CFU/m3) and the Gyn OR had the least at 3 CFU/dm2/hr (39 CFU/m3) and this was much higher compared to reported lower counts in general surgery of 67 CFU/m3 to the highest in transplant surgery of 123 CFU/m3.28
Moreover, the mean bacterial count of major OR is 94.63 CFU/dm2/hr (1,104.12 CFU/m3) during the active time was unacceptable compared to the standard set by Fisher’s index of microbial air contamination (>9.0 CFU/dm2 at rest and >91.0 CFU/dm2 in activity).22 When compared with other similar studies conducted in different parts of Ethiopia, it was found that the mean CFU of Gyn OR was unacceptable in Jimma,13 11 major OR during the passive time in Hawassa,29 and major OR before cleaning-fumigation during morning and evening time in Mekelle.30 A high degree of microbial contamination highlights the necessity for ongoing surveillance targeted at preventing HAIs and early detection of bacterial contamination levels.12
According to several studies done in operating rooms, there is a general correlation between total air count and the risk of infection; the risk of airborne infection was significant when counts were between 700 and 1,800 CFU/m3, and the risk appeared to be minimal when they were less than 180 CFU/m3.31 Our finding revealed that Major OR had the highest bacterial load of 1,625 CFU/m3 which was much higher when compared with studies conducted in other countries. For instance, in India Gyn OT-2 and 3 showed less bacterial CFU rate of air (6 CFU/m3)12 and the study by Shaw et al28 and Anjali et al32 reported mean colony counts of 78 CFU/m3 in major ORs and 255 CFU/m3 in Gyn OT, respectively. Moreover, in our study, the mean bacterial counts of major OR (1,104.12 CFU/m3) and Gyn OR (965 CFU/m3) were in the range 700–1,800 CFU/m3 and this implies a significant risk of airborne infection in both ORs. A much higher average indoor-air load was reported by Shiferaw et al,33 where OR critical zones during the active time and passive time were 4,124 CFU/m3 and 2,889 CFU/m3, respectively. The observed discrepancy in findings between different studies can be attributed to a number of factors, including the surveillance method (active or passive air sampling), sampling time (during surgery or at rest), ventilation system of the ORs, type of disinfectants used, and the sterilization techniques used.
The present study also revealed that the mean bacterial concentration recorded in MCR-2 (509.75 CFU/dm2/hr) and FCR-1 (509.38 CFU/dm2/hr) was found to be unacceptable compared to the acceptable range of 250–450 CFU/hr.22 In Jimma, lower mean aerobic colony counts of 465 CFU/hr and 461 CFU/hr were observed in Female room-1 and room-2, respectively.11 Although, the mean bacterial load (424.87 CFU/dm2/hr) recorded in the STR/MSR-1 was in the acceptable range it was somewhat higher compared to the reported 387.05 in Hawassa in 2015.29
Considering the indoor air bacterial load of surgical and maternity wards, the range was between 86 CFU/dm2/hr (1,127 CFU/m3) and 648 CFU/dm2/hr (8,492.6 CFU/m3) at the MW-3 and MSW-3, respectively. Our finding was lower than reported in Jimma (between 2,123 and 9,733 CFU/m3).34 But it was higher compared with the study in Gondar (range between 480 and 1,468 CFU/m3).35
According to the European Commission’s sanitary standards, a bacterial load of less than 50 CFU/m3 is considered to be “very low”, 50–100 CFU/m3 is considered to be “low”, 100–500 CFU/m3 is considered to be “intermediate”, 500–2,000 CFU/m3 is considered to be “high”, and more than 2,000 CFU/m3 is considered to be a “very high” load.36 The wards under investigation in the current study have “high” to “very high” bacterial load by these norms. A similar finding was observed in Jimma in 2015.37 High staff and visitor traffic, poor ventilation, lack of staff awareness, and frequent wound dressing that could lead to the shedding of bacteria, especially when those patients have SSIs, are all potential causes of the observed increased viable count in these wards.
Similarly, higher mean bacterial counts of 485.88 CFU/dm2/hr (6,367.62 CFU/m3) in MSW-3 and lower 137.25 CFU/dm2/hr (1,798.75 CFU/m3) in MW-3 were recorded in the morning and afternoon, respectively. This finding is in line with a study in Bahirdar where mean bacterial counts of 482.8 CFU/dm2 and 329 CFU/dm2 were recorded in the morning and the afternoon, respectively.34
Comparison of Indoor Bacterial Load Against the Standard
In this study,
Regarding time variation of sampled bacterial load, about 28 (43.75%) of bacterial counts recorded in the afternoon samples were unacceptable compared to the standard set by Fisher. This finding was in line with the study in Jimma11 that reported higher colony counts of all OTs in the afternoon, but it disagrees with studies done in Hawassa29 and Northern Ethiopia,30 which reported a higher proportion of unacceptable bacterial load taken in the morning than the afternoon. Possible reasons for the observed increment of bacterial counts in the afternoon could be due to an influence of temperature and humidity, improper cleaning of the rooms, or high visitor and student traffic.
Bacterial Profile Isolated from Indoor Air and Surfaces
In this study, an overall bacterial contamination rate of indoor air was found to be 54/128 (42.2%) and it was lower compared with the study in Bauchi, Nigeria, which was 85/150 (56.7%)39 but higher compared to that reported by Shaw et al28 of 25/250 (10%). Likewise, out of 30 cotton swabs taken from the surface of articles and equipment, 93.3% were positive for bacterial growth. This was much higher compared with the study conducted in India (48/111; 43%)12 and reported by Anjali et al32 (9/68; 13.2%).
When we look at the distribution of isolated bacterial species from indoor air, S. aureus was the predominant isolate (51.04%) followed by CoNS at 19.79%, and Bacillus sp was least isolated at 4.2%. This finding was in line with a study in Jimma,11 in Hawasa,29 and in Bulchi, Nigeria.39 On the contrary, the study in Gondar and Mekelle showed that CoNS was predominant followed by S. aureus.30,33 Likewise, Shaw et al28 reported CoNS as the commonest pathogen, followed by Micrococcus spp. and S. aureus. S. aureus is a potential pathogen able to cause skin and soft tissue infections. Similarly, CoNS and Streptococcus spp. are also important causes of SSIs. Hence, their identification from indoor air is a big threat putting patients highly susceptible to SSIs.
Our study revealed that S. aureus was isolated from all investigated OR sites and wards which is supported by a study in Nigeria.21 As stated by WHO, the amount of S. aureus that an individual has dispersed is indicated by the presence of the organism in the air of patient rooms and operating rooms. It is an effective way to identify the presence of potentially harmful dispersers. A 10-fold increase in the air count of S. aureus may result from the admission of a single heavy disperser to a hospital room with multiple beds, and this would be clearly visible on settle-plates.31 Given that S. aureus is a common normal flora of human skin and the nasopharynx, the surgical team could be the main source of the infection.13
When it comes to bacteria isolated from the surface of articles and equipment, Bacillus sp was found to be the major bacterial isolates (55%), followed by Klebsiella sp (26%). This finding is supported by the study in Nigeria,21 but previous studies in Sao Paulo (Brazil) and in Gaza showed a higher prevalence of S. aureus.35,40 Whereas Anjali et al32 reported CoNS species followed by Bacillus and Klebsiella species. The detection of a higher load of Bacillus sp from articles and equipment implies the presence of higher environmental contamination in investigated rooms and wards. The hands of HCWs could be easily contaminated during care-giving or from contact with environmental surfaces in close proximity to the patient are capable of spreading MDROs from one person to another,41,42 hence are more likely to transmit MDROs to patients. Thus, strategies to increase and monitor adherence are important components to reduce contamination and of MDRO control programs.42
The current study widened our understanding on the importance of monitoring microbial air contamination by providing ample evidence to suggest that potential pathogenic bacteria carried on indoor air as well as surfaces of patient caring equipment are the major source of HAIs and spread of MDROs. These pathogens possess the potential for prolonged survival on the surface of inanimate items/equipment and indoor air, capable of initiating infections, acquiring new resistant genes, and disseminating within the units and hospital environment.8,11 In addition, the presence of S. aureus in the air of all investigated rooms/wards and on the surfaces of equipment as evidenced by the current study, also by several previous studies, highlights the implication of this bacteria in posing gross contamination of hospital settings.
In general, poor microbial air quality within a hospital environment implies a huge potential for transmitting and/or spreading MDROs and a source of higher incidence of HAIs. Therefore, increased lengths of stay, extra medical costs, and mortality associated with MDROs and HAIs could simply be reverted by routine microbial surveillance of hospital environments and escalation of IPC measures.
Limitation of the Study
Due to resource constraints, the drug susceptibility pattern for the bacterial isolates has not been done.
Conclusion
A higher proportion of bacterial indoor air load recorded during the active time and the afternoon samples were far beyond the acceptable limit and this entails poor cleanliness of the investigated room/which might be a potential risk factor for the spread of MDROs and HAIs. Thus, there is a need for awareness creation of infection control.
According to standards set by Fisher’s IMA and the sanitary standards of the European Commission for non-industrial premises, the bacterial load of the investigated wards is considered “high” and this implies a potential risk of airborne infection for patients, visitors, and hospital employees.
An overall bacterial contamination rate on the surface of articles and equipment within the OR suggests ineffectual cleaning and decontamination of materials. This indicates that the patient care equipment and materials are not sterile and the patients can be infected with the pathogenic microorganisms present in these items. The bacteriological profiles of the isolates, such as S. aureus and CoNS, Bacillus sp, Klebsiella sp, P. aeruginosa and others, are troublesome as these bacteria are well-known causative agents of HAIs in highly sensitive hospital environments, including operating theater rooms and wards.
Recommendations
- Devising effective control strategies targeted on surface cleansing and sterilizing the air of hospital environment.
- Implement a multi-disciplinary process to monitor and improve HCP adherence to recommended practices for Standard and Contact Precautions.
- Follow recommended cleaning, disinfection and sterilization guidelines for maintaining patient care areas and equipment.
- Focus on cleaning and disinfecting frequently touched surfaces (eg, door and/or locker handlers, linen and patient cloth, chairs, light switch, operation coaches, trolleys, and sink) and equipment in the immediate vicinity of patients with powerful detergents and disinfectants.
- Periodic microbial surveillance of hospital environment is a paramant to implement and revise IPC measures.
- Further studies should be done on antimicrobial resistance patterns of the isolated bacteria.
Acknowledgment
We sincerely acknowledge Dire Dawa University, the Research Affairs Directorate office, and the College of Medicine and Health Science for their assistance and facilitation. We are also grateful to Dire Dawa health bureau and Dilchora Referral Hospital for letting us do the study. Our gratitude goes to the staff of Dire Dawa regional laboratory, Harare regional laboratory, and Ethiopia public health institute-microbiology units for their indispensable technical and material support.
Funding
The research, writing, and/or publication of this paper were all done without any financial assistance from any sources.
Disclosure
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this paper.
References
1. Rodriguez-Merchan EC. Risk factors of infection following total knee arthroplasty. J Orthop Surg. 2012;20(2):236–238. doi:10.1177/230949901202000220
2. Leaper DJ, Edmiston CE. World Health Organization: global guidelines for the prevention of sugical site infection. J Hosp Infect. 2017;95(2):135–136. doi:10.1016/j.jhin.2016.12.016
3. Gioffre A, Dragone M, Ammoscato I, et al. The importance of the airborne microorganism evaluation in the operating rooms: the biological risk for health care workers. G Ital Med Lav Ergon. 2007;29:743–745.
4. Dharan S, Pittet D. Environmental controls in operating theatres (review). J Hosp Infect. 2002;51(2):79–84. doi:10.1053/jhin.2002.1217
5. Imai E, Ueda M, Kanao K, et al. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. Am J Infect Control. 2008;36(10):727–731. doi:10.1016/j.ajic.2007.12.011
6. Fernstrom A, Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. J Pathog. 2013;2013:1–13. doi:10.1155/2013/493960
7. Landrin A, Bissery A, Kac G. Monitoring air sampling in operating theatres: can particle counting replace microbiological sampling? J Hosp Infect. 2005;61(1):27–29. doi:10.1016/j.jhin.2005.03.002
8. Okon KO, Osundi S, Dibal J, et al. Bacterial contamination of operating theatre and other specialized care unit in a tertiary hospital in Northeastern Nigeria. Afr J Microbiol Res. 2012;6(13):3092–3096.
9. Abubakar AS, Barma MM, Balla HJ, et al. Spectrum of bacterial isolates among intensive care patients in a tertiary hospital in northeastern Nigeria. Indian J Sci and Tech. 2014;2(6):42–46.
10. Mengistu H, Misganaw B, Elshaday A. Bacterial load and antibiotic susceptibility pattern of isolates in operating rooms at Hawassa University Referral Hospital, southern Ethiopia. JMA. 2015;8(1):1–6. doi:10.5897/JMA2015.0349
11. Chalachew G, Gebre K, Wondewosen T. Indoor air bacterial load and antibiotic susceptibility pattern of isolates in operating rooms and surgical wards at Jimma University Specialized Hospital, Southwest Ethiopia. Ethiop J Health Sci. 2011;21(1):9–17.
12. Kiranmai S, Madhavi K. Microbiological surveillance of operation theatres, intensive care units and labor room of a teaching hospital in Telangana, India. Int J Res Med Sci. 2016;4(12):5256–5260. doi:10.18203/2320-6012.ijrms20164190
13. Al-Benna S. Infection control in operating theatres. J Perioper Pract. 2012;22(10):318–322. doi:10.1177/175045891602201002
14. Napoli C, Marcotrigiano V, Montagna MT. Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theaters. BMC Public Health. 2012;12(1):594. doi:10.1186/1471-2458-12-594
15. Giraldi G, Montesano M, Napoli C, et al. Healthcare-associated infections due to multidrug-resistant organisms: a surveillance study on extra hospital stay and direct costs. Curr Pharmaceut Biotech. 2019;20(8):643–652. doi:10.2174/1389201020666190408095811
16. Macedo-Viñas M, De Angelis G, Rohner P, et al. Burden of meticillin-resistant Staphylococcus aureus infections at aSwiss University hospital: excess length of stay and costs. J Hosp Infect. 2013;84(2):132–137. doi:10.1016/j.jhin.2013.02.015
17. Cofsky R, Vangala K, Haag R, et al. The cost of antibiotic resistance: effect of resistance among Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudmonas aeruginosa on length of hospital stay. Infect Control Hosp Epidemiol. 2002;23(2):106–108. doi:10.1086/502018
18. Cosgrove SE. The Relationship between Antimicrobial Resistance and Patient Outcomes: mortality, Length of Hospital Stay, and Health Care Costs. Clin Infect Dis. 2006;42(Supplement_2):S82–89. doi:10.1086/499406
19. Fournier PE, Richet H, Weinstein RA. The Epidemiology and Control of Acinetobacter baumannii in Health Care Facilities. Clin Infect Dis. 2006;42(5):692–699. doi:10.1086/500202
20. Central statistical agency of Ethiopia, (CSA) 2007.
21. Hafeez JR, Zubair M, Anwar MS, Tayyib M, Husnain S. Microbiological surveillance of operation theatres and Icus of a Tertiary Care Hospital, Lahore. Biomedica. 2008;24(WC):99–102.
22. Pasquarella C, Pitzurra O, Savino A. The index of microbial air contamination. J Hosp Infect. 2000;46(4):241–256. doi:10.1053/jhin.2000.0820
23. Omelyansky VL. Manual in microbiology. Leningrad: USSR Academy of Sciences Moscow; 1940.
24. Cheesbrough M. District Laboratory Practice in Tropical Countries Volume II: Microbiology. Cambridge (UK): Cambridge university press; 2005: 19–26.
25. Jaffal AA, Nsanze H, Bener A, et al. Hospital airborne microbial pollution in a desert country. Environ Int. 1997;23(2):167–172. doi:10.1016/S0160-4120(97)00003-2
26. Gururajan G, Kaliyaperumal KA, Ramasamy B. Prevalence of extended spectrum beta lactamases in uropathogenic Escherichia coli and Klebsiella species in a Chennai Suburban Tertiary care hospital and its antibiogram pattern. Res J Microbiol. 2011;6(11):796–800. doi:10.3923/jm.2011.796.804
27. Ekhaise FO, Ighosewe OU, Ajaakpori OD. Hospital indoor airborne micro-flora in private and government owned hospitals in Benin City, Nigeria. World J Med Sci. 2008;3:34–38.
28. Shaw LF, Chen IH, Chen CS, et al. Factors influencing microbial colonies in the air of operating rooms. BMC Infect Dis. 2018;18(1):4. doi:10.1186/s12879-017-2928-1
29. Mengistu H, Mesfin W, Elshadie A. Intensive care units and operating rooms bacterial load and antibiotic susceptibility pattern. J Surg. 2016;4(2):60–64. doi:10.11648/j.js.20160402.21
30. Tesfaye T, Berhe Y, Gebreselassie K. Microbial contamination of operating theatre at Ayder Referral Hospital, Northern Ethiopia. IJPSR. 2015;6(10):1264–1267.
31. World Health Organization. Hospital-acquired infections: guidelines to laboratory methods. WHO Regional Publications European Series No. 4; 1978.
32. Anjali K, Anamika V, Mrithunjay K, Dalal AS, Kumar A. Environmental microbiological surveillance of operation theatres in a tertiary care hospital. IJCR. 2015;7(03):13977–13980.
33. Shiferaw T, Gebre-silasse L, Mulisa G, et al. Bacterial indoor-air load and its implications for healthcare-acquired infections in a teaching hospital in Ethiopia. Int J Infect Control. 2016;12:1–9. doi:10.3396/IJIC.v12i1.004.16
34. Getachew H, Derbie A, Mekonnen D. Surfaces and air bacteriology of selected wards at a referral hospital, Northwest Ethiopia: a cross-sectional study. Int J Microbiol. 2018;2018:2190787. doi:10.1155/2018/2190787
35. Gizaw Z, Gebrehiwot M, Yenew C. High bacterial load of indoor air in hospital wards: the case of University of Gondar teaching hospital, Northwest Ethiopia. Multidiscip Respir Med. 2016;11(1):24. doi:10.1186/s40248-016-0061-4
36. Commission of European Communities. Biological particles in indoor environments, European Collaborative Action - Indoor air quality and its impact on man, Report No. 12, CEC, Luxembourg. Available from: http://www.soer.justice.tas.gov.au/2003/source/94/index.php.
37. Fekadu S, Getachewu B. Microbiological assessment of indoor air of teaching hospital wards: a case of Jimma university specialized hospital. Ethiop J Health Sci. 2015;25(2):117–122. doi:10.4314/ejhs.v25i2.3
38. Qudiesat K, Abu-Elteen K, Elkarmi A, Hamad M, Abussaud M. Assessment of airborne pathogens in healthcare settings of Jordan. AJMR. 2009;3(2):066–076.
39. Mohammed A, Okon Kenneth O, Yusuf JB, et al. Bacterial contamination of operating theatres at tertiary hospital in Bauchi, Northeastern Nigeria. EJPMR. 2017;4(4):182–188.
40. Al Laham NA. Distribution and antimicrobial resistance pattern of bacteria isolated from operation theaters at Gaza Strip. J Al Azhar Univ Gaza Nat Sci. 2012;14:19–34.
41. Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of Nosocomial Pathogens on Hands After Contact With Environmental Surfaces Near Hospitalized Patients. Infect Control Hosp Epidemiol. 2004;25(2):164–167. doi:10.1086/502369
42. CDC. Guideline for hand hygiene in health-care settings. MMWR. 2002;51(16):1–44.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
