Back to Journals » OncoTargets and Therapy » Volume 13
B7-H6 Promotes Cell Proliferation, Migration and Invasion of Non-Hodgkin Lymphoma via Ras/MEK/ERK Pathway Based on Quantitative Phosphoproteomics Data
Authors Yang S, Yuan L , Wang Y, Zhu M, Wang J, Ke X
Received 9 April 2020
Accepted for publication 28 May 2020
Published 18 June 2020 Volume 2020:13 Pages 5795—5805
DOI https://doi.org/10.2147/OTT.S257512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Siyuan Yang, Lei Yuan, Yanfang Wang, Mingxia Zhu, Jing Wang, Xiaoyan Ke
Department of Hematology and Lymphoma Research Center, Peking University Third Hospital, Beijing 100191, People’s Republic of China
Correspondence: Xiaoyan Ke
Department of Hematology and Lymphoma Research Center, Peking University Third Hospital, Beijing 100191, People’s Republic of China
Tel/Fax +86-10-82266778
Email [email protected]
Purpose: B7 homologue 6 (B7-H6) has been found at an up-regulated level in multiple cancer cells and identified to be positively correlated with inferior clinical features. In non-Hodgkin lymphoma (NHL), however, the roles of B7-H6 and the underlying mechanism of action remain unclear. Through in vivo and in vitro experiments, the aim of this study was to explore the regulatory mechanism of B7-H6 in NHL in order to provide new therapeutic strategies that can potentially be applied in clinical practice.
Methods: The expression of B7-H6 in T-lymphoblastic lymphoma (TLBL), diffuse large B cell lymphoma (DLBCL) and lymph node reactive hyperplasia (LRH) tissues were compared by immunohistochemistry. A total of 10 NHL cell lines were screened by Western blot to evaluate the expression of B7-H6. The effects of B7-H6 knockdown on cell proliferation, migration and invasion of NHL cells were studied in vivo using a transplanted tumor mice model, and in vitro by Cell Counting Kit-8 (CCK-8) and Transwell assays. Quantitative phosphoproteomics was performed to identify the changes of protein phosphorylation and related pathways affected by B7-H6. The effects of B7-H6 on NHL were validated via B7-H6 overexpression and pathway inhibitor assays.
Results: The expression levels of B7-H6 in NHL cell lines, and TLBL and DLBCL tissues were significantly increased compared with those in the control groups. Inhibition of cell proliferation, migration and invasion was observed in Jurkat and Raji cells with B7-H6 knockdown. The ability of B7-H6 in promoting tumorigenesis was further validated by in vivo experiments. In addition, Ras and HIF-1 signaling pathways were shown to be significantly affected by B7-H6 through quantitative phosphorylation proteomics analysis. Ras/MEK/ERK pathway was verified to be significantly inhibited after B7-H6 knockdown by Western blot analysis. Strikingly, MEK inhibitor AZD8330 was found to have the ability to sufficiently inhibit Ras/MEK/ERK pathway, partially reverse cell proliferation and completely reverse cell migration and invasion induced by B7-H6.
Conclusion: B7-H6 promotes cell proliferation, migration and invasion in NHL via Ras/MEK/ERK pathway. Hence, B7-H6 or Ras/MEK/ERK pathway targeting may be used as potential therapeutics for treating NHL.
Keywords: B7-H6, non-Hodgkin lymphoma, proliferation, migration, invasion, MEK inhibitor
Introduction
Non-Hodgkin lymphoma (NHL), which accounts for approximately 90% of all lymphomas, has varied features that differ by geographical distributions, pathological types to clinical treatments and prognosis.1 Recently, the management of NHL has been greatly improved, due to significant advancements in new diagnostic methods and therapeutic approaches, especially targeted drugs and cellular immunotherapies.2 However, most NHL patients experience recurrence and drug resistance issues coupled with aggressive behaviors of the lymphoma. Hence, there is an urgent call in studying the underlying mechanism of NHL.
Also known as NCR3LG1, B7 homologue 6 (B7-H6) is a new member of the B7 family that is undetectable in normal human tissues but expressed in several malignant tumor cells.3 Studies have indicated that the expression of B7-H6 is highly correlated with inferior clinical features in various solid tumors, such as ovarian cancer, breast cancer and oral squamous cell carcinoma.4–6 The B7-H6 protein molecule contains several domains associated with signal transduction, such as the SH2 and SH3 domains.3,7 Our previous research have shown that, in B cell lymphoma, knockdown of B7-H6 could affect the phosphorylation of STAT3, inhibit proliferation and increase chemosensitivity.8 However, the regulatory mechanism of B7-H6 in NHL, especially in T cell lymphoma remains to be uncovered. In this study, the roles of B7-H6 in non-Hodgkin lymphoma, as well as the relevant associated signaling pathways were investigated in order to provide new insights on potential treatment strategies.
Materials and Methods
Immunohistochemistry
This study was approved by the Ethics Committee of Peking University Third Hospital, and informed consent was obtained from patients in accordance with the Declaration of Helsinki. All specimens were handled based on the standard procedures of deparaffinization, dehydration and antigen retrieval for immunohistochemical staining. B7-H6 primary antibody (Abcam, USA) was incubated at the concentration of 1:200. The immunoreactive scores (IRS) were recorded as described by Specht.9 The IRS were recorded according to the following formula: IRS= staining intensity (0, negative; 1, weak; 2, moderate; and 3, strong) × staining density (0, 0%; 1, <10%; 2, 10–50%; 3, 51–80%; and 4, >80%). Specimens were classified as negative (IRS= 0–3) or positive (IRS= 4–12).
Cell Lines and Cell Culture
The human lymphoma cell lines, ie Burkitt lymphoma CA46, Raji and Daudi cells, mantle cell lymphoma Maver, Z138 and Jeko-1 cells, DLBCL DB cells, T-acute lymphoblastic leukemia Jurkat (Clone E6-1) and Molt-4 cells, mature T cell lymphoma HuT 78 cells and human cervix carcinoma Hela cells were obtained from ATCC. Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood samples of healthy subjects with prior informed consent. CA46, Raji, Daudi, Z138, DB, Jurkat and Molt-4 cells were cultured in RPMI 1640 medium with 10% FBS; while Jeko-1 and HuT 78 cells were cultured in RPMI 1640 medium with 20% FBS (Gibco, USA). Maver and Hela cells were respectively cultured in IMDM and DMEM medium with 10% FBS. All cells were cultured based on standard procedures at 37 °C in a humidified chamber with 5% CO2.
Western Blotting
Total cell protein was extracted using RIPA lysis buffer containing phosphatase inhibitor and protease inhibitor. Protein samples at an equal loading quantity were separated by 10% SDS-PAGE before transferring onto nitrocellulose membranes for Western blotting. Transferred membranes were individually blocked in TBST containing 5% skimmed milk, followed by overnight incubation at 4°C with primary antibodies of B7-H6, GAPDH and β-actin (Abcam, USA); MEK1/2, phospho-MEK1/2, ERK1/2 and phospho-ERK1/2 (CST, USA). Immunodecorated membranes were washed with TBST buffer, followed by a 1-hour incubation step at room temperature with anti-rabbit or anti-mouse secondary antibodies and a TBST-washing step afterwards before fluorescent signal analysis using Odyssey infrared imaging system (LI-COR).
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total cell RNA was extracted by Trizol reagent (Invitrogen, USA) and transcribed into cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). The expression levels of mRNA were determined by RT-qPCR using SuperReal PreMix Color kit (Tiangen, China). The primer sequences of the RT-qPCR were as follows: B7-H6 forward, 5ʹ-CTTTACCCTGACTGCTGC-3ʹ; B7-H6 reverse, 5ʹ-ATATGAGGTGCTCTTTCTTC-3ʹ; GAPDH forward, 5ʹ-TCAAGGCTGAGAACGGGAAG-3ʹ; GAPDH reverse, 5ʹ-TCGCCCCACTTGATTTTGGA-3ʹ. The relative B7-H6 RNA expression was calculated using a comparative 2−ΔΔCt.
Lentivirus Infection and Plasmid Transfection
A small hairpin RNA (5ʹ-CATCTTCAGCCTATACTCCTCTCAA-3ʹ) that targets human B7-H6 (shB7-H6) and the corresponding negative control (shCtrl) sequence (5ʹ-TTCTCCGAACGTGTCACGTAA-3ʹ) were designed and synthesized by Hanbio Biotechnology (Hanbio, China). Jurkat and Raji cells were infected in the presence of 5 µg/mL polybrene (Sigma, USA) and screened by 2 μg/mL puromycin. Knockdown of B7-H6 was confirmed by qRT-PCR and Western blotting.
B7-H6 overexpression plasmid (hB7-H6) was purchased from Hanbio Biotechnology (Hanbio, China). Cells at a density of 2 × 106 cells/100 μL B7-H6 overexpression plasmid or negative control plasmid (hCtrl) in OPTI-MEM® medium were transfected using Super Electroporator NEPA21 Type II (NEPAGENE, Japan).
CCK-8 Assay
Cell proliferation was determined by CCK-8 assays (Dojindo, Japan). Briefly, cells at a density of 5000 cells/well were inoculated in 96-well plates and incubated for 0, 24, 48 and 72 hours at 37 °C in the presence of 5% CO2, followed by a further 2-hour incubation step with 10 μL of CCK-8 solution added. The OD values of the solution in each well were measured with a microplate reader at 450 nm.
Cell Migration and Invasion Assays
Twenty-four-well Transwell cell culture chambers (Corning, USA) or Matrigel (BD Biosciences, USA)-coated invasion chambers were used for cell migration and invasion assays. The upper chambers were inoculated with 5 × 105 (migration) or 3 × 105 (invasion) cells in 100 μL of RPMI 1640 medium; while the lower chambers were added with 600 μL complete medium, followed by incubation at 37 °C for 24 hours. The migrated cells in the lower chambers were collected. The invasive cells through matrigel at the bottom of the upper chambers were fixed with methyl alcohol before staining with 0.1% crystal violet. Cells were counted and photographed under five random microscopic fields.
Subcutaneous Xenograft Model
The protocol of this animal study was approved by the Institutional Review Board (IRB) of Peking University. The experimental procedures in handling animals were in strict adherence to the guidelines of animal welfare national standards (GB/T 35892–2018). Female NOD/SCID mice (4–5 weeks) were purchased from Beijing Vital River Laboratory Animal Technology Co Ltd. (Beijing, China). Cell suspensions (5 × 106 cells) of Jurkat/shB7-H6 or Jurkat/shCtrl were inoculated subcutaneously into the armpits of NOD/SCID mice. Graft diameters, long (a) and short (b) were measured weekly. Tumor volume (V) was calculated according to the following formula: V=a×b2/2. Mice were euthanized upon tumor development and the xenografts were stripped for weighing.
Quantitative Phosphoproteomic Analysis
Quantitative phosphoproteomic analysis was performed by PTM-BIO Company (Zhejiang, China) using the detailed protocols outlined in the Supplementary Materials and Methods. In brief, the procedures started with protein extraction and trypsin digestion, followed by TMT labeling, HPLC fractionation and affinity enrichment, and finally LC-MS/MS analysis, database search and bioinformatic analysis.
Statistical Analysis
SPSS 18.0 software (IBM, USA) was used for statistical analysis and Prism 6.02 software (GraphPad, USA) was used for drawing statistical charts. Data coincided with normal distribution were presented as mean ± standard deviation for each experiment; while differences between the two groups were calculated with two-tailed t-test. Conversely, data without normal distribution were presented as median (lower quartile, upper quartile) for each experiment; while differences between the two groups were calculated with Wilcoxon Signed-Rank test. P< 0.05 was considered statistically significant.
Results
B7-H6 Is Overexpressed in NHL Patients and Cell Lines
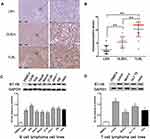
The expression levels of B7-H6 in pathological tissues of T-lymphoblastic lymphoma (TLBL), diffuse large B cell lymphoma (DLBCL) and lymph node reactive hyperplasia (LRH) were analyzed by immunohistochemistry (Figure 1A). The IRS indicated that B7-H6 was positively stained in 30% (3/10) of LRH, 81.3% (13/16) of DLBCL and 93.3% (14/15) of TLBL with a respective score of 2 (2–3.5), 6 (4–6) and 12 (9–12). The B7-H6 expression rates and the IRS scores in each group were found to be statistically different (Figure 1B).
In order to detect the expression of B7-H6 in NHL cells, the total protein samples extracted from 10 human NHL cell lines, including 7 B cell lymphoma cell lines (CA46, Raji, Daudi, Maver, Z138, Jeko-1 and DB) and 3 T cell lymphoma cell lines (Jurkat, Molt-4 and HuT 78) were subjected for Western blot analysis. The protein samples of PBMCs from healthy donors and human cervix carcinoma cell line Hela were used as negative and positive controls, respectively. As shown in Figure 1C and D, B7-H6 was overexpressed in all the NHL cell lines compared to that in negative control. Due to the relatively high expression levels of B7-H6, T cell lymphoma cell line Jurkat and B cell lymphoma cell line Raji were selected for further analysis in vitro.
Knockdown of B7-H6 Inhibits NHL Cell Proliferation, Migration and Invasion in vitro
To knockdown B7-H6, recombinant lentiviral shRNAs containing B7-H6 (shB7-H6) and vector (shCtrl) were used for cell transfection, followed by puromycin screening of targeted cells. The B7-H6 expression in transfected cells was analyzed by RT-qPCR and Western blot. The expression of B7-H6 in Jurkat and Raji cells was significantly decreased, both at RNA (Figure 2A) and protein (Figure 2B) levels, indicating that the knockdown was successful.
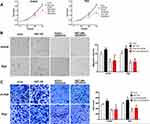
To identify whether knockdown of B7-H6 could impact cell proliferation, migration and invasion in NHL, CCK-8 and Transwell assays were performed. Cell growth curves (Figure 2C) indicated that the OD values of Jurkat/shB7-H6 cells and Raji/shB7-H6 cells at 48- and 72-hour were significantly lower than those of the corresponding shCtrl cells. Consistently, the migration rates and the invasion ability of Jurkat/shB7-H6 cells and Raji/shB7-H6 cells were both found to be significantly lower than those of the corresponding shCtrl cells (Figure 2D and E). Taken together, these data indicated that knockdown of B7-H6 could inhibit cell proliferation, migration and invasion.
Knockdown of B7-H6 Inhibits NHL Tumorigenesis in vivo
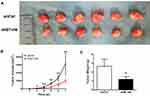
To explore the roles of B7-H6 on NHL cell proliferation in vivo, a subcutaneous xenograft model in mice was used. As shown in Figure 3B, the tumor volumes of Jurkat/shB7-H6 group were obviously decreased compared to those of Jurkat/shCtrl group from the third week onwards. The harvested tumors were photographed and weighed (Figure 3A and C) for direct comparison, and the results clearly indicated that knockdown of B7-H6 inhibited NHL tumorigenesis in vivo.
B7-H6 Alters Phosphorylation Patterns
To understand whether B7-H6 could change the phosphorylation patterns of downstream signaling and targets, quantitative phosphoproteomic analysis was performed according to the standard workflow (Figure 4A), where cell lysates from Jurkat/shB7-H6 and Jurkat/shCtrl cells analyzed by LC-MS/MS and bioinformatics. In quantifying protein phosphorylation, a fold-change ratio of more than 1.2 was considered as up-regulation; while fold-change ratio of less than 0.83 was considered as down-regulation. As shown in Figure 4B and C, 616 phosphosites in 410 proteins were found to be up-regulated; while 858 phosphosites in 553 proteins were observed to be down-regulated following the knockdown of B7-H6. GO analysis showed that the phosphorylated proteins were mainly involved in biological processes such as cell cycle, tissue development and material transportation (Figure 4D). KEGG pathways enrichment analysis based on the differential proteins showed that HIF-1 and Ras signaling pathways were significantly down-regulated following the knockdown of B7-H6 (Figure 4E).
 |
Figure 4 B7-H6 altered phosphorylation patterns. (A) Workflow of phosphoproteomic analysis. (B and C) Histogram and volcano plots of the quantified up-regulated and down-regulated phosphosites and proteins. (D) Bubble map of GO enrichment analysis of phosphoproteins based on biological process. (E) Up-regulated and down-regulated pathways of KEGG pathways enrichment analysis for altered phosphoproteins. |
B7-H6 Regulates the Phosphorylation of Ras/MEK/ERK Pathway
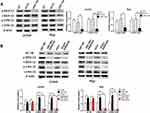
Previous studies have confirmed that HIF-1 could be affected by Ras signaling pathway.10,11 Thus, the upstream Ras signaling pathway attracted our attention for its essential role in the complicated signal network. In this study, Western blot was used to elucidate the changes in the phosphorylation levels of MEK and ERK, the key molecules in Ras signaling pathway. In order to uncover the correlation between Ras/MEK/ERK pathway and B7-H6, Jurkat and Raji cells transfected with B7-H6 overexpression (hB7-H6) or control (hCtrl) plasmids were incubated with MEK inhibitor AZD8330 (50nM) for 24 hours. Our results showed that, whilst knockdown of B7-H6 significantly decreased the phosphorylation levels of MEK and ERK compared with those of the control groups (Figure 5A), overexpression of B7-H6 significantly increased the phosphorylation levels of MEK and ERK (Figure 5B). Meanwhile, it was observed that the level of phosphorylation was attenuated by AZD8330 regardless of B7-H6 expression. Moreover, AZD8330 was found to be incapable of affecting the expression of B7-H6 (Figure 5B), indicating that B7-H6 may play a role as an upstream regulator in the activation Ras/MEK/ERK pathway.
MEK Inhibitor Reverses the B7-H6-Promoted Cell Proliferation, Migration and Invasion
To validate the roles of B7-H6 in promoting cell proliferation, migration and invasion, and to investigate whether Ras/MEK/ERK pathway was involved in the process, cells overexpressing B7-H6 (Jurkat/hB7-H6 and Raji/hB7-H6) and control cells (Jurkat/hCtrl and Raji/hCtrl) were treated with or without MEK inhibitor AZD8330 (50nM). CCK-8 assays revealed that overexpression of B7-H6 significantly enhanced cell proliferation in Jurkat and Raji cells at 48- and 72-hour, and that cell proliferation was significantly decreased by AZD8330 regardless of B7-H6 expression (Figure 6A). However, in the presence of AZD8330, B7-H6 overexpression significantly facilitated cell proliferation (Figure 6A), demonstrating that B7-H6-promoted cell proliferation could be partially reversed by AZD8330. Consistently, Transwell assays indicated that overexpression of B7-H6 significantly promoted cell migration and invasion in Jurkat and Raji cells, and that AZD8330 could significantly alleviate cell migration and invasion regardless of B7-H6 expression (Figure 6B and C). Additionally, B7-H6 overexpression was found to be unable to promote cell migration and invasion (Figure 6B and C) in the presence of AZD8330, indicating that the cell migration and invasion promoted by B7-H6 could be reversed completely by AZD8330.
Discussion
NHL is currently the 5th and 6th most common cancer type in males and females, respectively.12 Due to the increasing incidence, as well as the growing number of recurrent and refractory patients, NHL is indisputably a major health burden. In order to explore new therapeutic strategies, the underlying molecular mechanism of NHL oncogenesis and progression demand further studies. An up-regulated level of B7-H6 has been demonstrated in various human cancers, playing a role in impacting prognosis.13 Previously, we have found that knockdown of B7-H6, which was overexpressed in B cell lymphoma cell lines, inhibited the proliferation, migration and invasion of CA46 cell.8 However, the roles of B7-H6 in other NHL cells, especially in T cell lymphoma are poorly understood.
In this study, NHL patients and cell lines were screened and the mechanism of B7-H6 in NHL was studied using shRNA and overexpression plasmids. Our results showed that B7-H6 was high-expressed in DLBCL, TLBL tissues and all NHL cell lines, including B cell lymphoma and T cell lymphoma. Jurkat and Raji cells were chosen to explore the function of B7-H6 in NHL. The IRS scores in TLBL was found to be significantly higher than those in DLBCL, probably due to the more aggressive cancer behavior in TLBL. In agreement with our previous study, knockdown and overexpression assays in this study indicated that B7-H6 could act as an oncogene in T cell lymphoma in promoting cell proliferation, migration and invasion. These results are also consistent with solid tumor cells, such as hepatocellular carcinoma and glioma.14–16
The B7-H6 protein contains domains like SH2 and SH3 which are closely related to signal transduction pathways activated by protein tyrosine kinases.17,18 Our results revealed that B7-H6 altered phosphorylation patterns in Ras and HIF-1 signaling pathways. As a major signaling pathway activated by tyrosine kinases, aberrant Ras/MEK/ERK signaling, especially the translocation of activated ERK1/2 into the nucleus, is associated with multiple biological processes including survival, proliferation, migration and differentiation in cancer cells.19–23 Gene mutation in Ras, as well as changes in Ras signaling cascade have been detected in a variety of NHL, especially in chronic lymphocytic leukemia, T cell lymphoblastic leukemia/lymphoma, central nervous system lymphoma and cutaneous T cell lymphoma.24–27 Since HIF-1 usually plays a role as a downstream effector of Ras signaling,10,11 we selected Ras signaling pathway for validation. Our data demonstrated that the phosphorylation levels of MEK and ERK in Ras/MEK/ERK pathway are regulated by B7-H6 upstream, indicating that B7-H6 facilitates cell proliferation, migration and invasion in NHL through Ras/MEK/ERK pathway.
AZD8330, a MEK1/2 inhibitor known to be efficient in suppressing Ras/MEK/ERK pathway, has been shown to exhibit therapeutic potentials in advanced malignancies.28 We found that AZD8330 could sufficiently inhibit Ras/MEK/ERK pathway, partially reverse cell proliferation and completely reverse cell migration and invasion promoted by B7-H6. Our previous study has revealed that knockdown of B7-H6 decreased the phosphorylation of STAT3.8 Another study has shown that the specific sequences of STAT3 could be recognized and activated by ERK.29 These indicated that the regulations of B7-H6 in the biological processes of NHL are complex. Whilst Ras/MEK/ERK pathway plays an important role in cell migration and invasion; other transduction signals like STAT3 and HIF-1 may also participate in the regulation network. However, further studies are required to elucidate the mechanism.
In conclusion, we found that B7-H6 plays a role in activating Ras/MEK/ERK pathway, which then promotes cell proliferation, migration and invasion in NHL (Figure 7). Our data revealed an intrinsic mechanism in tumor development and progression by B7-H6, hence providing new insights on potential therapeutic strategies that target B7-H6 and Ras/MEK/ERK pathway in treating NHL.
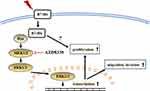 |
Figure 7 Diagrammatic drawing of this study. B7-H6 activates Ras/MEK/ERK pathway, thus promoting cell proliferation, migration and invasion in NHL. |
Conclusion
B7-H6 promotes cell proliferation, migration and invasion in NHL via Ras/MEK/ERK pathway. B7-H6 or Ras/MEK/ERK pathway targeting may be used as potential therapeutics for NHL.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 81670181).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Armitage JO, Gascoyne RD, Lunning MA, et al. Non-hodgkin lymphoma. Lancet. 2017;390(10091):298–310. doi:10.1016/S0140-6736(16)32407-2
2. Matsuki E, Younes A. Checkpoint inhibitors and other immune therapies for hodgkin and non-hodgkin lymphoma. Curr Treat Options Oncol. 2016;17(6):31. doi:10.1007/s11864-016-0401-9
3. Brandt CS, Baratin M, Yi EC, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206(7):1495–1503. doi:10.1084/jem.20090681
4. Zhou Y, Xu Y, Chen L, et al. B7-H6 expression correlates with cancer progression and patient’s survival in human ovarian cancer. Int J Clin Exp Pathol. 2015;8(8):9428–9433.
5. Sun J, Tao H, Li X, et al. Clinical significance of novel costimulatory molecule B7-H6 in human breast cancer. Oncol Lett. 2017;14(2):2405–2409. doi:10.3892/ol.2017.6417
6. Wang J, Jin X, Liu J, et al. The prognostic value of B7-H6 protein expression in human oral squamous cell carcinoma. J Oral Pathol Med. 2017;46(9):766–772. doi:10.1111/jop.12586
7. Flajnik MF, Tlapakova T, Criscitiello MF, et al. Evolution of the B7 family: co-evolution of B7H6 and NKp30, identification of a new B7 family member, B7H7, and of B7’s historical relationship with the MHC. Immunogenetics. 2012;64(8):571–590. doi:10.1007/s00251-012-0616-2
8. Wu F, Wang J, Ke X. Knockdown of B7-H6 inhibits tumor progression and enhances chemosensitivity in B-cell non-Hodgkin lymphoma. Int J Oncol. 2016;48(4):1561–1570. doi:10.3892/ijo.2016.3393
9. Specht E, Kaemmerer D, Sanger J, et al. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology. 2015;67(3):368–377. doi:10.1111/his.12662
10. Yoshikawa Y, Takano O, Kato I, et al. Ras inhibitors display an anti-metastatic effect by downregulation of lysyl oxidase through inhibition of the Ras-PI3K-Akt-HIF-1alpha pathway. Cancer Lett. 2017;410:82–91. doi:10.1016/j.canlet.2017.09.017
11. Lim JH, Lee ES, You HJ, et al. Ras-dependent induction of HIF-1alpha785 via the Raf/MEK/ERK pathway: a novel mechanism of Ras-mediated tumor promotion. Oncogene. 2004;23(58):9427–9431. doi:10.1038/sj.onc.1208003
12. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565
13. Hu Y, Zeng T, Xiao Z, et al. Immunological role and underlying mechanisms of B7-H6 in tumorigenesis. Clin Chim Acta. 2020;502:191–198. doi:10.1016/j.cca.2019.12.030
14. Li YM, Liu ZY, Li ZC, et al. Alterations of the immunologic co-stimulator B7 and TNFR families correlate with hepatocellular carcinoma prognosis and metastasis by inactivating STAT3. Int J Mol Sci. 2019;20:1.
15. Che F, Xie X, Wang L, et al. B7-H6 expression is induced by lipopolysaccharide and facilitates cancer invasion and metastasis in human gliomas. Int Immunopharmacol. 2018;59:318–327. doi:10.1016/j.intimp.2018.03.020
16. Jiang T, Wu W, Zhang H, et al. High expression of B7-H6 in human glioma tissues promotes tumor progression. Oncotarget. 2017;8(23):37435–37447. doi:10.18632/oncotarget.16391
17. Tinti M, Kiemer L, Costa S, et al. The SH2 domain interaction landscape. Cell Rep. 2013;3(4):1293–1305. doi:10.1016/j.celrep.2013.03.001
18. Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4(1):25–30. doi:10.1016/0959-437X(94)90087-6
19. Eblen ST. Extracellular-regulated kinases: signaling from Ras to ERK substrates to control biological outcomes. Adv Cancer Res. 2018;138:99–142.
20. Cristea S, Sage J. Is the canonical RAF/MEK/ERK signaling pathway a therapeutic target in SCLC? J Thor Oncol. 2016;11(8):1233–1241. doi:10.1016/j.jtho.2016.04.018
21. Suojun Z, Feng W, Dongsheng G, et al. Targeting Raf/MEK/ERK pathway in pituitary adenomas. Eur J Cancer. 2012;48(3):389–395. doi:10.1016/j.ejca.2011.11.002
22. Chung E, Kondo M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol Res. 2011;49(1–3):248–268. doi:10.1007/s12026-010-8187-5
23. Wang AX, Qi XY. Targeting RAS/RAF/MEK/ERK signaling in metastatic melanoma. IUBMB Life. 2013;65(9):748–758. doi:10.1002/iub.1193
24. Herling CD, Klaumunzer M, Rocha CK, et al. Complex karyotypes and KRAS and POT1 mutations impact outcome in CLL after chlorambucil-based chemotherapy or chemoimmunotherapy. Blood. 2016;128(3):395–404. doi:10.1182/blood-2016-01-691550
25. Tan Q, Brunetti L, Rousseaux MWC, et al. Loss of Capicua alters early T cell development and predisposes mice to T cell lymphoblastic leukemia/lymphoma. Proc Natl Acad Sci U S A. 2018;115(7):E1511–E1519. doi:10.1073/pnas.1716452115
26. Takashima Y, Sasaki Y, Hayano A, et al. Target amplicon exome-sequencing identifies promising diagnosis and prognostic markers involved in RTK-RAS and PI3K-AKT signaling as central oncopathways in primary central nervous system lymphoma. Oncotarget. 2018;9(44):27471–27486. doi:10.18632/oncotarget.25463
27. Kiessling MK, Oberholzer PA, Mondal C, et al. High-throughput mutation profiling of CTCL samples reveals KRAS and NRAS mutations sensitizing tumors toward inhibition of the RAS/RAF/MEK signaling cascade. Blood. 2011;117(8):2433–2440. doi:10.1182/blood-2010-09-305128
28. Cohen RB, Aamdal S, Nyakas M, et al. A Phase I dose-finding, safety and tolerability study of AZD8330 in patients with advanced malignancies. Eur J Cancer. 2013;49(7):1521–1529. doi:10.1016/j.ejca.2013.01.013
29. Kodama H, Fukuda K, Pan J, et al. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2000;279(4):H1635–1644. doi:10.1152/ajpheart.2000.279.4.H1635
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.