Back to Journals » Cancer Management and Research » Volume 11
B7-H3 Induces Ovarian Cancer Drugs Resistance Through An PI3K/AKT/BCL-2 Signaling Pathway
Received 6 July 2019
Accepted for publication 22 October 2019
Published 3 December 2019 Volume 2019:11 Pages 10205—10214
DOI https://doi.org/10.2147/CMAR.S222224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Li Zhou,1 Yangchun Zhao2
1Department of Gynecology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
Correspondence: Yangchun Zhao
Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou 310005, People’s Republic of China
Fax +86-0571-86288322
Email [email protected]
Purpose: This study was aimed to investigate the underlying mechanism of B7-H3 induced ovarian cancer proliferation and drugs resistance.
Materials and methods: We compared the expression of B7-H3 in ovarian tumor tissues from high-malignant or low-malignant patients by immunohistochemistry. We established B7-H3 overexpression and knockout ovarian cells by CRISPR-Cas9 technology and examined the expression of the PI3K/AKT/BCL-2 signals in tumor cells by Western blot or immunofluorescence. We detected the B7-H3 overexpression ovarian cancer cells drugs resistance by CCK8 cell proliferation analysis and Annexin V/PI staining. Tumor-bearing mice were used to investigate the anticancer effects of PI3K/AKT inhibitors in combination with B7-H3 neutralizing antibodies.
Results: Enhanced expression of B7-H3 was observed in ovarian tumor tissues from high-malignant patients compared to those from low-malignant patients. Notably, B7-H3 overexpression caused enhanced cells proliferation and chemo-resistance in vitro and in vivo through the activation of PI3K/AKT signaling pathways and up-regulation of BCL-2 protein. Combination of chemotherapeutic agents and B7-H3 neutralizing antibodies efficiently reverses the drugs resistance induced by B7-H3, resulting in improved anticancer effects in ovarian cancer.
Conclusion: B7-H3 expression induces the activation the PI3K/AKT signaling pathway and up-regulates BCL-2 in protein level, resulting in the sustained growth and chemo-resistance in ovarian cancer. Blockade of B7-H3 signals efficiently reverses the chemo-resistance, which provides an innovative target in ovarian cancer treatment.
Keywords: B7-H3, CD276, PI3K, AKT, BCL-2, ovarian cancer
Introduction
Ovarian cancer is one of the most common gynecologic carcinomas with a high risk of metastasis.1 Approximately 70% of ovarian cancer patients revealed peritoneal cavity metastasis in early diagnosis.2 Despite advances in surgical operations and systemic chemotherapy technology, the patients still suffered from the distant metastasis and drugs resistance development after standard treatment. Moreover, the underlying mechanism of ovarian cancer development still remains unclear and new therapies are urgent to improve the anticancer effects in clinical ovarian cancer treatment.
B7-H3 (CD276), a type I transmembrane protein belonging to the B7 family, is a glycoprotein consisting of 2 Ig-B7-H3 and 4 Ig-B7H3 isoforms in human.3 B7-H3 is extensively known as a checkpoint molecular which is expressed on many tissues as well as immune cells. The enhanced expression of B7-H3 could down-regulate the type I interferon γ by T cells and reduce the cytotoxicity activity of NK cells, resulting in the immune suppression.4 B7-H3 also has limited expression on many tissues, including breast, liver, urinary and lymphoid systems. However, the high level of B7-H3 expression was observed in a wide range of carcinomas, including the bladder cancer, brain cancer and prostate cancer.5–7 Previous reports indicated that the overexpression of B7-H3 contributes to tumor immune evasion and promotes tumor metastatic, resulting in a poor prognosis.8 Also, Qing Ge and his colleagues have reported that B7-H3 could promote multiple myeloma cell survival and proliferation through a ROS-dependent signaling pathway.9 Notably, B7-H3 is an attractive target for cancer immunotherapy due to its specific expression in various tumor tissues. B7-H3-specific monoclonal antibodies and CAR-T technologies reveal dramatic anticancer effects along with a good safety profiles, which provide new targets in cancer therapy.10 However, the underlying mechanisms and downstream signaling pathways of B7-H3 in tumor development still remain unclear. And the role of B7-H3 in ovarian cancer development still needs further investigation.
In our study, we firstly observed enhanced expression of B7-H3 in malignant ovarian cancer tissues and demonstrated the correlation between the B7-H3 and ovarian cancer drug resistance development. The overexpression of B7-H3 results in enhanced cells proliferation and sustained tumor growth in vitro and vivo though activation of PI3K/AKT pro-survival signaling pathway. More importantly, we further described the underlying mechanism of the tumor growth and drugs resistance through the B7-H3 molecule. We demonstrated that B7-H3 could induce cancer cells drug resistance through the activation of downstream anti-apoptosis protein, resulting in the poor prognosis of clinical chemotherapy. And blockade of B7-H3 significantly enhanced the anticancer effects of chemotherapeutic agents, which provides an innovative approach for clinical ovarian cancer treatment.
Materials And Methods
Cell Culture And Patients’ Samples
OVCAR-3 and A2780 human ovarian cancer cell line were obtained from the National Infrastructure of Cell Line Resources (Chinese Academy of Medical Sciences, Beijing, China) and were cultured in DMEM media supplemented with 10% of heat-inactivated fetal calf serum (FBS). All media were purchased from Gibco Inc (MA, USA). The FBS was purchased from Gibco Inc (MA, US) and heat-inactivated at 56°C for 10 mins prior use. Cells were maintained at 37°C with 5% CO2 in a humidified incubator.
For stable knock-out of B7-H3, 2×105 human ovarian cancer cells were seeded in wells of a 6-well plate. After 8 hrs, cells were transfected with 5 μg of a px459 vector expressing sgRNAs targeted B7-H3 using the Lipofectamine 3000 (Thermo Fisher Scientific Inc, MA, US) according to the manufacturer’s instructions. 72 hrs later, cells were treated with puromycin (1.5 μg/mL). Growing isolated clones were harvested using cloning cylinders (Corning, MA, US). Each single clone was detected for B7-H3 expression by Western blot.
For stable knock-out of BCL-2, 2×105 human ovarian cancer cells were seeded in wells of a 6-well plate. After 8 hrs, cells were transfected with 5 μg of a px459 vector expressing sgRNAs targeted BCL-2 using the Lipofectamine 3000 (Thermo Fisher Scientific Inc, MA, US) according to the manufacturer’s instructions. 72 hrs later, cells were treated with puromycin (1.5 μg/mL). Growing isolated clones were harvested using cloning cylinders (Corning, MA, US). Each single clone was detected for BCL-2 expression by Western blot.
For stable overexpression of B7-H3, B7-H3 (NM_025240) human tagged ORF clone lentiviral particle was purchased from OriGene (MD, USA). 5×104 human ovarian cancer cells were seeded in wells of a 24-well plate and after 12 hrs later infected with 20 μL of a lentiviral particle expressing either a vector or B7-H3 (NM_025240) according to the manufacturer’s instructions. 72 hrs later, cells were treated with puromycin (1.5 μg/mL) for 48 hrs. Growing isolated clones were harvested using cloning cylinders (Corning, MA, US). Each single clone was detected for B7-H3 expression by Western blot.
The ovarian tumor tissues were collected from Affiliated Hangzhou First People’s Hospital and fixed by formalin fixation in 2 hrs after surgery. Samples were divided into high- malignant (IV stage/FIGO stage) group and low-malignant (I stage) group. All researches have been carried out in accordance with the World Medical Association Declaration of Helsinki and approved by the ethics committee of the Second Affiliated Hospital of Zhejiang Chinese Medical University (XH20160213).
Regents
The following antibodies were used in this study: antibodies against GAPDH (CST, USA, 1:1000), B7-H3(CST, USA, 1:1000), BCL-2 (CST, USA, 1:1000), PI3K (Abcam, UK, 1:100), AKT (Abcam, UK, 1:100), p-PI3K (Abcam, UK, 1:100), p-AKT (Abcam, UK, 1:100). Cisplatin (CIS) was obtained from Solarbio (Beijing, China). Paclitaxel (PTX) was obtained from Solarbio (Beijing, China).
Cell Proliferation And Colony Formation Assay
Cell proliferation was measured using the Cell counting kit-8 (CCK8, Dojindo, Japan). Cells were grown in 96-well plates at a density of 5000 cells per well and were incubated for 24 hrs. Then, 15 μL of CCK8 solution was added to each well on 24, 48, 72 hrs. The absorbance of each sample was measured at 450 nm. For colony formation assay, 1000 cells were seeded into 6-well plates to form colonies within two weeks. Colonies were fixed with glutaraldehyde (6.0% v/v, Sorlarbio, Beijing, China), stained with crystal violet (0.5% w/v, Sorlarbio, Beijing, China), and counted using a stereomicroscope. All experiments were performed in triplicate and the average values were calculated.
Cell Apoptosis Assay
The apoptosis assays were conducted on flow cytometry (FCM). Before analysis, cells were treated by PTX and CIS for 48 hrs. Then, cells were harvested by a 5-min centrifugation at 300 g and resuspended in 200 μL binding buffer. And cells were stained by Cell apoptosis detection kit (BD, MA, USA) according to the manufacturer’s protocol. The data were analyzed by FlowJ Software 10.0.
Western Blot Analysis
Total proteins were extracted using the protein extraction kits (Solarbio, Beijing, China). And the samples were run by SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The nitrocellulose membranes were blocked with 5% skim milk for 60 mins at room temperature and incubated antibodies for 12–24 hrs. Nitrocellulose membranes were rinsed by TBST three times for 10 mins every time and HRP-labeled secondary antibody (1:3000, Solarbio, Beijing, China) for 2 hrs. After washed by TBST three times for 10 mins every time, detected proteins were visualized using an ECL kit (Thermo Fisher Scientific Inc, MA, US). GAPDH was used as an internal control. All experiments were repeated three times.
Immunofluorescence (IF)
For cell biology experiments, the cells on the cover slips were fixed with 4% paraformaldehyde for 10 mins. The cover slips and sections were rinsed 3 times with PBS for 5 mins each time. The fixed cells were permeabilized with 0.2% Triton X-100 in PBS for 5 mins and the cover slips and sections incubated with a blocking solution of 5% goat serum in PBS for 10 mins. The blocking solution was aspirated, and the cells and tissues were incubated with primary antibody overnight at 4 °C. The cover slips and sections were washed with PBS 3 times for 5 mins each time. The cover slips and sections were incubated with Alexa Fluor 488-conjugated and/or Alexa Fluor 594-conjugated secondary antibodies (1:200, Thermo Fisher Scientific Inc, MA, US) for 1 hr in a moist, dark environment. After washing with PBS, 10 μL of antifade mounting medium containing DAPI (Thermo Fisher Scientific Inc, MA, US) was added to the cover slips and sections for nuclear staining. The immunofluorescence intensity was calculated by Image J 2.0.
Immunohistochemistry (IHC)
For pathological tissues analysis, the patient pathological tissues were made into a tissue array including 30 pairs of cancer and paracancer tissues. The tissues were fixed with 4% paraformaldehyde for 72 hrs and were made into sections. The sections then underwent sodium citrate antigen retrieval and were cooled to room temperature. Hydrogen peroxide (3%) was used to block endogenous peroxidase activity and goat serum was used to block non-specific antigens. The sections were then incubated overnight at 4 °C with antibodies against B7-H3 (Abcam, USA, 1:100). After being rinsed with PBS, the sections were incubated with horseradish peroxidase-conjugated secondary antibody was applied at 37 °C for 40 mins and then incubated with diaminobenzidine solution. Finally, the nuclei were counterstained with Mayer’s hematoxylin. The intensity of positive cells was analyzed by image pro plus 6.0.
In Vivo Tumor Model And Tumorigenicity Assay
All our animal experiments were conducted in accordance with guidelines approved by the Institute Ethics Committee of the Second Affiliated Hospital of Zhejiang Chinese Medical University# (XH20160213). For assessment of in vivo tumor model, female NSG mice were purchased from Beijing HFK Bioscience Co (Beijing, China). Female NSG mice (4–6 weeks of age, 18–20 g) were randomly divided into the indicated groups. Mice (n = 6) in the groups were subcutaneously injected with 3 × 106 OVCAR-3 or A2780 cells. OVCAR-3 or A2780 cells derived subcutaneous model was used for the anti-B7-H3 and chemotherapeutic agents therapy. Tumor growth was monitored by measuring tumor volume (1/2 × length × width2) on indicated days.
For assessment of tumor formation abilities, 1×105 ovarian cancer cells were suspended in 100 μL of DMEM medium and Matrigel (BD Biosciences, Bedford, MA, USA) and subcutaneously transplanted into female NSG mice (4–6 weeks of age, 18–20 g). Tumor formation was weekly monitored. After 7–8 weeks, mice were sacrificed, and the tumor numbers were recorded.
Results
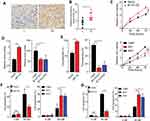
B7-H3 Facilitates Sustained Growth And Drugs Resistance Development In Ovarian Cancer
Previous evidence indicated that the B7-H3 expression is associated with the tumor growth and patients’ prognosis in several tumor types.11–13 Here, we observed increased expression of B7-H3 in those patients with IV stage (FIGO stage, high-malignant) compared to I stage (FIGO stage, low-malignant) (Figure 1A). This pattern was observed in multiple patients as evidenced by increased expression of B7-H3 in high-malignant ovarian cancer patients (Figure 1B, n=10), indicating that B7-H3 might be strictly correlated with the tumor stage in ovarian cancer. To further investigate the role of B7-H3, we established the B7-H3 overexpression ovarian cancer cell lines A2780 (Fig. S1A), B7-H3 knockdown ovarian cancer cell line A2780 (Fig. S1B), B7-H3 overexpression ovarian cancer cell lines OVCAR-3 (Fig. S1A) and B7-H3 knockdown ovarian cancer cell line OVCAR-3 (Fig. S1B). Intriguingly, the expression of B7-H3 significantly facilitated the cells proliferation of OVCAR-3 (Figure 1C) and A2780 (Fig. S1C). Additionally, B7-H3 overexpression also strengthened the colony formation (Figure 1D) and tumorigenesis (Figure 1E) capability whereas blockade of B7-H3 suppressed the phenomenon in ovarian cancer cells OVCAR-3 (Figure 1D and E). The same results were observed in A2780 cell line (Fig. S1D and E). On the basis of those data, we supposed that the expression of B7-H3 could facilitate the sustained growth and tumorigenesis capability in ovarian cancer cells, resulting in the poor prognosis of ovarian cancer patients. The tumor sustained growth is also accompanied by the drug resistance in various tumor types, resulting in the chemo-resistance and intensive tumor progressions. CIS and PTX are the traditional chemotherapeutic agents in clinical ovarian cancer treatment. Of note, the overexpression of B7-H3 also retarded the cells apoptosis induced by CIS and PTX in OVCAR-3 cells (Figure 1F and G) and A2780 cells (Fig. S1F and G), suggesting that B7-H3 could also promote the drug resistance in ovarian cancer cells.
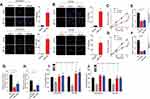
B7-H3 Induces The Tumor Sustained Growth Through The Activation Of PI3K/AKT Signals
PI3K/AKT signaling pathway serves as the classic downstream signal of various cell surface molecules in tumor cells, which is tightly associated with cell proliferation, tumor stemness regulation and drugs resistance development.14,15 Previous report also indicated that the AKT is correlative in the B7-H3 induced tumor metastasis in bladder cancer cells.16 Therefore, we evaluated the phosphorylation of PI3K and AKT in B7-H3 overexpression ovarian cancer cells and found that the expression of phospho-PI3K and phospho-AKT was increased in the B7-H3 overexpression OVCAR-3 and A2780 cells compared to the vector (Figure 2A, B and Fig. S2A, B), indicating that the activation of PI3K/AKT signaling pathway in B7-H3 overexpression cancer cells. Also, reduced phospho-PI3K and phospho-AKT expression were observed in B7-H3 knockdown cancer cells (Fig. S2C and D), indicating that B7-H3 positively regulates the PI3K/AKT signal in ovarian cancer. To further demonstrate the role of PI3K/AKT signal in B7-H3 induced tumor progressions, the PI3K inhibitor LY294002 and AKT inhibitor MK-2206, which could efficiently suppress p-PI3K and p-AKT expression (Fig. S2E–H), were used to treat the B7-H3 overexpression ovarian cancer cells. We found that blockade of PI3K or AKT efficiently suppressed the cells proliferation (Figure 2C and D). Furthermore, the enhanced colony formation and tumorigenesis capability of B7-H3 overexpression ovarian cancer cells were retarded by LY294002 and MK-2206 (Figure 2E–H), indicating that blockade of PI3K/AKT signal resulted in the suppression of tumor growth induced by B7-H3. Additionally, adding LY294002 and MK-2206 also effectively reversed the resistance of B7-H3 overexpression OVCAR-3 and A2780 cells to PTX and CIS (Figure 2I and J). These results suggested that PI3K/AKT may work as the down-stream of B7-H3 axis in tumor sustained growth and acquisition of drug resistance in ovarian cancer cells.
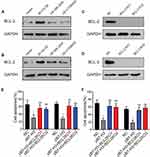
B7-H3 Triggers The BCL-2 Expression To Induce Drug Resistance
We have demonstrated that the B3-H7 could regulate the tumor sustained growth through the activation of pro-survival PI3K/AKT signaling pathway. However, PI3K/AKT signal is not capable of regulating the anti-apoptosis process of tumor cells to chemotherapy directly. It has been reported that the AKT downstream protein BCL-2 participated in anti-apoptosis process of various cancer cells, leading to reduced cytotoxicity and drug resistance.17,18 Herein, we detected the expression of BCL-2 in B7-H3 overexpression ovarian cancer cells and found that BCL-2 was significantly up-regulated in B7-H3 overexpression OVCAR-3 and A2780 cells compared with the Vector (Figure 3A and B). More importantly, the protein expression of BCL-2 was suppressed in those B7-H3 overexpression cancer cells treated with LY294002 or MK-2206 (Figure 3A and B), indicating that BCL-2 serves as the downstream molecule of PI3K/AKT, which was activated in B7-H3 overexpression ovarian cancer cells. To further demonstrate the role of BCL-2 in the drugs resistance development induced by B7-H3, we knockout the BCL-2 in B7-H3 overexpression OVCAR-3 (Figure 3C) and A2780 (Figure 3D) cell lines by CRISPR-Cas9. Intriguingly, BCL-2 knockout efficiently reversed the OVCAR-3 and A2780 resistance to PTX and CIS induced by B7-H3 (Figure 3E and F). Together, those results suggested that B7-H3 up-regulated the expression of AKT downstream molecule BCL-2 to induce the ovarian cancer drugs resistance development.
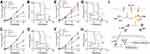
Blockade Of B7-H3 Signal Reverses Chemotherapeutic Drugs Resistance In Ovarian Cancer In Vivo
In order to evaluate the B7-H3 induced drug resistance in vivo, we generated the xenograft mouse model using OVCAR-3 cells injected into NSG mice subcutaneously. Then, the mice were treated with PBS, chemotherapeutic drugs, anti-B7-H3 neutralizing antibodies and anti-B7-H3 neutralizing antibodies combined with chemotherapeutic drugs. The tumor-bearing mice data revealed that anti-B7-H3 or PTX treatment slightly suppressed tumor growth whereas the combination of PTX and anti-B7-H3 neutralizing antibodies significantly reduced the tumor volume (Figure 4A) and prolonged the survival time (Figure 4B). Consistently, the combination of CIS and anti-B7-H3 neutralizing antibodies also revealed enhanced anticancer effects compared to CIS group (Figure 4C and D). More importantly, we generated the B7-H3 overexpression xenograft mouse model using B7-H3 overexpression OVCAR-3 cells injected into NSG mice subcutaneously. Then, the mice were treated with control IgG, chemotherapeutic drugs, anti-B7-H3 neutralizing antibodies and anti-B7-H3 neutralizing antibodies combined with chemotherapeutic drugs. Notably, the chemotherapeutic drugs treatment was not capable of suppressing the tumor growth efficiently compared to the control IgG group, indicating that B7-H3 expression induced the drugs resistance in ovarian cancer. However, blockade of B7-H3 by anti-B7-H3 neutralizing antibodies significantly reversed the PTX drugs resistance caused by B7-H3 (Figure 4E) and prolonged the survival time of tumor-bearing mice (Figure 4G). Consistently, the combination of CIS and anti-B7-H3 neutralizing antibodies also revealed enhanced anticancer effects compared to CIS group (Figure 4F and H). Taken together, those data suggested that blockade of B7-H3 by anti-B7-H3 neutralizing antibodies could effectively reverse the drugs resistance and enhance the anticancer effects of chemotherapeutic agents, which provides an innovative approach for clinical ovarian cancer therapy.
Discussion
Accumulating evidence indicates that B7-H3 is present abundantly in several tumor types and participates in the immunosuppression and tumor progressions.19–21 In the present study, we observed restricted expression of B7-H3 in tumor tissues from low-malignant patients, but enriched expression in those ovarian tumor tissues from high-malignant patients. The expression of B7-H3 also induces the stem-like phenotypes and drugs resistance of ovarian cancer cells in vitro and vivo, which is consistent with precious reports in prostatic cancer and bladder cancer. More importantly, we found the activation of PI3K/AKT signaling pathway induced by B7-H3, resulting in the enhanced stem-like phenotypes of ovarian cancer cells. Additionally, the AKT downstream anti-apoptosis protein BCL-2 was up-regulated and caused the drugs resistance in ovarian cancer treatment. Combination of chemotherapeutic agents and B7-H3 neutralizing antibodies could efficiently reverse the drugs resistance and suppress tumor growth, resulting in superior anticancer effects (Figure 4I).
Tumor drugs resistance development is regarded as the major cause of clinical chemotherapy in ovarian cancer treatment. The development of drugs resistance is associated with various factors, including the overexpression of multi-drugs resistance proteins P-gp, the activation of pro-survival signaling pathways and anti-apoptosis proteins, hypoxia, tumor microenvironment and so on.22–24 Previous reports indicated that some CD133 or ALDH positive cancer cells might reveal enhanced capability of sustained growth and drug resistance.25,26 However, the membranous markers for those drugs resistant cancer cells still remain to be investigated. B7-H3 is a member of immune modulators which is expressed on the membrane surface of various cell types. However, the role of B7-H3 in tumor progression still remains unclear. Accumulating evidence indicated that B7-H3 participates in the metastasis and stemness regulation in some tumor cells.27–29 B7-H3 could facilitate the cancer migration and invasion through the activation of AKT/STAT3 signals in bladder cells. Also, the expression of B7-H3 has been proved to be associated with the sustained cancer cells proliferation and poor prognosis of brain cancer and breast cancer.16 However, the underlying mechanism of B7-H3 to induce tumor cells progressions still remains unclear. And the role of B7-H3 in tumor drugs resistance also remains to be investigated.
Based on the limitations of previous reports, we further described the role of B7-H3 in tumor-associated progressions. Firstly, we expounded the relationship between B7-H3 and ovarian cancer and further demonstrated that the excessive expression of B7-H3 facilitated the ovarian cancer development. Second, the determination of causal association between B7-H3 expression and drugs resistance development was observed in our study. Previous reports focused on the role B7-H3 in tumor growth, metastasis or immune modulation.30–32 Here, we first demonstrated B7-H3 could participate in the drugs resistance development through the activation of downstream BCL-2 protein. Third, we further demonstrated the downstream pro-survival PI3K/AKT signaling pathway induced by B7-H3, resulting in the sustained growth and poor prognosis in ovarian cancer. Finally, the application of B7-H3 neutralizing antibody; efficiently reversed the drug resistance in ovarian cancer cells, which provides new targets for clinical treatment. Furthermore, the investigation of the B7-H3 level in ovarian cancer tissues or serums might serve as a potential biomarker for ovarian cancer diagnosis or drugs resistance development.
In conclusion, we demonstrated that high increasing B7-H3 expression is associated with the sustained growth and drugs resistance development in patients with ovarian cancer. The tumor progression is induced by the B7-H3/PI3K/AKT/BCL-2 signaling pathway. Inhibitors targeting B7-H3 might serve as a potential strategy in chemo-resistant ovarian cancer treatment.
Acknowledgments
This study was supported by the Basic Public Welfare Research Projects of Zhejiang province (LQ18H040003). All ethical approval documents can be viewed in the Supplemental material: The approval from the Human Tissue and Animal Ethics Committee and patient’s written informed consent.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. doi:10.1016/j.bpobgyn.2016.08.006
2. Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii61–viii65. doi:10.1093/annonc/mdx443
3. Wang Z, Wang Z, Zhang C, et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018;109(9):2697–2705. doi:10.1111/cas.13744
4. Luo L, Zhu G, Xu H, et al. B7-H3 promotes pathogenesis of autoimmune disease and inflammation by regulating the activity of different T cell subsets. PLoS One. 2015;10(6):e0130126. doi:10.1371/journal.pone.0130126
5. Ma W, Ma J, Ma P, et al. Targeting immunotherapy for bladder cancer using anti-CD3x B7-H3 bispecific antibody. Cancer Med. 2018;7(10):5167–5177. doi:10.1002/cam4.1775
6. Zhou Z, Luther N, G M I, et al. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J Neurooncol. 2013;111(3):257–264. doi:10.1007/s11060-012-1021-2
7. Kreymborg K, Haak S, Murali R, et al. Ablation of B7-H3 but not B7-H4 results in highly increased tumor burden in a murine model of spontaneous prostate cancer. Cancer Immunol Res. 2015;3(8):849–854. doi:10.1158/2326-6066.CIR-15-0100
8. Picarda E, Ohaegbulam KC, Zang X. Molecular pathways: targeting B7-H3 (CD276) for human cancer immunotherapy. Clin Cancer Res. 2016;22(14):3425–3431. doi:10.1158/1078-0432.CCR-15-2428
9. Lin L, Cao L, Liu Y, et al. B7-H3 promotes multiple myeloma cell survival and proliferation by ROS-dependent activation of Src/STAT3 and c-Cbl-mediated degradation of SOCS3. Leukemia. 2018;33(6):1475–1486.
10. Majzner RG, Theruvath JL, Nellan A, et al. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res. 2019;25(8):2560–2574. doi:10.1158/1078-0432.CCR-18-0432
11. He L, Li Z. B7-H3 and its role in bone cancers. Pathol Res Pract. 2019;215:152420. doi:10.1016/j.prp.2019.04.012
12. Ma J, Shang T, Ma P, et al. Bispecific anti-CD3 x anti-B7-H3 antibody mediates T cell cytotoxic ability to human melanoma in vitro and in vivo. Invest New Drugs. 2019;37:1036–1043. doi:10.1007/s10637-018-00719-7
13. Han S, Shi X, Liu L, et al. Roles of B7-H3 in cervical cancer and its prognostic value. J Cancer. 2018;9(15):2612–2624. doi:10.7150/jca.24959
14. Zhang F, Li K, Yao X, et al. A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer. EBioMedicine. 2019;44:311–321. doi:10.1016/j.ebiom.2019.05.003
15. Ling C, Wang X, Zhu J, et al. MicroRNA-4286 promotes cell proliferation, migration, and invasion via PTEN regulation of the PI3K/Akt pathway in non-small cell lung cancer. Cancer Med. 2019;8:3520–3531. doi:10.1002/cam4.2019.8.issue-7
16. Li Y, Guo G, Song J, et al. B7-H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J Cancer. 2017;8(5):816–824. doi:10.7150/jca.17759
17. Yang T, Xu F, Sheng Y, et al. A targeted proteomics approach to the quantitative analysis of ERK/Bcl-2-mediated anti-apoptosis and multi-drug resistance in breast cancer. Anal Bioanal Chem. 2016;408(26):7491–7503. doi:10.1007/s00216-016-9847-7
18. P L S, Sasano H, Gao H. Bcl-2 family in non-small cell lung cancer: its prognostic and therapeutic implications. Pathol Int. 2017;67(3):121–130. doi:10.1111/pin.12507
19. Carvajal-Hausdorf D, Altan M, Velcheti V, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung cancer (SCLC). J Immunother Cancer. 2019;7(1):65. doi:10.1186/s40425-019-0540-1
20. Zhao J, Meng Z, Xie C, et al. B7-H3 is regulated by BRD4 and promotes TLR4 expression in pancreatic ductal adenocarcinoma. Int J Biochem Cell Biol. 2019;108:84–91. doi:10.1016/j.biocel.2019.01.011
21. Zhang C, Zhang Z, Li F, et al. Large-scale analysis reveals the specific clinical and immune features of B7-H3 in glioma. Oncoimmunology. 2018;7(11):e1461304. doi:10.1080/2162402X.2018.1461304
22. Seguin L, Desgrosellier JS, Weis SM, et al. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25(4):234–240. doi:10.1016/j.tcb.2014.12.006
23. Manoochehri Khoshinani H, Afshar S, Najafi R. Hypoxia: a double-edged sword in cancer therapy. Cancer Invest. 2016;34(10):536–545. doi:10.1080/07357907.2016.1245317
24. Gabellini C, Trisciuoglio D, Del Bufalo D. Non-canonical roles of Bcl-2 and Bcl-xL proteins: relevance of BH4 domain. Carcinogenesis. 2017;38(6):579–587. doi:10.1093/carcin/bgx016
25. Matsushita M, Mori Y, Uchiumi K, et al. PTPRK suppresses progression and chemo-resistance of colon cancer cells via direct inhibition of pro-oncogenic CD133. FEBS Open Bio. 2019;9(5):935–946. doi:10.1002/feb4.2019.9.issue-5
26. Duan JJ, Cai J, Guo YF, et al. ALDH1A3, a metabolic target for cancer diagnosis and therapy. Int J Cancer. 2016;139(5):965–975. doi:10.1002/ijc.30091
27. Yu TT, Zhang T, Lu X, et al. B7-H3 promotes metastasis, proliferation, and epithelial-mesenchymal transition in lung adenocarcinoma. Onco Targets Ther. 2018;11:4693–4700. doi:10.2147/OTT.S169811
28. Han S, Wang Y, Shi X, et al. Negative roles of B7-H3 and B7-H4 in the microenvironment of cervical cancer. Exp Cell Res. 2018;371(1):222–230. doi:10.1016/j.yexcr.2018.08.014
29. Flem-Karlsen K, Fodstad O, Tan M, et al. B7-H3 in cancer – beyond immune regulation. Trends Cancer. 2018;4(6):401–404. doi:10.1016/j.trecan.2018.03.010
30. Shi T, Ma Y, Cao L, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10(4):308. doi:10.1038/s41419-019-1549-6
31. Janakiram M, Shah UA, Liu W, et al. The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol Rev. 2017;276(1):26–39. doi:10.1111/imr.12521
32. Kang FB, Wang L, Jia HC, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15:45. doi:10.1186/s12935-015-0195-z
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.