Back to Archived Journals » Core Evidence » Volume 11
Azilsartan medoxomil in the management of hypertension: an evidence-based review of its place in therapy
Authors Angeloni E
Received 8 October 2015
Accepted for publication 21 February 2016
Published 5 April 2016 Volume 2016:11 Pages 1—10
DOI https://doi.org/10.2147/CE.S81776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Emiliano Angeloni
Cardiovascular Pathophysiology and Imaging, Sapienza Università di Roma, Rome, Italy
Background: Azilsartan (AZI) is a relatively new angiotensin receptor blocker available for the treatment of any stage of hypertension, which was eventually given in combination with chlorthalidone (CLT).
Objective: To review pharmacology and clinical role of AZI monotherapy and AZI/CLT or AZI/amlodipine combination therapies for hypertension management.
Methods: PubMed, Embase, and Cochrane Library were searched using search terms “azilsartan”, “chlorthalidone,” “pharmacology,” “pharmacokinetics,” “pharmacodynamics,” “pharmacoeconomics,” and “cost-effectiveness.” To obtain other relevant information, US Food and Drug Association as well as manufacturer prescribing information were also reviewed.
Results: Randomized controlled trials demonstrated AZI to be superior to other sartans, such as valsartan, olmesartan, and candesartan, in terms of 24-hour ambulatory blood pressure monitoring (ABPM) reduction with respect. That beneficial effect of azilsartan was also associated with similar safety profiles. When compared to other antihypertensive drugs, azilsartan was found to be superior to any angiotensin-converting enzyme inhibitor, including ramipril, in terms of ABPM results, and noninferior to amlodipine in terms of sleep-BP control. The association of AZI and CLT was then found to be superior to other sartans + thiazide combination therapies in terms of both BP lowering and goal achievement. The combination of AZI and amlodipine has also been tested in clinical trials, but compared only with placebo, demonstrating its superiority in terms of efficacy and similarity in terms of safety.
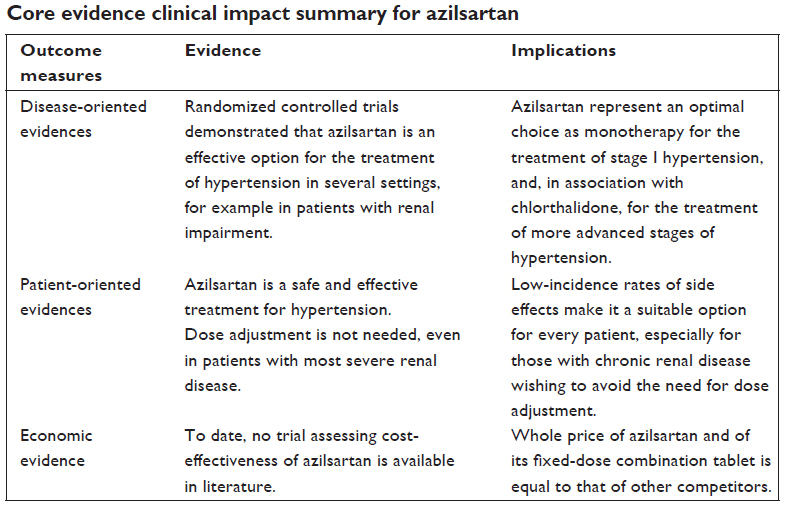
Conclusion: Azilsartan is a safe and effective treatment option for every stage of hypertension, both alone or in fixed-dose combination tablets with chlorthalidone or amlodipine. Beneficial effects of AZI were also noted in patients with any degree of renal impairment. In addition, safety profiles of AZI were similar to that of the placebo.
Keywords: hypertension, pharmacology, azilsartan, blood pressure, pharmacokinetics, cost-effectiveness
Introduction
According to the 2015 AHA statistical report,1 over 75% of the population aged at least 40 years has elevated blood pressure (BP) levels (65.3% among those aged 40–59 years, and 84.3% among those aged >59 years). Furthermore, only 60% of patients achieve good BP control, and despite the decreasing cardiovascular (CV) morbidity, BP is still a leading cause of death in Western countries, affecting nearly 230 of every 100,000 individuals.1 In addition, the annual direct and indirect cost of CV diseases in the United States is an estimated US$320.1 billion. This includes US$195.6 billion in expenditures (direct costs, which include the cost of physicians and other professionals, hospital services, prescribed medication, and home health care, but not the cost of nursing home care) and US$124.5 billion in lost future productivity attributed to premature cardiovascular disease and stroke mortality (indirect costs).1
Hypertension is one of the leading risk factors for ischemic heart disease, stroke, heart failure, and renal dysfunction.2 Thus, management of hypertension should be targeted not only for BP control but also for the reduction of overall cardiovascular and renal morbidity and mortality.3 In these settings, the lack of medical success is one of the many reasons triggering the development of new antihypertensive agents.
Due to their beneficial effects in reducing both cardiovascular morbidity and mortality, angiotensin receptor blockers (ARBs) alone or in combination are considered among the best available therapeutic options for the treatment of hypertension even in patients with compelling indications, such as heart failure, diabetes, and previous myocardial infarction.3 In addition, the use of fixed-dose combination therapies demonstrated the potential to increase patient adherence4 and overall clinical effect.5
Nonpeptide antagonists of the angiotensin II type 1 (AT1) receptor constitute a very useful and widely prescribed class of antihypertensive drugs.6 After the US Food and Drug Administration approved losartan in 1995,7 a host of other AT1 receptor antagonists were rapidly introduced in clinical practice.
Beneficial effects of ARBs are supposed to be mediated mainly by mechanisms independent from BP reduction, and include endothelial modulation, renoprotection, and reduction of fibrosis.6,8,9 However, protection against target organ damage and improvement of clinical outcomes is still considered to be largely mediated by the BP decrease correlated to ARBs administration.8,10
Clinically available sartans are known to have several differences in terms of plasma half-life, intensity of AT1 blockade, and slope of the BP-lowering dose–response curve.9,11 However, clinical studies always failed to identify an ARB more effective than others in terms of BP lowering.12
Ultimately, a large body of literature13–16 prompted the comparison of clinical safety and effectiveness of different ARBs; hence, the aim of this paper was to review latest evidences about the use of azilsartan, alone or in fixed-dose combination (FDC) regimens, in comparison with other antihypertensive drugs.
Pharmacology of azilsartan
Mechanism of action
Azilsartan is a selective blocker of AT1 receptors that prevents angiotensin II binding, resulting in vasodilation and decrease in the effects of aldosterone, because of the presence of such receptors in the vascular smooth muscle and in the adrenal gland.17,18 With respect to other ARBs, azilsartan is highly selective for the AT1 receptor and not the AT2 receptor.19
Pharmacokinetics
Azilsartan medoxomil is a prodrug subject to hydrolyzation of its active moiety, azilsartan, at the level of the gastrointestinal tract. Azilsartan reaches its peak plasma concentration between 1.5 and 3 hours following oral administration. Coadministration with food does not affect bioavailability, which is approximately 58%.18 Metabolization of azilsartan occurs in the liver via cytochrome P450 (CYP) 2C9, resulting in the formation of a nonactive metabolite, M-II (formed by O-dealkylation). Metabolization to a lesser extent is then provided by CYP2B6 and CYP2C8, resulting in the formation of another inactive metabolite, M-I (formed by decarboxylation). Azilsartan is primarily excreted by the kidney, as inactive metabolites, with a clearance of 2.3 mL/min. The elimination half-life is approximately 11 hours, with steady-state plasma concentrations reached 5 days after oral administration.18 In conclusion, the dose range of 20–320 mg does not need any adjustment based on patient’s age, sex, race, or degree of renal/hepatic impairment.
Drug interactions
Drug interactions of azilsartan with caffeine, antacid, warfarin, digoxin, tolbutamide, glyburide, metformin, pioglitazone, chlorthalidone, amlodipine, dextromethorphan, midazolam, and fexofenandine have been studied; any other significant interactions have also been observed. In another drug interaction study, azilsartan clearance was reduced when coadministered with fluconazole (a CYP2C9 inhibitor), but not when coadministered with ketoconazole (a CYP3A4/5 inhibitor).
It is well known that angiotensin II increases the glomerular filtration rate by means of efferent arteriole vasoconstriction, therefore reduced angiotensin II binding caused by azilsartan slightly decreases the glomerular filtration rate because of efferent arteriole vasodilation. Given that, nonsteroidal anti-inflammatory agents and COX-2 inhibitors cause prerenal acute renal failure by blocking prostaglandin production, which also alters local glomerular arteriolar perfusion,20 the use of these agents concurrently with azilsartan may increase the risk of renal function deterioration.
Experimental evidences
Several studies investigated pleiotropic effects of azilsartan and their effectiveness in treating pathological conditions underlying hypertension.
For example, an insulin-sensitizing effect of azilsartan has been demonstrated in obese rats, regardless of food intake and body weight increase, introducing a possible role of azilsartan in the treatment of metabolic syndrome.21,22 Another beneficial effect of azilsartan was also confirmed in animal models, with studies showing that azilsartan medoxomil, independently of BP lowering, offers preventive and therapeutic vasculoprotection in diabetes-induced cerebrovascular remodeling and myogenic dysfunction.23
In addition to the vasculoprotective effect, reduced left ventricular wall thickness and hypertrophy were also demonstrated, leading to increased cardiac output after aortic banding compared with controls, thus suggesting a favorable biological effect on the hearts of obese, insulin-resistant mice subjected to LV pressure overload.24 Beneficial effects on left ventricular remodeling regardless of BP lowering have also been seen in mice after creation of an anterior myocardial infarction.25
Azilsartan has also been claimed to have renoprotective effects, in terms of reducing proteinuria,26 albuminuria, and nephrinuria along with reduced tubular cast formation and glomerular injury.27
Besides this, azilsartan has also been advocated for its anti-inflammatory effects, such as reducing plasma monocyte chemoattractant protein-1 levels28 and increasing the anti-inflammatory cytokine IL-10 levels.29 Therefore, by reducing vascular inflammation, azilsartan also exerts beneficial effects in terms of endothelial restoration, thus preventing its dysfunction;30 for example, reducing tumor necrosis factor-α and IL-1β levels, and upregulating the vascular endothelial growth factor.31 In addition, via the suppression of plasminogen activator inhibitor type-I expression, azilsartan also may attenuate the evolution of atherosclerotic plaques vulnerable to rupture.32
Clinical evidences
Comparison of azilsartan vs other sartans
Trials comparing clinical safety and efficacy of azilsartan with that of other antihypertensive drugs are listed in Table 1.
Bakris et al14 published the first study investigating the antihypertensive efficacy and safety of azilsartan in 2011. It was a placebo-controlled trial involving a total of 1,275 patients who were randomized to placebo, azilsartan 20, 40, or 80 mg daily, or olmesartan 40 mg daily for 6 weeks. Twenty four-hour mean systolic BP (SBP; measured by 24 hours ambulatory blood pressure monitoring [ABPM]) was found to be significantly reduced in all azilsartan groups (−12.2, −13.5, and −14.6 mmHg) and in the olmesartan 40 mg group (−12.6 mmHg). Reductions were significantly greater with azilsartan 80 mg than olmesartan 40 mg (P=0.038), while azilsartan 40 mg was not inferior to olmesartan 40 mg. Safety profiles of both drugs were similar to placebo, although statistical comparison was not performed.
Another double-blind, randomized, placebo-controlled trial13 compared azilsartan with olmesartan and valsartan. A total of 1,285 patients were randomized to placebo, azilsartan 40–80 mg daily, olmesartan 40 mg daily, or valsartan 320 mg daily for 6 weeks. Placebo-adjusted ABPM-SBP was lowered by azilsartan (−14.3 mmHg) significantly more than by valsartan (−10.0 mmHg; P<0.001) and olmesartan (−11.7 mmHg; P=0.009). Safety profiles were not statistically investigated, nonetheless the raw data reported show similar results between study groups.
Comparison of azilsartan and valsartan has been further investigated by Sica et al15 in another double-blind, randomized trial including 984 patients randomized to placebo, azilsartan 20 mg titrated to 40 mg, 40 mg titrated to 80 mg, or valsartan 80 mg titrated to 320 mg for 24 weeks. Azilsartan 40 and 80 mg lowered 24-hour mean SBP (−14.9 and −15.3 mmHg, respectively) more than valsartan 320 mg (−11.3 mmHg; P<0.001 for both comparisons).
Furthermore, safety and efficacy of azilsartan (20–40 mg) has also been compared to candesartan (8–12 mg) in a 16-week randomized controlled trial involving 622 patients.16 Results demonstrated a significant BP reduction among patients administered azilsartan (–12.4 mmHg diastolic blood pressure [DBP] and –21.8 mmHg SBP) in comparison with those receiving candesartan (–9.8 mmHg DBP and –17.5 mmHg SBP). This analysis of sitting BP was further confirmed by ABPM findings. The most common adverse effect was nasopharyngitis (interesting 18% vs 16% patients, P= nonsignificant), and safety profiles were also similar between treatment groups.
Use of azilsartan in patients with renal impairment
The use of azilsartan in patients with renal impairment has received special focus. First, Preston et al33 demonstrated that no dose adjustment is needed for patients with mild, moderate, or even severe chronic kidney disease, as well as those with end-stage renal disease requiring hemodialysis, when administering azilsartan 40 mg. Indeed, they found that patients with renal impairment had increased accumulation of the major metabolite of azilsartan (called M-II), which is pharmacologically inactive; thus this increase was not considered important in dose selection in subjects with renal disease.
Then, Kusuyama et al34 published a retrospective study investigating 17 hemodialysis patients receiving azilsartan 20 or 40 mg. After 6 months of therapy, ABPM revealed significant BP decrease (–19.6 mmHg), along with significant reductions of serum noradrenaline (–198.4 pg/mL) and left ventricular indexed mass (–6 g/m2).
Comparison of azilsartan vs other antihypertensive drugs
Monotherapy with azilsartan 20 mg (forced titrated to 80 mg) has been compared to ramipril 2.5 mg (forced titrated to 10 mg) in a recent randomized, double-blind, controlled trial investigating changes in SBP after 24 weeks of treatment among 884 hypertensive patients.35 The main finding was that azilsartan significantly decreased SBP (–21.2 mmHg) much more than ramipril (–12.2 mmHg; P<0.001). Adverse effects leading to treatment discontinuation were more likely to occur among patients administered ramipril (4.8%) than those administered azilsartan (3.1%), but this trend did not reach statistical significance.
A recent multicenter, randomized, controlled trial by Kario et al,36 aimed to compare minimum dosage of azilsartan (20 mg) and amlodipine (5 mg) for the treatment of stage I and II hypertension in 668 patients. The primary end point was control rate of sleep-ABPM after 8 weeks of therapy; no significant difference was found between study groups, despite a nonstatistically significant trend favoring amlodipine (34.7% vs 30%), especially among patients aged over 60 years.
Another study, named EARLY registry, comparing angiotensin-converting enzyme (ACE) inhibitors therapy with ARB therapy (by means of azilsartan as first-line therapy) in patients with newly diagnosed hypertension has been recently published.37 Data from this prospective, “real-world” registry were used to compare achievement of BP control (<140/90 mmHg) between patients administered azilsartan (n=789) and those administered any other ACE inhibitor (n=364) as monotherapy. The authors concluded that in newly diagnosed hypertensive patients, azilsartan monotherapy provided superior BP control with a similar safety profile compared with ACE inhibitors.
In addition, a recent meta-analysis38 compared azilsartan 40 mg vs any other control therapy (including placebo). End points estimated were SBP and DBP reductions both in clinical and ambulatory monitoring settings; statistically significant reductions were found always favoring azilsartan.
Comparison of azilsartan + chlortalidone vs other combination therapies
Trials comparing clinical safety and efficacy of azilsartan/chlorthalidone with that of other combination therapies for the treatment of stage II hypertension are listed in Table 2.
The first investigation dealing with combination therapies involving azilsartan was a double-blind, randomized, placebo-controlled trial by Kipnes et al39 investigating DBP reduction after 32-week treatment with azilsartan 40 mg (forced titrated to 80 mg) with or without the adjunct of chlorthalidone 25 mg if required, to reach target BP. Among the 418 patients randomized to different treatment strategies, mean changes in SBP/DBP from baseline were –23/–16 mmHg. The most common adverse events, irrespective of treatment, were dizziness (8.9%) and headache (7.2%), while serious adverse events were reported in only eight patients (1.9%). Mean DBP was maintained through the reversal phase in patients receiving azilsartan monotherapy, but increased with placebo (difference: –7.8 mmHg; P<0.001).
Efficacy and safety of azilsartan/chlorthalidone FDC therapy has been compared with that of the individual monotherapies in a double-blind factorial study40 involving a total of 1,714 patients with grade II hypertension randomized to azilsartan 0, 20, 40, or 80 mg and/or chlorthalidone 0, 12.5, or 25 mg. The primary efficacy end point (change of mean ABPM-SBP from baseline to 8 weeks) was –28.9 mmHg for the pooled azilsartan/chlorthalidone 40/25 and 80/25 mg FDC (similar between 40/25 and 80/25 mg), significantly exceeding that of azilsartan 80 mg and chlorthalidone 25 mg monotherapies (P<0.001 for both comparisons). In addition, despite African–American patients having been previously demonstrated to be less responsive to azilsartan alone,41 treatment with azilsartan/chlorthalidone FDC resulted in a similar magnitude of BP reduction in this subset of patients. Discontinuation rates and elevations in serum creatinine were dose-dependent and occurred more often in the azilsartan/chlorthalidone FDC groups (0.6%–5% compared to 0.1% in monotherapy groups).
Moreover, the efficacy of azilsartan/chlorthalidone FDC therapy force titrated to highest dosage (80/25 mg) has been compared to olmesartan/hydrochlorothiazide FDC therapy force titrated to 40/25 mg in a trial42 comprising a total of 1,071 patients with stage II hypertension randomly assigned to different treatment strategies. After 12 weeks, mean ABPM-SBP reductions were significantly greater in both the azilsartan/chlorthalidone arms rather than in the olmesartan/hydrochlorothiazide arm (–42.5, –44.5, and –37.1 mmHg, respectively; P<0.001 for all comparisons). Adverse events leading to drug discontinuation occurred in 7.9%, 14.5%, and 7.1% of the patients, respectively, and hence the authors concluded that the azilsartan/chlorthalidone combination was more effective in reducing BP than the olmesartan/hydrochlorothiazide combination, even if the approximate equivalent dose of chlorthalidone 25 mg is hydrochlorothiazide 50 mg; therefore, it is possible that unequal potency doses were being compared.
One more randomized, placebo-controlled, double-blind trial compared the efficacy of azilsartan/chlorthalidone and azilsartan/hydrochlorothiazide combinations.43 A total of 609 patients with stage II primary hypertension were randomized to receive 12.5 mg of chlorthalidone or hydrochlorothiazide in addition to azilsartan 40 mg for 4 weeks, and diuretics were titrated up to 25 mg for another 4 weeks if BP was not controlled. Target clinical BP (<140/90 mmHg for participants without diabetes or chronic kidney disease, otherwise <130/80 mmHg) represented the main end point of the study. Results showed that the association of azilsartan/ chlorthalidone provided greater reduction in SBP than the combination of azilsartan with hydrochlorothiazide (–31.5 mmHg vs –29.5 mmHg, P<0.001). The percentage of patients achieving target BP after 6 weeks of treatment was greater for the chlorthalidone vs hydrochlorothiazide combination (64.1% vs 45.9%; P<0.001). Drug discontinuation due to adverse events was not statistically significantly different between groups (9.3% vs 7.3%; P=0.38). It is also important to note that the approximate equivalent dose of chlorthalidone 25 mg is hydrochlorothiazide 50 mg; while the latter study compared unequal dosage of such diuretics.43
Furthermore, study 491-CLD-301 compared the efficacy of once-daily FDC of azilsartan/chlorthalidone 20/12.5 or 40/25 mg with a FDC of olmesartan/hydrochlorothiazide 20/12.5 mg among a total of 1,085 patients randomized to different treatment strategies.44 After the first 4 weeks of treatment, subjects achieving both target SBP and diastolic BP (<140/90 mmHg for subjects without diabetes or chronic kidney diseases, otherwise <130/80 mmHg) continued to receive their starting dose for the duration of the study. For subjects who did not achieve target BP, the following dose titrations were prescribed: azilsartan/chlorthalidone 40/25 mg, 80/25 mg and olmesartan/hydrochlorothiazide 40/25 mg. After 8 weeks of treatment, SBP reductions in both azilsartan/chlorthalidone treatment groups (–33 to –38 mmHg) were significantly (P<0.05) greater than in the olmesartan/hydrochlorothiazide groups (–27 to –32 mmHg). The most common side effects in the azilsartan/chlorthalidone group included serum creatinine elevation (2.5% in the higher dosage group), fatigue (3.8% in the higher dosage group), and hypotension (1.1% in the higher dosage group). It is again important to point out that the approximate equivalent dose of chlorthalidone 25 mg is hydrochlorothiazide 50 mg, despite only hydrochlorothiazide 25 mg having been tested in this study.
More recent trials compared azilsartan with amlodipine. The first among them was a multicenter, randomized, double-blind trial investigating 603 patients randomized for 8 weeks to receive azilsartan 20 mg and amlodipine 2.5 or 5 mg either alone or in a fixed-dose combination fashion.45 Both DBP (–22.3 and –19.2 mmHg; P<0.0001 vs other regimens) and SBP (–35.3 and –31.4 mmHg; P<0.0001 vs other regimens) reductions were significantly greater for patients administered the fixed-dose combination therapies. In addition, BP goal achievement rate was higher for patients taking the fixed combinations (56.4% and 41.7% vs 19.9%, 24%, and 11.8%). Common adverse effects were nasopharyngitis (8%) and dizziness (2.7%); overall tolerability was similar between study groups, and serious adverse effects were registered in only two patients (0.3%). Another randomized, placebo-controlled, double-blind trial compared efficacy of amlodipine 5 mg associated with placebo, azilsartan 40 mg or azilsartan 80 mg among 566 patients with stage II hypertension.46 Reductions of SBP (measured by 24-hour ABPM) were significantly higher for patients receiving the addition of azilsartan (–25 and –14 mmHg; P<0.0001 for both comparisons), as were DPB reductions (–15 and –7 mmHg; P<0.0001 for both comparisons). The latter findings were found to be independent of age, sex, and body mass index. In addition, 6-week BP goal achievement was greater among patients receiving azilsartan (49.2% and 46.4% vs 25.1%; P<0.0001). Incidence of adverse events was similar between groups (40%–48%), and severe adverse events were recorded rarely (0.5%–1%); of note, peripheral edema was less common in patients taking the combination therapies (3% vs 8%).
Furthermore, clinical studies also revealed a 30% reduction in total mortality in chronic heart failure.47
Overall, the association of azilsartan and chlorthalidone has been demonstrated to lower BP much more than olmesartan and hydrochlorothiazide; furthermore, little difference was noted between the 40 and 80 mg dosage of azilsartan.
Side effects
According to the manufacturer, more than 4,000 patients were evaluated in premarketing clinical trials when treated with azilsartan or azilsartan plus chlorthalidone for 6 months to 1-year.18,48 Both were generally well tolerated, and the adverse events that occurred were frequently mild and transient. Common side effects included dizziness (8.9%) and fatigue (2%).48 Only 1.7% and 0.3% of patients reported hypotension and syncope episodes, respectively.
Side effects causing therapy cessation were only seen in 8.3% of patients administered the azilsartan/chlorthalidone, in 3.2% of patients receiving azilsartan, and in 3.2% of patients receiving chlorthalidone. Increase of creatinine levels (3.6%) and dizziness (2.3%) were noted as the most frequent events causing treatment discontinuation.
The FDC of azilsartan/chlorthalidone showed a safety profile similar to that of placebo.
Because of the well-known pharmacologic effect of the renin–angiotensin–aldosterone system blockade, both ARBs and ACE inhibitors are known to potentially result in increased creatinine levels, often related to the magnitude of BP decrease. Of note, the incidence of consecutive increases of creatinine >50% from baseline was 2.0% in patients receiving azilsartan/chlorthalidone FDC compared to 0.4% and 0.3% with azilsartan and chlorthalidone alone, respectively. Mean increases in blood urea nitrogen were observed with azilsartan/chlorthalidone (5.3 mg/dL) compared to azilsartan (1.5 mg/dL) and chlorthalidone (2.5 mg/dL) alone.
In addition, based on the manufacturer recommendations, anuria is the only contraindication to the use of azilsartan/chlorthalidone. An additional issue preventing the use of azilsartan/chlorthalidone should be pregnancy (because of fetal and neonatal morbidity and death when renin–angiotensin system blockers are administered during the second and the third trimester48). In addition, patients who are volume- or salt-depleted may be more sensitive to the hypotensive effect of azilsartan. Along with the enhanced hypotensive effect in volume- or salt-depleted patients, it needs to be pointed out that hypokalemia is a dose-dependent adverse reaction correlated with chlorthalidone, which may be exacerbated by digitalis coadministration. As a counterpoint, azilsartan may attenuate chlorthalidone-related hypokalemia.
Other adverse events registered during clinical trials along with efficacy evaluations have been explained earlier in this section. These side effects were recorded mainly during the trial, in the absence of any poststudy surveillance.
Pharmacoeconomics/cost-effectiveness
A literature search found that no formal cost-effectiveness study has been performed to date for azilsartan. The average cost of azilsartan is slightly higher compared to other antihypertensive drugs. On the other hand, the average wholesale price of azilsartan/chlorthalidone for a 30-day supply is similar, and often cheaper, than other ARB/thiazide combinations available on the market.49
Conclusion
Randomized controlled trials demonstrated that azilsartan is superior, in terms of 24-hour ambulatory BP monitoring reduction, with respect to other sartans, such as valsartan, olmesartan, and candesartan. In addition, safety profiles of the latter drugs were similar and not statistically different from placebo.
Moreover, beneficial effects of azilsartan were also noted in patients with any degree of renal impairment, even in case of patients with end-stage renal disease; with anuria being the only absolute contraindication to the association of azilsartan plus chlorthalidone. With respect to other antihypertensive medications, azilsartan was found to be superior to any ACE inhibitor in terms of ABPM results and noninferior to amlodipine in terms of sleep-BP control.
Furthermore, the association of azilsartan and chlorthalidone was then found to be superior to other combination therapies, including a sartan plus thiazide combination, in terms of both BP lowering and BP goal achievement. Of note, it has to be pointed out that those comparisons were carried out between unequal dosages of thiazide and chlorthalidone, favoring the latter; hence, this may be a bias affecting results of such comparisons.
Besides this, the other available combination of azilsartan with amlodipine has also been tested in clinical trials. That comparison was only made vs placebo, demonstrating its superiority in terms of efficacy and similar tolerability.
Conclusions coming from drug interactions studies should result in paying special attention when administering azilsartan with fluconazole, which is a CYP2C9 inhibitor. Moreover, given that nonsteroidal anti-inflammatory agents and COX-2 inhibitors cause prerenal acute renal failure by blocking prostaglandin production, further altering local glomerular arteriolar perfusion, the concomitant use of these agents with azilsartan may increase the risk of renal dysfunction. Many other studies demonstrated no interaction with caffeine, antacid, warfarin, digoxin, tolbutamide, glyburide, metformin, pioglitazone, chlorthalidone, amlodipine, dextromethorphan, midazolam, and fexofenandine.
Many studies investigated the pleiotropic effects of azilsartan and showed that it may be effective in ameliorating several pathological patterns underlying hypertension.
In conclusion, azilsartan is a safe and effective treatment option for every stage of hypertension, both alone or in fixed-dose combination tablets with chlorthalidone or amlodipine. Azilsartan demonstrated a good and stable BP improvement, free from significant complications, even in a titrate-to-target approach. This was found both in case of azilsartan monotherapy or fixed-dose combination therapies with chlorthalidone or amlodipine. In addition, several experimental evidences indicate an interesting series of pleiotropic actions resulting in different beneficial effects in terms of renal, and endothelial function, and metabolic homeostasis.
On the other hand, it has to be disclosed that many other antihypertensive drugs cheaper than azilsartan have been found to show efficient BP control. In addition, older drugs have been tested in more large trials, thus data demonstrating safety and efficacy of azilsartan are expected to be corroborated, in the near future, from further clinical studies.
Disclosure
The author reports no conflicts of interest in this work.
References
Heart Disease and Stroke Statistics – 2015 update. A report from the American Heart Association. Circulation. 2015;131:e29–e322. | |
Centers for Disease Control and Prevention. High Blood Pressure Facts. Atlanta, GA: Centers for Disease Control and Prevention; 2011. Available from: http://www.cdc.gov/bloodpressure/facts.htm. Accessed July 24, 2011. | |
National Institutes of Health; National Heart, Lung, and Blood Institute. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Bethesda, MD: US Department of Health and Human Services; 2004. Available from: http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed August 24, 2011. | |
Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010;55(2):399–407. | |
Angeloni E, Vitaterna A, Lombardo P, Michele P. Single-pill combination therapy in the initial treatment of marked hypertension: a propensity-matched analysis. Clin Exp Hypertens. 2015;37:404–410. | |
Ram CV. Angiotensin receptor blockers: current status and future prospects. Am J Med. 2008;121(8):656–663. | |
Duncia JV, Chiu AT, Carini DJ, et al. The discovery of potent nonpeptide angiotensin II receptor antagonists: a new class of potent antihypertensives. J Med Chem. 1990;33(5):1312–1329. | |
Griffin KA, Bidani AK. Progression of renal disease: renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol. 2006;1(5):1054–1065. | |
Unger T. Significance of angiotensin type 1 receptor blockade: why are angiotensin II receptor blockers different? Am J Cardiol. 1999;84(10A):9S–15S. | |
Staessen JA, Richart T, Wang Z, Thijs L. Implications of recently published trials of blood pressure-lowering drugs in hypertensive or high-risk patients. Hypertension. 2010;55(4):819–831. | |
Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103(6):904–912. | |
Giles TD, Oparil S, Silfani TN, Wang A, Walker JF. Comparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertension. J Clin Hypertens (Greenwich). 2007;9(3):187–195. | |
White WB, Weber MA, Sica D, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. 2011;57(3):413–420. | |
Bakris GL, Sica D, Weber M, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens. 2011;13(2):81–88. | |
Sica D, White WB, Weber MA, et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring. J Clin Hypertens. 2011;13(7):467–472. | |
Rakugi H, Enya K, Sugiura K, Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I–II essential hypertension: a randomized, doubleblind clinical study. Hypertens Res. 2012;35:552–558. | |
Appendix to Clinical Pharmacology Review: Edarbi®. Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s). Silver Spring, MD: Food and Drug Administration; 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/ 2011/200796Orig1s000ClinPharmR.pdf. Accessed July 26, 2011. | |
Edarbi® [prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2012. Available from: http://www.edarbi.com/. Accessed February 22, 2016. | |
Ojima M, Igata H, Tanaka M, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther. 2011;336(3):801–808. | |
Agrawal M, Swartz R. Acute renal failure. Am Fam Physician. 2000;61(7):2077–2088. | |
Zhao M, Li Y, Wang J, et al. Azilsartan treatment improves insulin sensitivity in obese spontaneously hypertensive Koletsky rats. Diabetes Obes Metab. 2011;13:1123–1129. | |
Hye Khan MA, Neckar J, Haines J, Imig JD. Azilsartan improves glycemic status and reduces kidney damage in zucker diabetic fatty rats. Am J Hypertens. 2014;27(8):1087–1095. | |
Abdelsaid M, Coucha M, Ergul A. Cerebrovasculoprotective Effects of azilsartan medoxomil in diabetes. Transl Res. 2014;164(5):424–432. | |
Tarikuz Zaman AK, McLean DL, Sobel BE. The efficacy and tolerability of azilsartan in obese insulin-resistant mice with left ventricular pressure overload. J Cardiovasc Pharmacol. 2013;62(4):381–387. | |
Nakamura Y, Suzuki S, Saitoh S, Takeishi Y. New angiotensin II type 1 receptor blocker, azilsartan, attenuates cardiac remodeling after myocardial infarction. Biol Pharm Bull. 2013;36(8):1326–1331. | |
Kusumoto K, Igata H, Ojima M, et al. Antihypertensive, insulin-sensitising and renoprotective effects of a novel, potent and long-acting angiotensin II type 1 receptor blocker, azilsartan medoxomil, in rat and dog models. Eur J Pharmacol. 2011;669:84–93. | |
Khan AH, Neckar J, Cummens B, Wahl GM, Imig JD. Azilsartan decreases renal and cardiovascular injury in the spontaneously hypertensive obese rat. Cardiovasc Drugs Ther. 2014;28:313–322. | |
Jin C, O’Boyle S, Kleven DT, Pollock JS, Pollock DM, White JJ. Antihypertensive and anti-inflammatory actions of combined azilsartan and chlorthalidone in Dahl salt-sensitive rats on a high-fat, high-salt diet. Clin Exp Pharmacol Physiol. 2014;41:579–588. | |
Araújo AA, Varela H, Brito GA, et al. Azilsartan increases levels of IL-10, down-regulates MMP-2, MMP-9, RANKL/RANK. Cathepsin K and up-regulates OPG in an experimental periodontitis model. PLoS One. 2014;9(5):e96750. | |
Matsumoto S, Shimabukuro M, Fukuda D, et al. Azilsartan, an angiotensin II type 1 receptor blocker, restores endothelial function by reducing vascular inflammation and by increasing the phosphorylation ratio Ser1177/Thr497 of endothelial nitric oxide synthase in diabetic mice. Cardiovasc Diabetol. 2014;13:30–40. | |
Araújo AA, Varela H, Medeiros CA, et al. Azilsartan reduced TNF-α and IL-1β levels, increased IL-10 levels and upregulated VEGF, FGF, KGF, and TGF-α in an oral mucositis model. PLoS One. 2015;10(2):e0116799. | |
French CJ, Zaman AK, Sobel BE. The angiotensin receptor blocker, azilsartan medoxomil (TAK-491), suppresses vascular wall expression of plasminogen activator inhibitor type-I protein potentially facilitating the stabilization of atherosclerotic plaques. J Cardiovasc Pharmacol. 2011;58(2):143–148. | |
Preston RA, Karim A, Dudkowski C, et al. Single-center evaluation of the single-dose pharmacokinetics of the angiotensin II receptor antagonist azilsartan medoxomil in renal impairment. Clin Pharmacokinet. 2013;52(5):347–358. | |
Kusuyama T, Ogata H, Takeshita H, et al. Effects of azilsartan compared to other angiotensin receptor blockers on left ventricular hypertrophy and the sympathetic nervous system in hemodialysis patients. Ther Apher Dial. 2014;18(5):398–403. | |
Bonner G, Bakris GL, Sica D, et al. Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor ramipril. J Human Hypertens. 2013;27:479–486. | |
Kario K, Hoshide S. Age-related difference in the sleep pressure-lowering effect between an angiotensin II receptor blocker and a calcium channel blocker in asian hypertensives. The ACS1 study. Hypertension. 2015;65:729–735. | |
Schmieder RE, Potthoff SA, Bramlage P, et al. Patients with newly diagnosed hypertension treated with the renin angiotensin receptor blocker azilsartan medoxomil vs angiotensin-converting enzyme inhibitors: the prospective early registry. J Clin Hypertens. 2015;17(12):947–953. | |
Takagi H, Mizuno Y, Niwa M, Goto SN, Umemoto T. A meta-analysis of randomized controlled trials of azilsartan therapy for blood pressure reduction. Hypertens Res. 2014;37(5):432–437. | |
Kipnes MS, Handley A, Lloyd E, Barger B, Roberts A. Safety, tolerability, and efficacy of azilsartan medoxomil with or without chlorthalidone during and after 8 months of treatment for hypertension. J Clin Hypertens. 2015;17(3):183–192. | |
Sica D, Bakris GL, White WB, et al. Blood pressure lowering efficacy of the fixed dose combination of azilsartan and chlorthalidone: a factorial study. J Clin Hypertens. 2012;14(5):284–292. | |
Divisional Memo: Azilsartan medoxomil (Edarbi) for hypertension. Center for Drug Evaluation and Research. Medical Review(s). Silver Spring, MD: Food and Drug Administration; 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/200796Orig1s000MedR.pdf. Accessed February 22, 2016. | |
Cushman WC, Bakris GL, White WB, et al. Azilsartan medoxomil plus chlorthalidone reduces blood pressure more effectively than olmesartan plus hydrochlorthiazide in stage 2 systolic hypertension. Hypertension. 2012;60(2):310–318. | |
Addendum to Clinical Review of NDA 202–331 (Edarbyclor®). Center for Drug Evaluation and Research. Medical Review(s). Silver Spring, MD: Food and Drug Administration; 2011. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/202331Orig1s000MedR.pdf. Accessed February 22, 2016. | |
Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med. 2012;125(12):1229.e1–1229.e10. | |
Rakugi H, Nakata E, Sasaki E, Kagawa T. Evaluation of the efficacy and tolerability of fixed-dose combination therapy of azilsartan and amlodipine besylate in Japanese patients with grade I to II essential hypertension. Clin Ther. 2014;36:711–721. | |
Weber MA, White WB, Sica D, et al. Effects of combining azilsartan medoxomil with amlodipine in patients with stage 2 hypertension. Blood Press Monit. 2014;19:90–97. | |
Roush GC, Ernst ME, Kostis JB, Kaur R, Sica DA. Not just chlorthalidone: evidence-based, single tablet, diuretic alternatives to hydrochlorothiazide for hypertension. Curr Hypertens Rep. 2015; 17(4):540. | |
Edarbyclor® [prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2012. Available from: http://www.edarbi.com/. Accessed February 22, 2016. | |
Medication Average Wholesale Price. Thomson Reuters Micromedex Clinical Evidence Solutions. Thomson Reuters; c2011. RED BOOK Drug References; c2011 [cited July 8, 2011]. Available from: http://thomsonreuters.com/products_services/healthcare/healthcare_products/clinical_deci_support/micromedex_clinical_evidence_sols/med_safety_solutions/red_book/. Accessed February 22, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



