Back to Journals » OncoTargets and Therapy » Volume 10
Axl promotes the proliferation, invasion and migration of Wilms’ tumor and can be used as a prognostic factor
Authors Zhu S, Liu G, Fu W, Hu J, Fu K, Jia W
Received 11 November 2016
Accepted for publication 5 January 2017
Published 16 February 2017 Volume 2017:10 Pages 955—963
DOI https://doi.org/10.2147/OTT.S127419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
Shibo Zhu, Guochang Liu, Wen Fu, Jinhua Hu, Kai Fu, Wei Jia
Department of Pediatric Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, People’s Republic of China
Purpose: Overexpression of Axl has been reported in many tumors, where it promotes tumorigenesis and progression, as well as correlates with the prognosis of different malignancies. However, Axl expression and its function have rarely been reported in Wilms’ tumor (WT). This study aimed to reveal the clinical significance of Axl expression in patients with WT and determine its mechanisms.
Materials and methods: We analyzed the expression of Axl and its correlations with various clinicopathological features in 72 WT tissues and 72 adjacent non-cancerous tissues by immunohistochemistry. Cox proportional hazards regression models were used to investigate the correlations between Axl expression and the prognosis of WT patients. Fresh frozen samples from 20 WT patients were examined using Western blotting (WB) and real-time quantitative polymerase chain reaction (RT-qPCR). In WT cell line, after Axl knockdown by sh-Axl and growth arrest-specific 6 (Gas6) stimulation, the cell proliferation, migration and invasion abilities were detected by methyl-thiazolyl-tetrazolium (MTT), clone-forming, wound-healing and transwell assays. Meanwhile, the tumor-forming ability was tested on nude mice xenograft models. Finally, the expression of several proteins in signal pathways was quantified by WB assays.
Results: Compared with the adjacent non-cancerous tissues, the expression of Axl was significantly higher in WT tissues (P<0.05). High expression of Axl was associated with tumor recurrence or lung metastasis of WT patients and was a prognostic factor for WT patients (P<0.05). In vitro assays, the proliferation, migration and invasion of WT cells decreased with Axl knockdown and significantly increased with Axl activation by Gas6 (P<0.05). In vivo assays, the ability of tumorigenicity in WT cells reduced dramatically after Axl knockout (P<0.05). Moreover, PI3K–Akt pathway proteins decreased with Axl knockdown.
Conclusion: Our results suggest that Axl is highly expressed in WT and is a prognostic factor, which could promote the progression of WT in vitro and in vivo. It may also be a potential biomarker for WT.
Keywords: Wilms’ tumor, Axl, prognosis, proliferation, invasion, migration
Introduction
Wilms’ tumor (WT) is one of the most common pediatric tumors, accounting for ~8% of all pediatric cancers.1 Although significant advances in combined treatments have improved the survival rates of 90% of patients with localized disease, a number of patients still have a bad prognosis because of metastasis or recurrence.2 It is important to investigate the molecular factors of WT in order to improve its accuracy of diagnosis and direct the treatment in the clinic, especially in the patients who have bad prognosis.
The advances in research have led to the discovery of biomarkers that are associated with tumor occurrence and progression, such as the expression of SIX1 and SIX2 and dysregulation of CTNNB1.3 As we all know, receptor tyrosine kinases (RTKs) mediate important cellular processes in tumorigenesis and progression in many tumors, such as pancreatic cancer, ovarian cancer and hepatocellular carcinoma.4–6 Axl, along with Tyro3 and Mer, is a member of the TAM family of RTKs and was first identified as a transforming gene in chronic myeloid leukemia.7 The ligand for Axl, growth arrest-specific 6 (Gas6), is a vitamin K-dependent protein that binds Axl with high affinity.8 Axl activation and signaling by Gas6 have been implicated in multiple cellular responses, including cell survival, proliferation and migration.9 However, Axl expression and its function have rarely been reported in WT. In this study, we aimed to reveal the Axl expression on clinical samples, analyzed its correlation with clinicopathological features and investigated its mechanisms.
Materials and methods
Clinical samples and follow-up
Paired WT tissues and adjacent non-cancerous tissues were collected from the Guangzhou Women and Children’s Medical Center. A total of 72 cases of formalin-fixed, paraffin-embedded (FFPE) biopsy specimens of primary WT obtained between 2010 and 2015 were analyzed. A total of 20 cases of fresh samples of WT and paired non-tumor tissues from surgical resection were frozen in liquid nitrogen and stored at −80°C for RNA and protein extraction. This study was approved by the Institutional Research Ethics Committee of Guangzhou Medical University, and written informed consent was obtained from all participants. Follow-up was performed every 2–3 months during the first year after surgery until 2016.2
WT cell line
The WT cell line was established from a fresh tumor sample of a WT patient in our hospital. After surgical removal, the tissue was rinsed in calcium-free Hanks’ solution containing penicillin. Then, it was minced into fragments and digested into single cells using 0.25% trypsin–ethylenediaminetetraacetic acid (EDTA) solution. The suspension was centrifuged at 1,000× g for 5 min, and then the single cells were cultured in a culture flask containing Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA, USA), supplemented with 15% fetal bovine serum (FBS; Gibco Carlsbad, CA, USA) and 100 U/mL penicillin/streptomycin solution (Gibco Carlsbad, CA, USA), at 37°C in a humidified 5% CO2 atmosphere.
Lentivirus transfection of WT cells
The short hairpin RNA (shRNA) that inhibits the Axl expression was designed and synthesized by ShangHai SBO Medical Biotechnology Company (Shanghai, China). The sequences are F: TAGTACCAGTGTTTGGTGTTTCTTCCTGTCAAAACACCAAACACTGGTACTGTTTTTTTC and R: TCGAGAAAAAAAGTACCAGTGTTTGGTGTTTTGACAGGAAGAAACACCAAACACTGGTACTGA. Then, the sh-Axl was inserted into the lentiviral vector pLL3.7 with T4 DNA ligases, and the vectors were transformed into Escherichia coli stbl3, which were resistant to ampicillin. Then, the correct vectors were cotransfected with psPAX2 and pMD2.G (GenePharma, Shanghai, China) in 293T cells using Xtreme (Roche, South San Francisco, CA, USA). Infectious lentiviruses were harvested at 48 h post-transfection and filtered through 0.45 μm polyvinylidene fluoride (PVDF) filters. Finally, these viruses were transfected into WT cells, and the transfected efficiency was detected using real-time quantitative polymerase chain reaction (RT-qPCR).
RNA extraction, complementary DNA (cDNA) synthesis and quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from the WT tumor tissues, and the adjacent normal tissues were matched using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). Briefly, all samples were treated with Trizol followed by chloroform. The mixture was centrifuged at 14,000× g for 10 min at 4°C, and 700 μL of 75% ethanol was added to the aqueous layer. Finally, the purified RNA was diluted with 30 μL of RNase-free water. cDNA synthesis was performed with 2 μg total RNA using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara, Otsu, Shiga, Japan) for the next qPCR of Axl according to the manufacturer’s instructions. The Axl primers (F: TCAAGGTGGCTGTGAAGACGA, R: CGTTCAGAACCCTGGAAACAGAC) and GAPDH primers (F: GCACCGTCAAGGCTGAGAAC, R: TGGTGAAGACGCCAGTGGA) were obtained from Takara (Takara, Dalian, China). A qPCR was performed using the SYBR Premix Ex Taq II kit (Takara, Otsu, Shiga, Japan) and the Applied Biosystems 7500 Fluorescent Quantitative PCR system (Applied Biosystems Life Technologies, Foster, CA, USA). The mixtures were incubated at 95°C for 30 s, followed by 40 amplification cycles of 95°C for 5 s and 60°C for 34 s. The comparative cycle threshold method was used to quantify the relative expression levels of messenger RNA (mRNA). Expression levels of the housekeeping gene GAPDH were used to normalize the expression levels of the genes of interest. The relative mRNA levels were calculated based on the cycling threshold (Ct) values and normalized using the relative housekeeping gene expression. Each sample was assayed in triplicate.
Western blotting (WB)
The WT cells, stably transfected WT cells and 40 frozen tissues (20 tumor samples and 20 adjacent samples) were collected for protein extraction. Total proteins were isolated, and the protein concentration was measured. Identical quantities of proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose filter membranes. After an incubation with antibodies against Akt (Ser 473) (1:1,000; Cell Signaling Technology [CST], Danvers, MA, USA), Axl (1:1,000; CST Danvers, MA, USA), PI3K (1:1,000; CST, Danvers, MA, USA) and P70S6K (1:1,000; CST, Danvers, MA, USA) were used, and antibodies against GAPDH were used as an internal control. After incubation with horseradish peroxide (HRP)-conjugated secondary antibody, the signals were detected using chemiluminescence imaging system (Bio-Rad, Hercules, CA, USA).
Methyl-thiazolyl-tetrazolium (MTT) assay in vitro
The cells in different groups were cultured in a 96-well plate at the density of 2,000 cells per well. Then, 0.15 mg MTT (Kaiji, Taizhou, China) was added to the medium and cultured for 4 h. Finally, the supernatant was removed and 150 μL dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO, USA) was added into each well to dissolve the formazan crystals. The absorbance at 490 nm was detected with a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Wound-healing assays in vitro
The WT cells in different groups were cultured in a 6-well plate and then scratched to create two parallel wounds by using a 100 μL pipette tip at 70% confluence. The cells were then washed with phosphate-buffered saline (PBS) and incubated in serum-free medium at 37°C for 24 h. Pictures were taken every 8 h, using a Leica DMI4000B microscope (Leica, Wetzlar, Germany).
Transwell assays in vitro
Matrigel™ Matrix (BD Biosciences, San Jose, CA, USA) was diluted 1:7 using serum-free basal medium, and each 50 μL Matrigel Matrix dilution was added to an upper chamber (8 μm pore size) of transwell inserts (Corning, Lowell, MA, USA). Then, 100 μL WT cell (2×105/mL) suspensions from different groups were seeded in the upper chambers in 24-well plates and cultured in serum-free basal medium. A total of 500 μL of the medium with 10% FBS was added to the lower chambers. After 24 h, cells were removed using cotton swabs in the upper chambers. The inserts were washed three times with PBS, and cells that invaded to the bottom surface of the insert were fixed with 4% paraformaldehyde and stained using 1% crystal violet. Then, the cells were counted under the microscope.
Clone formation assay in vitro
The WT cells (400 cells/well) were seeded in 6-well plates. The cells were cultured for ~10 days and fixed with 4% paraformaldehyde. After washing, the plates were air-dried and the total number of clones (>50 cells/clone) was counted.
In vivo nude mice models
Male BALB/C nude mice (5–6 weeks of age, 16–18 g) were purchased from the Experiment Animal Center of Guangdong province and maintained in a pathogen-free facility. Mice were randomly divided into two groups in subcutaneous tumor models, which included control and sh-Axl group. Then, 2×106 WT cells in a volume of 100 μL were injected subcutaneously in one flank of the mice. The mice were observed and the tumors that developed on the mice were recorded every 3 days. The study was approved by the Experimental Animal Ethics Committee of Guangzhou Medical University and conducted in compliance with the Protocols of Care and Use of Experimental Animals developed by Guangzhou Medical University Experimental Animal Administration Committee.
Immunohistochemistry
An Envision two-step assay was used for the immunohistochemistry stain for Axl in WT and duplex kidney sections. Briefly, after baking at 65°C for 2 h, the sections (4 μm thickness) were dewaxed in xylene, hydrated using a graded series of alcohols (100%, 95% and 85%) and rinsed with PBS. Antigenic retrieval was performed by submerging in citric acid (pH 6.0) and microwaving. To block any nonspecific binding, the sections were treated using a 0.3% hydrogen peroxide solution for 15 min. Then, the sections were incubated overnight at 4°C using Axl antibody (1:100, CST, Danvers, MA, USA) and examined using HRP Envision Systems (Dako, Shanghai, China). Finally, the sections were visualized after counterstaining with hematoxylin. Staining results were scored semi-quantitatively based on the combined percentage (five-tiered algorithm for positive cells [0: 0%; 1: <25%; 2: 25%–50%; 3: 56%–75%; 4: >75%]) and intensity of staining (four-tiered system [0: negative; 1: weak; 2: moderate; 3: strong]), and the scores were tabulated as the expression index (percentage positive × intensity). The index scores of three pathologists were averaged to obtain the final expression index. For Axl grading, high expression was defined as a score of 2 or more; scores <2 were defined as low expression.
Statistical analysis
The correlations between the patients’ clinicopathological features and Axl expression were examined using the chi-square test or analysis of variance (ANOVA) testing. Survival curves were plotted using the Kaplan–Meier method, and differences between the survival curves were tested using the log-rank test. Cox’s proportional hazards model was adopted for the univariate and multivariate analyses of the prognostic factors. A two-tailed P-value of <0.05 was considered statistically significant. Statistical analyses were conducted using SPSS 13.0 software (SPSS, USA).
Results
Axl was overexpressed in WT compared with adjacent tissues, which promoted the lung metastasis
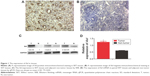
To detect the expression of Axl in WT, immunohistochemistry, WB and RT-qPCR assays were performed using clinical WT and adjacent non-tumor samples. First, the immunohistochemistry assays showed that of 72 WT samples, Axl was highly expressed in 49 cases with the average score of 3.15 (according to the calculation method mentioned earlier), and had low expression in 23 cases with the score of 0.96. Only 35 cases were highly expressed in the 72 cases of adjacent non-cancerous tissues, with an average score of 3.62, and 27 cases of low expression with the score of 0.65. There were significant differences between the WT group and the adjacent non-cancerous group (P<0.05) (Table 1). The representative immunohistochemistry results of high and low expressions are shown in Figure 1A and B. After analyzing the expression of Axl and clinicopathological features of 72 WT patients, Axl expression was positively correlated with tumor recurrence or lung metastasis, and the patients with higher Axl expression developed more recurrence or lung metastasis (P<0.05). However, Axl expression exhibited no significant relationship with other clinical features, including gender, age, tumor size or histological type (Table 2). Then, WB assays were used to test the 20 fresh frozen WT and adjacent tissue samples. As shown in Figure 1C, the expression of protein Axl in fresh tumor samples was higher than that in adjacent non-cancerous samples by WB, as well. Furthermore, RT-qPCR showed that the expression of Axl (Axl mRNA) was more in tumor samples than in adjacent samples, significantly (Figure 1D). Moreover, the univariate Cox regression analysis was performed to check the effect of Axl expression (from immunohistochemistry assays) on prognosis of WT patients (Table 3). The expression of Axl and the local recurrence or lung metastasis in WT significantly correlated with patient prognosis (P<0.05). The high Axl expression in WT was an independent predictor factor for the prognosis of WT patients (P<0.05).
  | Table 1 Axl expression in WT and matched adjacent non-cancerous tissues |
  | Table 2 The relationship between the clinicopathological features and Axl expression of WT patients |
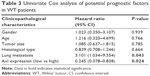  | Table 3 Univariate Cox analysis of potential prognostic factors in WT patients |
Axl increased the proliferation of WT cells in vitro
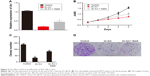
To assess the effect of Axl on the proliferation of WT cells, we first established a WT cell line from the fresh tumor tissue of a WT patient. Then, the cells were knocked down by lentiviral sh-Axl (sequence can be seen in “Materials and methods” section). Then, rhGas6 was added to the stably sh-Axl knocked down cells to activate the expression of Axl. The efficiency of treatments mentioned earlier is shown in Figure 2A. The rates of cell viability were quantified by MTT assays, and the results demonstrated that the Axl knock down significantly decreased the cell proliferation (P<0.05). However, after Gas6 stimulation, cell proliferation increased (P<0.05) (Figure 2B). The clone assays were used to verify the earlier results. The clone number of sh-Axl cells was dramatically lower than the control cells (P<0.05). The sh-Axl cells with Gas6 stimulation had an increased number of clones, compared with sh-Axl cells alone (Figure 2C and D). The earlier results demonstrated that the Axl expression could increase the cell proliferation of WT cells.
Axl improved the migration and invasion of WT cells in vitro
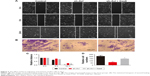
Considering that high Axl expression could influence the metastasis of WT patients, transwell and wound-healing assays were used to confirm the relation between Axl expression and migration and invasion in vitro. As shown in Figure 3A and C, the stable Axl reduction increased the healing distances of control cells and decreased the healing distances after Gas6 stimulation (P<0.05). In Figure 3B and D, the invading cells through the pores in sh-Axl group were much less than in the control group. After Gas6 stimulation, the invading cells were much more than in the sh-Axl group (P<0.05). These results illustrated that the lower Axl expression exhibited a less ability to migrate and invade WT cells in vitro.
Axl aggrandized the ability of WT cells for tumorigenicity in vivo
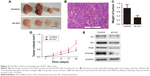
The xenograft models of nude mice were used to test the effect of Axl expression on tumor-forming ability. Only four mice developed subcutaneous tumors in the two groups (control group and sh-Axl group) (Figure 4A), and the formed tumors are shown in Figure 4B. However, the weight of tumors developed in the control group was much more than that in the sh-Axl group (Figure 4C). The tumor volume in the sh-Axl group was smaller than that in the control group (Figure 4D). Collectively, high Axl expression promoted the WT tumorigenicity in vivo.
The effect of Axl in WT could be through Akt signaling pathway
As known, the RTK could function in tumors through several pathways.6–8 Here, Western blot analysis was performed to determine the possible functional pathways in WT. The expression level of Axl after Axl knockdown was shown, and the proteins of Akt, PI3K and P70S6K in the Akt signaling pathway were decreased consequently (Figure 4E). This revealed that the effect of Axl in WT possibly worked through PI3K–Akt pathway and the expression of its downstream proteins.
Discussion
WT is the most common pediatric renal tumor, with a prevalence of 1:10,000 in children younger than 15 years of age.3 Generally, the prognosis of WT depends on many factors in clinics, including patients’ age, tumor stage, histological subtype and preoperative tumor volume.10–13 As well, the molecular factors about the prognosis of WT patients are of great importance. In this study, we investigated the expression and functions of Axl in WT, as it plays an important role in tumor development and progression due to its involvement in cell survival and growth in many cancers, such as osteosarcoma,14 mesothelioma15 and breast cancer.16 Our study first reported that the expression of Axl was much higher in WT than in adjacent non-tumor tissues. We revealed that high expression of Axl was consistent with the recurrence and lung metastasis of WT patients, and the patients who experienced high Axl expression had shorter survival time. Moreover, the high Axl expression is an independent risk factor.
As Ou et al15 reported, activation of Axl can enhance cell proliferation in mesothelioma cells. In cutaneous squamous cell carcinoma, Axl could downregulate the proapoptotic Bcl-2 family members to promote survival.17 Furthermore, in pancreatic ductal adenocarcinoma, overexpression of Axl promotes tumor cell invasion and survival.4 However, how does Axl influence the cancer progression of WT?
In our research, we found that the higher Axl expression increased the proliferation, migration and invasion of WT cells in vitro. It enhanced the tumor-forming ability of WT cells in vivo. Therefore, Axl could influence the progression of WT in many ways.
As we all know, the Akt pathway, which is an important downstream pathway of Axl, plays a crucial role in protein synthesis, cell survival and proliferation of tumor cells.18 After testing the signal proteins in Akt pathway, the expressions of PI3K, Akt, 4EBP1 and P70S6K were dramatically reduced after Axl knockdown. Consequently, the effect of Axl on proliferation, invasion and migration of WT cells probably works through the Akt pathway. However, there are still a few limitations in this study. Firstly, the cell line was established from a WT patient. Considering the biodiversity of tumors, more cells should be tested to confirm these results. Secondly, we only investigated the downstream proteins. It would be worthwhile further examining other possible mechanisms.
Conclusion
This study first revealed the high expression of Axl in WT and the correlation between the high Axl expression and prognosis of WT patients. The high Axl expression is a prognostic factor in WT. Axl promoted the proliferation, migration and invasion of WT. Furthermore, Axl enhanced tumorigenicity of WT cells. It probably works through the Akt pathway.
Acknowledgments
This manuscript was supported by the Guangdong Provincial Department of Science and Technology Foundation, China (Grant No 2016A020215009).
Disclosure
The authors report no conflicts of interest in this work.
References
Al-Hussain T, Ali A, Akhtar M. Wilms tumor: an update. Adv Anat Pathol. 2014;21(3):166–173. | ||
Routh JC, Grundy PE, Anderson JR, et al. B7-h1 as a biomarker for therapy failure in patients with favorable histology Wilms’ tumor. J Urol. 2013;189(4):1487–1492. | ||
Charlton J, Pavasovic V, Pritchard-Jones K. Biomarkers to detect Wilms tumors in pediatric patients: where are we now? Future Oncol. 2015;11(15):2221–2234. | ||
Song X, Wang H, Logsdon CD, et al. Overexpression of receptor tyrosine kinase Axl promotes tumor cell invasion and survival in pancreatic ductal adenocarcinoma. Cancer. 2011;117(4):734–743. | ||
Rankin EB, Fuh KC, Taylor TE, et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70(19):7570–7579. | ||
He L, Zhang J, Jiang L, et al. Differential expression of Axl in hepatocellular carcinoma and correlation with tumor lymphatic metastasis. Mol Carcinog. 2010;49(10):882–891. | ||
O’Bryan JP, Frye RA, Cogswell PC, et al. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991;11(10):5016–5031. | ||
Stitt TN, Conn G, Gore M, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995;80(4):661–670. | ||
Korshunov VA. Axl-dependent signalling: a clinical update. Clin Sci. 2012;122(8):361–368. | ||
Krishnan J, Pietras J, Nachmann M, et al. Adult Wilms’ tumor with a unique presentation of high-grade fever, photophobia, and headache. Rev Urol. 2012;14(1–2):31–34. | ||
Isidor B, Bourdeaut F, Lafon D, et al. Wilms’ tumor in patients with 9q22.3 microdeletion syndrome suggests a role for ptch1 in nephroblastomas. Eur J Hum Genet. 2013;21(7):784–787. | ||
Yadav YK, Sharma U, Gupta K, et al. Squamous predominant teratoid Wilms’ tumor. J Lab Physicians. 2012;4(1):50–52. | ||
Pluciennik E, Nowakowska M, Wujcicka WI, et al. Genetic alterations of wwox in Wilms’ tumor are involved in its carcinogenesis. Oncol Rep. 2012;28(4):1417–1422. | ||
Han J, Tian R, Yong B, et al. Gas6/Axl mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013;435(3):493–500. | ||
Ou WB, Corson JM, Flynn DL, et al. AXL regulates mesothelioma proliferation and invasiveness. Oncogene. 2011;30(14):1643–1652. | ||
Wang C, Jin H, Wang N, et al. Gas6/Axl axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3β/β-catenin signaling. Theranostics. 2016;6(8):1205–1219. | ||
Papadakis ES, Cichon MA, Vyas JJ, et al. Axl promotes cutaneous squamous cell carcinoma survival through negative regulation of pro-apoptotic Bcl-2 family members. J Invest Dermatol. 2011;131(2):509–517. | ||
Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021–1031. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.