Back to Journals » Open Access Journal of Contraception » Volume 5
Axillary migration of an Implanon® contraceptive rod: case report
Authors Berhe Y , Hagos G, Wall L
Received 28 March 2014
Accepted for publication 8 May 2014
Published 11 August 2014 Volume 2014:5 Pages 49—51
DOI https://doi.org/10.2147/OAJC.S65016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Yibrah Berhe,1 Girmay Hagos,2 L Lewis Wall1
1Department of Obstetrics and Gynecology, 2Department of Surgery, Ayder Referral Hospital, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
Abstract: Implanon®, a single-rod subdermal etonogestrel contraceptive implant, is usually found immediately adjacent to its insertion site. Although Implanon has been known to migrate, movement of the device is usually less than 2 cm from the site of insertion, and more distant migration has rarely been described. We report a patient whose Implanon was found near her left axilla over 12 cm proximal to the insertion scar. It was successfully removed without complications.
Keywords: Implanon, device migration, contraceptive complications
Case report
A 30-year-old primiparous woman had a spontaneous vaginal delivery of a 3,000 g live-born male infant with good perinatal outcome 3 years prior to presentation. Six weeks after delivery, in January 2011, she returned to her local health care institution requesting contraception. An Implanon® contraceptive rod (Merck and Co, Inc., Whitehouse Station, NJ, USA) was inserted into her left arm without apparent complications. She was amenorrheic after the insertion, but was reassured about the absence of menstruation. She reported that the Implanon had never been palpable after insertion, and she complained of pain in the upper arm. In January 2014, 3 years after the initial device insertion, she returned to her health care institution requesting removal of the Implanon, as she wished to become pregnant again. The health care providers at her local institution were not able to palpate the Implanon, and she was sent to Ayder Referral Hospital (College of Health Sciences, Mekelle, Ethiopia) for consultation.
At our institution, the device was not palpable in the expected location. The incision used for insertion of the contraceptive rod was clearly visible, and the location of the incision appeared to be appropriate. The patient complained of tenderness to palpation near her left axilla. The Implanon was subsequently identified in the left axillary region during an ultrasound examination using a linear array transducer.
The patient was taken to the operating room. After the successful induction of general anesthesia, the Implanon was palpable 12 cm above the site of insertion (Figure 1). The Implanon was removed through a linear incision and was located along the left anterior axillary line beneath the skin in the investing fascia of the brachialis muscle (Figure 2).
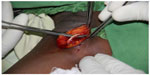  | Figure 2 Implanon® (Merck and Co, Inc., Whitehouse Station, NJ, USA) device in situ at the time of surgical removal at the axillary border. |
Discussion
Implanon is a long-acting contraceptive device that releases progestin slowly and continuously from a single, thin, nonbiodegradable rod, which is implanted subdermally.1 The rod is 40 mm in length and 2 mm in diameter and contains etonogestrel within an ethylene vinyl acetate copolymer membrane, which controls the rate of release. Implanon was approved by the US Food and Drug Administration for use in the US in 2006, and it is currently the most effective contraceptive available, with a yearly failure rate of 0.05% and a very low rate of complications.1 Millions of women around the world are using this form of long-acting reversible contraception.1 The most commonly reported complications of device insertion are malfunctions leading to retention of the implant in the insertion needle, bleeding, hematoma formation, and difficult implantation; complications associated with Implanon removal include implant breakage, difficulty locating the rod, and difficult device removal secondary to deep implantation or fixation of the implant to adjacent tissues.1
Significant migration of an Implanon is rare. A prospective study of 100 women who had an Implanon inserted found only small degrees of migration.2 Of the 87 patients seen 1 year after insertion, 39 showed no evidence of migration at all, while the remaining 48 patients showed only minor degrees of device migration. The vast majority of migrations (number =44) occurred caudally, with only four moving up the arm in a cranial direction. In only one case (cranial displacement) was movement over 2 cm from the insertion site noted.2 Evans et al3 reported significant movement of two Implanon rods in a cranial direction, 7.3 cm and 11.5 cm from the original implantation site in each patient. In both cases, the Implanon had been inserted through the same incision at the time of removal of a previously placed Norplant® (Hoechst Marion Roussel, Uxbridge, UK), a similar contraceptive system consisting of six individual rods that are inserted separately. The authors noted that Norplant removal using the “U” technique involves blunt dissection of the subcutaneous tissue, and they speculated that the tissue mobilization resulting at the surgical site during this procedure was probably the major contributory factor in the subsequent migration of the Implanon rods. They abandoned this technique in such cases in favor of inserting the new contraceptive system into the contralateral arm to prevent future migratory events.3 Prosch et al4 reported 21 cases of nonpalpable Implanon implants over 4 years in 2008. Only two cases involved substantial migration, both in the cranial direction (4 cm and 8 cm, respectively).4
We have no good explanation for the migration of the Implanon rod in this patient. The insertion took place postpartum and did not involve removal of a previously-inserted implantable contraceptive system. The scar indicates that implantation occurred in the appropriate location in her left arm; however, Implanon is usually inserted through a small puncture using the insertion needle, rather than the type of incision shown in Figure 1. It may be that whoever inserted the Implanon used an idiosyncratic insertion technique (such as that described by Evans et al3), and that this accounts for the unusual placement of the device in our case.
An implanted Implanon can usually be taken out through a small incision over the end of the rod, which is then grasped with a forceps and removed.1 However, in some cases, a dense fibrous sheath forms over the rod.1 Because this rod was in a most unusual location in the axilla, and because we were uncertain as to the other anatomic structures that might be involved in this particular case, the decision was made to remove the implant under general anesthesia in the operating room. At the time of surgical removal, the implant was not deep, but it was located in her axilla, over 12 cm from the insertion site. While faulty insertion technique cannot be ruled out in this case, it is difficult to see how the Implanon could have been inserted this close to the axilla even had a deliberate attempt been made to do so. We conclude that this is an exceptionally rare case of distant migration of an implantable contraceptive rod. Clinicians who are unable to locate a previously-implanted contraceptive device of this kind should keep the possibility of distant migration in mind, and investigate the case appropriately.5 It is likely that the improved technique for implantation of these contraceptive rods (marketed as Nexplanon® or Implanon NXT®; Merck and Co, Inc.), which involves a redesigned implantation cannula and a more “fool-proof” design, will eliminate complications such as the one described here.6 Clinicians who provide contraceptive services should, however, be aware that Implanon may on occasion show up in unexpected locations that are far removed from the site of insertion.
Disclosure
The authors report no conflicts of interest in this work.
References
Grentzer J, McNicholas C, Peipert JF. Use of the etonogestrel-releasing contraceptive implant. Expert Rev Obstet Gynecol. 2013;8(4):337–344. | |
Ismail H, Mansour D, Singh M. Migration of Implanon. J Fam Plann Reprod Health Care. 2006;32(3):157–159. | |
Evans R, Holman R, Lindsay E. Migration of Implanon: two case reports. J Fam Plann Reprod Health Care. 2005;31(1):71–72. | |
Prosch H, Walter RM, Westermayer V, Mostbeck GH. Sonografische Lokalisation nicht tastbarer Implanon®-Hormonimplantate. [Sonographic localization of non-palpable Implanon hormone implants]. Ultraschall Med. 2008;29 (Suppl 5):239–244. German. | |
Shulman LP, Gabriel H. Management and localization strategies for the nonpalpable Implanon rod. Contraception. 2006;73(4):325–330. | |
Mommers E, Blum GF, Gent TG, Peters KP, Sørdal TS, Marintcheva- Petrova M. Nexplanon, a radiopaque etonogestrel implant in combination with a next-generation applicator: 3-year results of a noncomparative multicenter trial. Am J Obstet Gynecol. 2012;207(5):388.e1–e6. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

