Back to Journals » Nature and Science of Sleep » Volume 11
Autonomic Arousals as Surrogates for Cortical Arousals Caused by Respiratory Events: A Methodological Optimization Study in the Diagnosis of Sleep Breathing Disorders
Authors Mayer P, Herrero Babiloni A , Aubé JL, Kaddaha Z, Marshansky S , Rompré PH, Jobin V, Lavigne GJ
Received 15 October 2019
Accepted for publication 4 December 2019
Published 19 December 2019 Volume 2019:11 Pages 423—431
DOI https://doi.org/10.2147/NSS.S234703
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven A Shea
Pierre Mayer,1 Alberto Herrero Babiloni,1–4 Jean-Louis Aubé,1 Zeina Kaddaha,1 Serguei Marshansky,1 Pierre H Rompré,3 Vincent Jobin,1 Gilles J Lavigne1–4
1Centre Hospitalier de l’Universite de Montreal (CHUM), Faculty of Medicine, University of Montreal, Montreal, QC, Canada; 2Research Center, Sacre-Coeur Hospital, Montreal University, Montreal, QC, Canada; 3Faculty of Dentistry, University of Montreal, Montreal, QC, Canada; 4Division of Experimental Medicine, McGill University, Montreal, QC, Canada
Correspondence: Alberto Herrero Babiloni
Hopital du Sacre-Coeur de Montreal, CEAMS, 5400 Boul Gouin O, Montreal QC H4J 1C5, Canada
Tel +514-338-2222
Fax +514-238-2531
Email [email protected]
Introduction: Portable monitoring (PM) is an alternative to laboratory polysomnography (PSG) for diagnosing obstructive sleep apnea (OSA). However, PM tends to underestimate the apnea-hypopnea index (AHI), as it does not identify non-desaturating events associated with electroencephalographic (EEG) arousal. The objectives were to explore heart rate acceleration (HRa) and decrease in pulse transit time (PTT) as surrogates to EEG arousal for non-desaturating hypopnea and respiratory effort-related arousal (RERA), and to estimate cut-off values for their use with both total sleep time (TST), the standard method for PSG, and total recording time (TRT), the usual method for PM.
Methods: Twenty-four consecutive individuals with suspected OSA were studied with PSG. Calculated outcomes were: AHI, respiratory disturbance index with EEG arousal (RDIe) and autonomic arousal by HRa (RDI-HRa) and PTT decreases (RDI-PTT) at different time cut-offs. Using RDIe as reference, Bland Altman and intraclass coefficient of correlation (ICC) were used to calculate agreement between indexes, and receiver operating curves (ROC) for sensitivity/specificity of the different cut-offs.
Results: Autonomic arousals, limited to respiratory events, were present in 36% of non-desaturating hypopneas and 29% of RERAs. Using TST, RDI-HRa of 10 bpm (ICC= 0.89) and RDI-PTT with a decrease of −15 msec (ICC=0.90) agreed better with RDIe. With TRT, the RDI-HRa of 5 bpm agreed better with the RDIe (ICC=0.89). Bland–Altman plots showed mean differences of 1.53 between RDI-HRa10-TST and RDIe and 0.89 between RDI-HRa5-TRT and RDIe.
Conclusion: Autonomic arousals (HRa and PTT) may be a suitable proxy of EEG arousals associated with respiratory events, using both TST and TRT. Therefore, they could potentially help to capture borderline symptomatic patients and to monitor treatment outcomes.
Keywords: sleep apnea, polysomnography, portable monitoring, autonomic nervous system, cardiovascular system, severity of illness index
Introduction
It is estimated that in North America, up to 15% of the general adult population have obstructive sleep apnea (OSA) and approximately 80% of individuals with moderate to severe OSA have not been clinically diagnosed.1,2 OSA can be associated with daytime somnolence, cognitive symptoms, hypertension, heart disease and stroke.3,4 Currently, to diagnose and categorize OSA according to its severity, the most common-utilized index is the apnea-hypopnea index (AHI). Yet, this index does not account for electroencephalography (EEG) arousals, which do disrupt sleep and contribute to sleep fragmentation.5 The Respiratory Disturbance Index (RDI) is another index that unlike AHI, also accounts for respiratory-effort related arousals (RERAs), which are arousals from sleep that do not technically meet the definitions of apneas or hypopneas.6 Nowadays, the gold standard technique for diagnosing OSA is in-laboratory polysomnography (PSG). However, due to the lack of broad accessibility and high cost of PSG, the American Academy of Sleep Medicine (AASM) recommends the use of unattended portable monitoring (PM) as an acceptable alternative for diagnosing OSA in the appropriate clinical settings.7
The use of PM implies the absence of EEG and electromyography (EMG) channels, thus being unable to identify EEG arousals. Therefore, PM focuses on analyzing desaturating respiratory events and consequently, an inaccurate magnitude of AHI may be obtained. Another potentially critical issue is that PM analyzes the number of events using the total recording time (TRT) as a denominator rather than the total sleep time (TST), which is the parameter employed while analyzing PSG data. Then, there is also possible that the severity of OSA can be miscalculated and that mild sleep apnea cases are omitted or under-identified. Such limitations may explain why PM is not routinely recommended in clinical practice when the probability of sleep breathing disorders is clinically low or questionable.
Past studies have shown that changes in autonomic variables during sleep can aid in the diagnosis of sleep breathing disorders.8 These markers, which are easily recognized and collected in PSG and PM, reflect the sympathetic tone variation and can contribute to identifying respiratory events with or without EEG arousal. Of these autonomic variables, pulse transit time (PTT) and heart rate acceleration (HRa) have shown promising results. PTT represents the vascular tone and measures indirectly the beat to beat pressure change,9 assessing the time from the R-wave on electrocardiogram (EKG) to the corresponding pulse shock wave at the finger. Thus, a respiratory event with arousal causes an augmentation in vascular tone, making the pulse wave travel faster and consequently reducing the pulse transit time. HRa is another variable that can be observed with an arousal or after an obstructive breathing event without arousal, and it has been shown that the increase in HR is strongly correlated with arousal duration.10,11 Some studies have already explored and compared HRa and/or PTT as possible surrogates for EEG arousal in healthy patients and also in sleep breathing disorders.12,13 One of those studies estimated that the values agreeing best with an EEG arousal were 10 bpm for HRa and a drop of 15 ms for PTT,12 with PTT presenting a slightly better correlation to EEG arousals than HRa. These findings support the use of autonomic arousals as possible surrogates to EEG recordings (absent in PM) thus potentially improving OSA’s diagnosis with PM and providing more insight about sleep disorders in PSG data. In fact, a very recent study investigated the use of HRa to improve the accuracy of PM using ≥6bpm as a cut-off, obtaining improved agreement between PSG and PM with the use of this autonomic marker.14 Although promising, these markers are still not standardized for clinical settings and an optimization of their methodology is necessary to generalize their clinical use.
Therefore, the overall goals of this methodological optimization study were to evaluate the potential use of HRa and PTT as possible surrogates to EEG arousal for non-desaturating hypopneas and RERAs, as well as to evaluate the use of different cut-off points to optimize their application, using both the TST (variable used with full laboratory PSG) and TRT (variable used with PM). More precisely, the study objectives were to i) investigate which HRa and PTT threshold best identifies respiratory events associated to an EEG arousal; ii) and to assess the correlation between RDIe (RDI calculated using EEG arousals) and the possible surrogate indexes RDI-HRa (RDI calculated using HRa) and RDI-PTT (RDI-HRa).
Methodology
Participants
Twenty-four individuals over 18 years of age referred for suspected OSA were investigated consecutively in a sleep laboratory in a tertiary hospital sleep center from June 2012 to October 2012. The CHUM ethics committee approved this study. All participants provided written informed consent, which was conducted in accordance with the Declaration of Helsinki.
Procedures
For in-laboratory polysomnography procedures, standard sleep variables were monitored: Electroencephalogram (EEG) recordings of C4/A1, C3/A2, O1/A2, FZ/A1; electrooculogram (EOG) for eye movements (R-EOG/A1, L-EOG/A1), electromyogram (EMG) for chin and right and left anterior tibialis, and electrocardiogram (EKG) for heart rate (bipolar). A body position sensor (Braebon Medical Corp, Canada) attached to a thoracic belt was used to monitor body position. Oxygen saturation was measured by pulse oximetry (OxiMax, Nellcor Puritan Bennett [Melville] Ltd, Canada). Tidal airflow was monitored with thermistor and a nasal/oronasal pressure cannula (low flow 0.03 Hz, and high flow 100 Hz, Braebon Medical Corp, Canada). Respiratory efforts were measured by respiratory-induced plethysmography belts placed around the thorax and abdomen. Participants were also monitored with a video camera and a microphone placed at the suprasternal notch to record snoring.
Analysis
PSGs were performed by trained sleep technicians with G3 Sleepware software (Philips, Respironics). The technicians were blinded to experimental conditions and scored sleep stages and respiratory events separately. The sleep stages were scored according to the AASM 2007 recommendations.15 EEG arousals during sleep stages N1, N2, N3 or REM were scored if there was an abrupt shift of EEG frequency including alpha, theta and/or frequencies greater than 16 Hz for at least 3 s, with at least 10 s of stable sleep preceding the change. To score arousals in REM sleep, a concurrent increase in submental EMG for at least 1 s was considered. EEG arousals included those following movement, breathing events or those that occur spontaneously. Other sleep variables included total sleep time, sleep latency (number of minutes from lights out until the first epoch scored as sleep), sleep efficiency (total sleep time as a percentage of time in bed), number of awakenings during sleep, and time spent in each sleep stage (non-rapid eye movement N1, N2, N3 and REM sleep). Respiratory events were scored manually according to the updated AASM 2012 recommendations.16 Events were scored as RERAs if there was a sequence of breaths lasting at least 10 s characterized by flattening of the inspiratory portion of the nasal pressure waveform leading to arousal from sleep, when the event did not meet criteria for an apnea or hypopnea. Events were scored as hypopneas based on a decrease of at least 30% of flow and they were subdivided according to the following individual criteria: desaturation ≥3% and/or the presence of EEG arousals and/or the presence of autonomic arousals.
AHITST, AHITRT (with desaturating events only) and RDIe (mixed apnea + central apnea + obstructive apnea + hypopnea-desaturated + hypopnea non-desaturated with EEG arousal + RERA with EEG arousal multiplied by 60 and divided by total sleep time) were calculated. HRa was scored as an increase of 5, 8, 10, 12, 15 and heart beats per minute (bpm) on EKG less than 20-s post-respiratory event. Then, RDI-HRa indexes were obtained for each of those cut-off points. PTT was defined as the time value between the highest peak of the R wave of the EKG and the 25% of the amplitude of the pulse wave, with an average value of 250 ms (± 2 ms),17 and it was measured by G3 Sleepware software (Philips Respironics). PTT was scored as a decrease of 5, 10, 15 and 20 msec. Then, RDI-PTT indexes were obtained for each of those cut-off points. These indexes were calculated using both TST and TRT. Therefore, RDI arousal with TST = mixed apnea + central apnea + obstructive apnea + hypopnea desaturated + hypopnea non-desaturated with EEG arousal + RERA with autonomic arousal multiplied by 60 and divided by total sleep time, while RDI arousal with TRT = mixed apnea + central apnea + obstructive apnea + hypopnea desaturated + hypopnea non-desaturated with EEG arousal + RERA with autonomic arousal multiplied by 60 and divided by total recoding time.
Statistical Analyses
A receiver operating characteristic curve (ROC) was employed to identify the HRa increase and PTT decrease that captured more non-desaturating hypopneas and RERAs associated with EEG arousals. Sensitivity and specificity were calculated for the different cut-off points using RDIe as a reference.
Bland–Altman (B-A) plots were used to assess the agreement between RDIe and the different cut-off points for both RDI-HRa and RDI-PTT. The limits of agreement were defined as the interval of the mean difference ± 1.96 x standard deviation (SD). Intraclass coefficients of correlation (ICC) to assess agreement between indexes, and Pearson R2 values were also produced to serve as comparisons. These analyses were done with SPSS statistical software version 23.
Results
The studied population constituted of 10 females and 14 males with a mean age of 48.4±15.6 (SD). None had diabetes or coronary artery disease, 20% had hypertension and 8.3% were using beta-blockers. Participants were mildly symptomatic with a mean Epworth Sleepiness Scale (EES) of 8.3 ± 6.1 (SD) (Table 1).
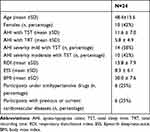 |
Table 1 Demographic and Clinical Characteristics of the Studied Sample |
A total of 2671 respiratory events were scored, of which 1762 events consisted of hypopnea and 561 RERAs. Of these respiratory events, 1341 hypopneas were non-desaturating, 714 presented with EEG arousal and 512 presented with an autonomous response. Therefore, autonomic arousals exclusive to respiratory events were observed in only 36% of non-desaturating hypopneas and 29% of RERAs (Table 2).
 |
Table 2 Description of the Scored Hypopneas and Respiratory Effort Related Arousals in Respect to Electroencephalogram Arousals from Twenty-Four Consecutive in-Laboratory Polysomnography Participants |
The mean respiratory indexes were AHITST 11.6 ± 7.0 (SD), AHITRT 5.8 ± 4.9 and RDIe 13.8 ± 7.9. The ICC correlation between AHITST and AHITRT was low at 0.47. All the desaturating hypopneas were excluded from our analysis on surrogate arousals (Table 3). Non-desaturating hypopneas and RERAs were subdivided into two categories: associated with EEG arousals, and associated with autonomic arousals, either by HRa or PTT, at different cut-offs previously described (Table 4). A heart rate acceleration of 10 bpm yielded to a mean (95% confidence interval) sensitivity of 74.3% (71.5–76.9) and a specificity of 41.3% (38.1–44.9), while less bpm yielded to higher sensitivity but lower specificity. A PTT decrease of −15 msec yielded to more balanced values, with higher sensitivity but lower specificity than −20 msec (Table 5) (Figure 1).
 |
Table 3 Total Number of Non-Desaturating Hypopneas and RERAs Associated with EEG Arousals and Autonomic Arousals for the Twenty-Four Consecutive in-Laboratory Polysomnography Participants |
 |
Table 5 Means and 95% Confidence Intervals Sensitivity and Specificity Values for the Different Heart Rate Acceleration (HRa) and Pulse Transit Time (PTT) Cut-Offs Calculated with Total Sleep Time |
 |
Figure 1 Sensitivity and specificity calculated for the different heart rate acceleration (HRa) and pulse transit time (PTT) cut-offs calculated with total sleep time. |
When using TST, it was found that RDI-HRa calculated with 10 bpm as a cut-off point correlated the best with RDIe with an ICC of 0.89. A similar ICC, 0.90, was present with RDI-PTT of –15msec (Table 6). Moreover, the Bland–Altman plot analysis showed a mean difference of only 1.53 between RDI-HRa10-TST and RDIe (Figure 2).
 |
Table 6 Bland–Altman Analyses Comparing Different Cut-Offs for Autonomic Arousals to RDIe |
 |
Figure 2 Bland–Altman plot demonstrating the mean difference between RDI-HRa10 vs RDIe in total sleep time (top) and RDI-HRa5 vs RDIe in total recorded time (bottom). |
When using TRT, a HRa rise of 5 bpm in TRT correlated the best with RDIe with an ICC of 0.89 (Table 6). The mean difference showed by Bland–Altman plots was only 0.89 between RDI-HRa5-TRT and RDIe (Figure 2). Levels of agreement and correlation for RDI-PTT were poor.
Discussion
The results of this study suggest that in a mild to moderate OSA population, HRa increase and PTT decrease can be potentially considered non-invasive surrogates for EEG arousals associated with non-desaturating hypopnea and RERAs scored in PSG. From our analyses, it appears that using the thresholds of 10 bpm for HRa and −15 msec for PTT yielded to better agreement with RDIe for in-laboratory PSG using TST as a denominator. When using TRT as a proxy to PM, an HRa threshold of 5 bpm agrees better with the RDI found on PSG with AASM standards, with an ICC of 0.89. Finally, a poor AHI correlation between TST and TRT was observed, confirming the role of the denominator (TRT) for the underestimation of AHI in PM.
For the diagnosis of OSA, unattended PM are progressively becoming more popular in everyday practice because of their accessibility and low cost compared to in-laboratory polysomnography for the diagnosis of obstructive sleep apnea. For example, scoring an in-laboratory PSG for EEG arousals can take a minimum of 2 hrs per patient. It is tedious, subjective and requires trained technicians.18 For that reason, PM is currently indicated for patients with high probability of having moderate to severe obstructive sleep apnea.7 However, one of its main limitations is the inability to identify events scored by EEG arousal, which leads to the underestimation of the sleep breathing disorder severity. Furthermore, the use of AHI, which is used to categorize the severity of OSA in PM, does not take into account RERAs.19 As it was observed in the present study, in patients with suspected mild to moderate OSA, the AHI based on desaturation criteria only calculated with TRT was 48% inferior in comparison to the AHI identified on TST based on AASM 2012 criteria (it is important to consider that we are using TRT as a proxy or an estimation of their application in PM devices, thus existing the potential of discordance with the actual test), in accordance with other results in the literature.14 Therefore, autonomic arousals, especially the ones that are characterized by HRa, are worth to explore due to their simple obtention and their accessibility in PM. Although PTT appears to be an interesting marker as well, adaptation of current PM is needed due to the necessity of a specific software and the 3 EKG derivation required to measure the time decrease.
In the study performed by Lachapelle et al, a cut-off point of ≥6 bpm for HRa yielded to a better diagnostic accuracy of PM when compared to PSG in patients with mild to moderate OSA.14 From our analyses, it seems that for in-laboratory PSG (using TST as denominator) the cut-off point 10 bpm for HRa and −15 msec for PTT presented better agreement with the reference RDIe, while using TRT as a proxy for PM, 5 bpm for HRa agreed better with RDIe, in agreement with the previously mentioned study.14 Although further validation of these cut-off points are warranted with larger samples, these results suppose initial evidence in favor of the utilization of these thresholds in research and eventually in clinical settings. Another interesting finding in this study is that 36% of non-desaturating hypopneas and 29% of RERAs identified were associated with an autonomic arousal but not an EEG arousal (Table 2). Thus, by the present standard, these respiratory events that are accompanied by physiological consequences would be ignored by the scoring of in-laboratory PSG. The clinical implication of these “orphan” respiratory events is yet to be determined. It is suggested that repetitive post-event tachycardia may contribute to the cardiovascular morbidity in obstructive sleep apnea.10 This reasoning leads toward the need of further investigations assessing the relevance of respiratory events associated with EEG arousals in contrast to those associated with autonomic arousals, and to evaluate which one has more the strongest clinical importance in relation to the cardiovascular risk on morbidity and mortality.
In addition to HRa and PTT, other autonomic arousal markers have been explored. An example is the use of pulse wave amplitude (PWA) drop, which was also tested as a surrogate to score EEG arousal events on PM.20 In one study, 312 subjects with all the different degrees of OSA severity were analyzed, and it was concluded that the addition of PWA drops to the definition of hypopnea as a surrogate for EEG arousal could provide a more comprehensive diagnosis of OSA. Similarly, another study considered hyperventilation following flow reduction as another potential surrogate for EEG arousals.21 Nevertheless, the authors of this study concluded that in severe OSA, the use of this marker as a surrogate did not present an additional value to OSA diagnosis with PSG nor PM. Our study showed the potential benefit of using HRa and PTT autonomic arousals in mild to moderate OSA, but future research could benefit from exploring the impact of different autonomic arousals in OSA classification and severity.
This study has obvious limitations. We recognize that the modest sample size of our study may have underpowered and limited our results. For instance, sub-analysis based on age, sex, medication intake, and comorbidities were not planned due to the small sample size. Moreover, the use of autonomic markers has also some limitations, as it can be influenced by the cardiovascular system disorders. In fact, beta-blockers were being taken by 8% of our population, which could have influenced our results.
Conclusion
The results of this methodological optimization study highlight the possible use of autonomic arousals as surrogates for EEG arousals. Such variables may be potentially used for complementing current PSG scoring and/or capturing mild OSA or borderline symptomatic cases in patients evaluated with PM. Larger sample size studies are necessary to confirm the clinical applicability and relevance of these findings.
Abbreviations
AASM, American Association Sleep Medicine; AHI, apnea-hypopnea index; EEG, electroencephalography; EKG, electrocardiogram; EOG, electrooculogram; EES, Epworth Sleepiness Scale; HRa, heart rate acceleration; ICC, iIntraclass coefficient of correlation; OSA, obstructive sleep apnea; RDIe, respiratory disturbance index with EEG arousal; RDI-HRa, respiratory disturbance index with autonomic arousal by heart rate acceleration; RDI-PTT, respiratory disturbance index with autonomic arousal by pulse transit time; RERA, respiratory effort-related arousal; ROC, receiver operating curves; PM, portable monitors; PTT, pulse transit time; PSG, Polysomnography; PWA, pulse wave amplitude; SD, standard deviation; TRT, total recording time; TST, total sleep time.
Acknowledgment
The authors would like to thank Lauren Kvasnicka, MPH, for her contribution and assistance in the development of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi:10.1093/sleep/20.9.705
2. Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108(5):246–249.
3. Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7(2):161–166. doi:10.1007/s11910-007-0012-8
4. Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi:10.1378/chest.127.6.2076
5. Bonnet MH, Doghramji K, Roehrs T, et al. The scoring of arousal in sleep: reliability, validity, and alternatives. J Clin Sleep Med. 2007;3(2):133–145.
6. Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159(2):502–507. doi:10.1164/ajrccm.159.2.9804051
7. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747.
8. Pepin JL, Tamisier R, Borel JC, Baguet JP, Levy P. A critical review of peripheral arterial tone and pulse transit time as indirect diagnostic methods for detecting sleep disordered breathing and characterizing sleep structure. Curr Opin Pulm Med. 2009;15(6):550–558. doi:10.1097/MCP.0b013e3283318585
9. Gribbin B, Steptoe A, Sleight P. Pulse wave velocity as a measure of blood pressure change. Psychophysiology. 1976;13(1):86–90. doi:10.1111/psyp.1976.13.issue-1
10. Azarbarzin A, Ostrowski M, Moussavi Z, Hanly P, Younes M. Contribution of arousal from sleep to postevent tachycardia in patients with obstructive sleep apnea. Sleep. 2013;36(6):881–889. doi:10.5665/sleep.2716
11. Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014;37(4):645–653. doi:10.5665/sleep.3560
12. Pitson DJ, Stradling JR. Autonomic markers of arousal during sleep in patients undergoing investigation for obstructive sleep apnoea, their relationship to EEG arousals, respiratory events and subjective sleepiness. J Sleep Res. 1998;7(1):53–59. doi:10.1046/j.1365-2869.1998.00092.x
13. Chakrabarti B, Emegbo S, Craig S, Duffy N, O’Reilly J. Pulse transit time changes in subjects exhibiting sleep disordered breathing. Respir Med. 2017;122:18–22. doi:10.1016/j.rmed.2016.11.014
14. Lachapelle P, Cascon J, Pamidi S, Kimoff RJ. Accuracy of portable devices in sleep apnea using oximetry-derived heart rate increases as a surrogate arousal marker. Sleep Breath. 2019;23(2):483–492. doi:10.1007/s11325-018-1708-5
15. Ruehland WR, O’Donoghue FJ, Pierce RJ, et al. The 2007 AASM recommendations for EEG electrode placement in polysomnography: impact on sleep and cortical arousal scoring. Sleep. 2011;34(1):73–81. doi:10.1093/sleep/34.1.73
16. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep Apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi:10.5664/jcsm.2172
17. Pitson D, Chhina N, Knijn S, van Herwaaden M, Stradling J. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects. Clin Sci. 1994;87(2):269–273. doi:10.1042/cs0870269
18. Drinnan MJ, Murray A, Griffiths CJ, Gibson GJ. Interobserver variability in recognizing arousal in respiratory sleep disorders. Am J Respir Crit Care Med. 1998;158(2):358–362. doi:10.1164/ajrccm.158.2.9705035
19. Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781–787. doi:10.1378/chest.104.3.781
20. Vat S, Haba-Rubio J, Tafti M, Tobback N, Andries D, Heinzer R. Scoring criteria for portable monitor recordings: a comparison of four hypopnoea definitions in a population-based cohort. Thorax. 2015;70(11):1047–1053. doi:10.1136/thoraxjnl-2014-205982
21. Masa JF, Corral J, Gomez de Terreros J, et al. Significance of including a surrogate arousal for sleep apnea-hypopnea syndrome diagnosis by respiratory polygraphy. Sleep. 2013;36(2):249–257. doi:10.5665/sleep.2384
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

