Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Autologous Infusion of Bone Marrow and Mesenchymal Stromal Cells in Patients with Chronic Obstructive Pulmonary Disease: Phase I Randomized Clinical Trial
Authors Squassoni SD, Sekiya EJ, Fiss E, Lapa MS, Cayetano DS, Nascimento F , Alves A, Machado NC, Escaramboni B , Lívero FAR , Malagutti-Ferreira MJ, Soares MR , dos Santos Figueiredo FW, Kramer BKN, Zago PMJJ, Ribeiro-Paes JT
Received 21 August 2021
Accepted for publication 6 December 2021
Published 29 December 2021 Volume 2021:16 Pages 3561—3574
DOI https://doi.org/10.2147/COPD.S332613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Selma Denis Squassoni, 1 Eliseo Joji Sekiya, 2,* Elie Fiss, 1, 3 Monica Silveira Lapa, 1 Daniela dos Santos Cayetano, 2 Flávia Nascimento, 2 Adelson Alves, 2 Nadine Cristina Machado, 1 Bruna Escaramboni, 4 Francislaine Aparecida dos Reis Lívero, 5 Maria José Malagutti-Ferreira, 4 Murilo Racy Soares, 6 Francisco Winter dos Santos Figueiredo, 1 Beatriz Kimberly Nath Kramer, 4 Priscila Megda João Job Zago, 5 João Tadeu Ribeiro-Paes 4,*
1ABC Medical School, São Paulo, SP, Brazil; 2São Lucas Research and Education Institute (IEP-Sao Lucas), TechLife, São Paulo, SP, Brazil; 3Hospital Alemão Oswaldo Cruz, São Paulo, SP, Brazil; 4São Paulo State University (UNESP), Assis, SP, Brazil; 5Paranaense University (UNIPAR), Umuarama, PR, Brazil; 6Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, University of São Paulo (USP), Ribeirão Preto, SP, Brazil
*These authors contributed equally to this work
Correspondence: João Tadeu Ribeiro-Paes
Department of Biotechnology, São Paulo State University - UNESP, Avenida Dom Antônio, 2100, Assis, SP, 19806-900, Brazil
Tel +55 11 986453163
Email [email protected]
Eliseo Joji Sekiya
IEP-São Lucas - TechLife, Avenida Arnolfo de Azevedo, 108 - Pacaembu, São Paulo, SP, 01236-030, Brazil
Tel +55 11 99293-8826
Email [email protected]
Background and Objectives: Chronic obstructive pulmonary disease (COPD) is characterized by the destruction of alveolar walls, chronic inflammation and persistent respiratory symptoms. There is no curative clinical treatment for COPD. In this context, cell-based therapy is a promising therapeutic alternative for COPD. Thus, in this open, controlled and randomized Phase I Clinical Trial, we aimed to assess the safety of the infusion of autologous bone marrow mononuclear cells (BMMC), adipose-derived mesenchymal stromal cells (ADSC) and, especially, the safety of concomitant infusion (co-infusion) of BMMC and ADSC as a new therapeutic alternative for COPD. The rationale for co-infusion of BMMC and ADSC is based on the hypothesis of an additive or synergistic therapeutic effect resulting from this association.
Methods: To achieve the proposed objectives, twenty patients with moderate-to-severe COPD were randomly divided into four groups: control group – patients receiving conventional treatment; BMMC group – patients receiving only BMMC; ADSC group – patients receiving only ADSC, and co-infusion group – patients receiving the concomitant infusion of BMMC and ADSC. Patients were assessed for pulmonary function, biochemical profile, and quality of life over a 12 months follow-up.
Results: No adverse events were detected immediately after the infusion of BMMC, ADSC or co-infusion. In the 12-month follow-up, no causal relationship was established between adverse events and cell therapy procedures. Regarding the efficacy, the BMMC group showed an increase in forced expiratory volume (FEV1) and diffusing capacity for carbon monoxide (DLCO). Co-infusion group showed a DLCO, and gas exchange improvement and a better quality of life.
Conclusion: The results obtained allow us to conclude that cell-based therapy with co-infusion of BMMC and ADSC is a safe procedure and a promising therapeutic for COPD. However, additional studies with a greater number of patients are needed before randomized and controlled Phase III clinical trials can be implemented.
Keywords: COPD, cell therapy, stem cells, mesenchymal stem cells, concomitant infusion, co-infusion
Graphical Abstract:
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable disease characterized by persistent respiratory symptoms and airflow limitation due to airway and/or alveolar abnormalities. These abnormalities are usually progressive and associated with an enhanced chronic inflammatory response in the airways and lung, generally caused by exposure to noxious particles and gases.1 From the epidemiological viewpoint, COPD represents a serious public health problem and a major therapeutic challenge for pulmonologists and general practitioners.2–4
Despite significant advances in new therapeutic and rehabilitation approaches, there is, to date, no curative clinical treatment for COPD/emphysema. In this scenario, cell-based therapies with bone marrow mononuclear cells (BMMC) or mesenchymal stromal/stem cells (MSC) are promising therapeutic approaches for COPD. Thus, a number of studies with cell-based therapies in preclinical experimental models of COPD have been published, as widely discussed in several comprehensive and consistent reviews.5–22
Cell-based therapy in animal models supported the development of clinical trials for COPD/emphysema. The first clinical trial was published by Ribeiro-Paes et al.23 In this pioneering study, a pool of BMMC-hematopoietic stem cells was infused immediately after bone marrow aspiration from the iliac crest. No directly related adverse events were recorded during a follow-up of 12 months (Clinical Trials NCT 01110252). Complementing this study, Stessuk et al2 have performed a 3-year clinical follow-up of the same patients. Moreover, in 2013, Weiss et al24 reported a placebo-controlled randomized trial, sponsored by Osiris Therapeutics Inc. (Columbia, MD, USA). In this study, 62 patients received repeated MSC infusions and a 2-year follow-up (Clinical Trials NCT 00683722). Stolk et al25 reported an interesting Phase I, prospective, open-label study for autologous MSC infusion in patients with severe emphysema (ClinicalTrials NCT 01306513). Next, Oliveira et al26 have published an innovative Phase I, prospective, patient-blinded, randomized, placebo-controlled clinical trial (ClinicalTrials NCT 04018729) in which one-way endobronchial valve insertion (EBV) was associated with the infusion of MSC. Recently, Le Thi Bich et al27 have published a pilot clinical study using allogenic, umbilical cord-derived mesenchymal cells (US-MSC) to treat patients with moderate and severe COPD and, next, another group of researchers from Vietnam28 published a Phase I/II study (ClinicalTrials NCT04433104) to evaluate the safety and efficacy of UC-MSC in patients with moderate-to-severe COPD.
Morphological regeneration of the lung parenchyma in preclinical animal models and improvement of pulmonary function parameters in response to BMMC or MSC-based therapy in patients with COPD has been attributed to a local paracrine effect that results from the modulation of the inflammatory response and release of cytokines that can stimulate tissue regeneration.11,14,16,17,19,20,29–40 However, there is no previous report in the literature on the use of concomitant infusion (co-infusion) of BMMC and ADSC as a therapeutic alternative in COPD. Therefore, it is important to evaluate the safety of the procedure, as well as the potential efficacy resulting from the association of these two cell types and possibly other associations such as BMMC and umbilical cord stromal cells (UC-MSC).
Based on these findings and considering the properties and mechanisms of action inherent to BMMC and MSC, we hypothesized that the concomitant infusion (co-infusion) of BMMC and MSC would result in an additive or synergistic effect of neoangiogenesis stimulation and a decrease in the inflammatory process, which could improve lung function or, at least, delay disease progression. Thus, the goal of this study was to test the safety of the procedure as a primary endpoint, and secondarily, the efficacy resulting from the co-infusion of BMMC and MSC in patients with moderate-to-severe COPD.
Methods
Study Design and Sampling
This was a randomized, open-controlled Phase I clinical trial with twenty patients enrolled at the pulmonology outpatient clinic of Faculdade de Medicina ABC (ABC Medical School – Brazil) and Instituto Chico Anysio (Rio de Janeiro – Brazil). The sample consisted of 20 patients with Grade 3-COPD according to the GOLD criterion.1 The detailed protocol for randomization and masking is described in Supplementary Data S1.
The study protocol was conducted in accordance with the Declaration of Helsinki, approved by the National Commission of Ethics in Research (CONEP – Brazil) registered with code CAAE 303490140.0000.0082 and registered at ClinicalTrials.gov with code NCT 02412332. Patients who fit the inclusion criteria were invited to participate in the study and sign the Informed Consent Form (ICF). The inclusion and follow-up of patients in the study occurred from 07/10/2015 to 06/12/2017, and it should be noted that, due to the inclusion and exclusion criteria adopted, to select the 20 patients included in the study, it was necessary to perform a clinical and laboratory evaluation of 140 patients.
Patients Inclusion and Exclusion Criteria
The inclusion criteria were: age 40 ≤ 70 years, optimized treatment for COPD grade 3, forced expiratory volume in the first second (FEV1) 30% ≤ 50% of predicted, and smoking cessation for at least six months.
The exclusion criteria were: absence of emphysema on chest tomography; active pulmonary or extrapulmonary infection or history of infection <3 months ago; previous history of coronary disease and/or severe ventricular dysfunction (ejection fraction < 55%); presence of pulmonary hypertension; domiciliary oxygen therapy, advanced renal failure (serum creatinine greater than 1.5 mg/dL) and liver failure CHILD B or C; immunosuppressive or infectious diseases detected; patients with known malignancies or collagen diseases; psycho-social problems, drug and/or alcohol abuse and not obedient to the established medical protocol; no family acceptance; pregnancy or at risk of pregnancy.
Randomization and Masking
Twenty patients with grade 3 COPD according to the GOLD criterion were allocated to four groups with five individuals in each group. In order to avoid selection bias, the study participants underwent a random allocation process performed by independent researchers who did not participate in the study. The detailed protocol for randomization and masking is described in Supplementary Data S1.
Clinical Study Groups
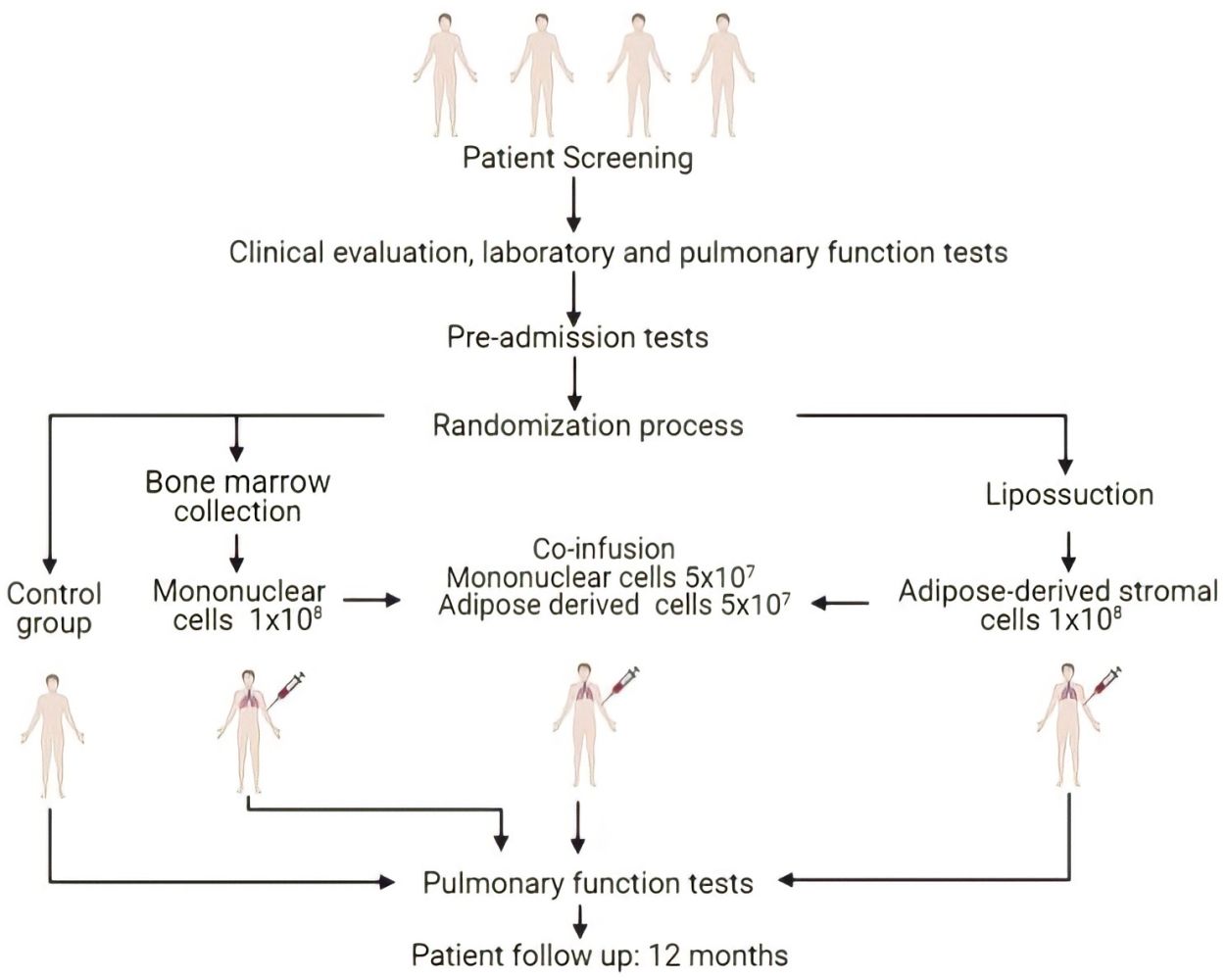
The patients were divided into 4 distinct groups defined by randomized draw (with 5 individuals in each group): control Group – patients maintained conventional clinical treatment for COPD; BMMC Group – patients received a pool of bone marrow mononuclear cell (BMMC); ADSC Group – patients received infusion of adipose-derived mesenchymal stromal/stem cells (ADSC) and in the co-infusion Group – patients received concomitant infusion (co-infusion) of ADSC and BMMC (Figure 1). The infusion of BMMC, ADSC and the co-infusion was performed using the middle brachial vein. The total number of cells per infusion was 1 × 108, and in patients who received the co-infusion, 5 × 107 of each cell type was infused. None of the patients were in use of noninvasive mechanical ventilation. All the patients were currently in use of optimized Long Acting Muscarinic Antagonist (LAMA) + Long Acting B2 Adrenergic Agonists (LABA) and inhaled corticosteroids. Only two patients were in a pulmonary rehabilitation program.
 |
Figure 1 Schematic representation of sample selection and experimental design. |
The primary outcome of this study was the safety assessment of the intervention procedures, from collection to 12-month follow-up after cell infusion. Therefore, the parameters evaluated were cardiopulmonary tests, dyspnea, liver and kidney function, tumor activity markers, and hematological alterations in the peripheral blood. These and other parameters evaluated over the 12-month follow-up of patients are detailed in Supplementary Table 1.
Isolation of Bone Marrow Mononuclear Cells (BMMC)
BMMC collections were performed according to Thomas and Storb41 with minor modifications, under sedation and local anesthesia, with punctures in the posterior iliac crest; the marrow was aspirated using 20-mL syringes heparinized with 250 U/mL heparin (Liquemin; Hoffmann LaRoche, GrenzachWyhlen, Germany), totaling 120 mL. The separation of mononuclear cells was performed by automated processing using the SEPAX 2 S-100 Cell Processing System (GE-Healthcare-Chicago-Illinois, USA) in a closed system, using the disposable kit SEPAX CS900, Ficoll-Paque, USA Premium reagent (1.077 g/mL, GE Healthcare), and washing solution composed of human albumin (Alburex 20 – CSL Behring Pennsylvania, USA) diluted to 5% in saline solution (Beker, São Paulo, Brazil). At the end of automated processing, a 0.5 mL sample of the buffy coat was separated for cell counting, viability tests, flow cytometry immunophenotyping, and blood culture, as described by Aktas et al.42
Isolation and Proliferation of Adipose-Derived Mesenchymal Stromal Cells (ADSC)
The method of obtaining adipose tissue was liposuction by a plastic surgeon. The isolation and proliferation ADSC were performed in the laboratory under GMP conditions, according to the procedure described by Zuk et al.43
The adipose tissue was extensively washed with DPBS (Gibco, New York, USA) until complete removal of the blood present in the material. A 0.075% collagenase solution was added. The enzyme digested product was centrifuged (400g for 10 minutes) and the pellet obtained was resuspended in low glucose DMEM (Gibco, New York, USA) plus 10% fetal bovine serum (HyClone, Utah, USA), Normocin (InvivoGen, Toulouse, France) and antibiotic-antimycotic (Gibco, New York, USA). The cells were seeded in culture flasks for initial cultivation and kept in a controlled environment at 37° C and 5% CO2. The culture medium was changed every 3–4 days until reaching 80% confluence, with trypsinization and cell counting at each passage until reaching a cell concentration of 1 × 108 cells, in a maximum of 3 passages. Samples of cultured cells were tested to negative microbiological control, viability, cell dosage, characterization by flow cytometry, cell differentiation in vitro and cytogenetic analysis as Quality Control Tests before delivery for administration to the patients.
Phenotype Characterization and Viability of BMMC and ADSC
Immunophenotyping of ADSC and BMMC was performed by flow cytometry analysis in a FACSCalibur 4-color cytometer (Becton Dickinson, NJ, USA). Samples of the ADSC population were evaluated, from isolation to third or fourth passage using the monoclonal antibodies cluster of differentiation CD73, CD90, CD34, CD45 (Becton Dickinson), CD105, HLA-DR, CD19 (Biolegend, San Diego, CA, USA), and CD11b (Exbio, Vestec, Czech Republic). The BMMCs were evaluated using the same equipment (FACSCalibur) and the markers CD34 and CD45 (Becton Dickinson). Cell counting and viability were measured by trypan blue exclusion assay using a Neubauer chamber.
Cellular Differentiation and Cytogenetic Analysis of ADSC
Cellular differentiation into osteogenic, chondrogenic and adipogenic lineages was induced in vitro using specific kits StemPro (Gibco, New York, USA), according to the manufacturer’s instructions. Osteogenic differentiation was confirmed by Alizarin Red staining, chondrogenic differentiation with Alcian Blue and adipogenic differentiation with Oil Red O. All dyes were from Sigma Aldrich, St. Louis, MO, USA. Cytogenetic analysis was performed from the second or third passage, by G-banding technique, as described by Borgonovo et al.44 Only cultured cells that showed normal results after evaluation of at least 20 metaphases per fixed material were used.
Laboratory and Pulmonary Function Tests
Laboratory tests and lung function exams were performed following the methodologies standardized by the FMABC (Brazil). Clinical parameters were evaluated pre-procedure and during the clinical follow-up of patients. The patients underwent hematology, biochemistry, serology, kidney and liver function, tumor marker evaluation, and radiological exams. The control and treated groups were evaluated periodically for 12 months (Supplementary Table 1).
Pulmonary Function Tests
Whole body spirometry and plethysmography were performed with constant volume and variable pressure on a Medgraphics Plethysmography (Elite Series Corporation Washington, DC, USA). Evaluated parameters were as follows: total lung capacity (TLC); forced vital capacity (FVC); FEV1; FEV1/FVC ratio; residual volume (RV); RV/TLC; airway resistance (Raw); diffusion lung capacity for carbon monoxide (DLCO); alveolar volume (AV); DLCO/VA; and inspiratory capacity (IC). Results have been evaluated using the reference for the Brazilian population.45
Cardiopulmonary Test
An incremental test (Cardiopulmonary Testing) was performed on a treadmill coupled to an electrocardiograph (Micromed, São Paulo, Brazil), with an initial speed of 3.0 km/h, inclination of 1% and increment of 0.5 km/h every minute. The parameter used was oxygen consumption at the peak of exercise (VO2 peak).
Six-Minute Walk Test (6MWT)
The 6MWT was conducted according to the guidelines proposed by the American Thoracic Society– ATS Statement: Guidelines for the Six-Minute Walk Test as described by Sciurba et al.46
Quality of Life Measure
The quality of life questionnaire (QLQ) was measured by the Saint George’s Respiratory Questionnaire (SGRQ) that is specific and widely used for obstructive respiratory diseases and was initially validated in Brazil in 2000.47 At every visit were asked to complete a QLQ SGRQ, this questionnaire provides score to evaluate three domains: symptoms, physical activity, and the psychosocial impact of the respiratory disease, aiming at better detection of clinical improvements. The responses were evaluated for the total questionnaire and for each of its domains considering that a 4% reduction or a 10% reduction in each domain between visits was a significant improvement. The patient progression in terms of QL was calculated relatively to the preceding evaluation score, as described above for the 6MWT.
Statistical Analysis
Qualitative characteristics were described by scores and presented as the median and 25–75% of the score range limits. Data were also shown by values relative to their basal ones before treatment: variation (Delta-Δ%) of pulmonary function, gas exchange, 6MWT, QLQ and cardiopulmonary test evaluated over 12 months follow-up for each group, compared to the baseline data. Quantitative characteristics are described by medians and their respective confidence intervals (95% CI). When applicable, Stata 11.0 (Stata Corp., College Station, TX, USA) was used for analysis. The data obtained after application of the SGRQ were analyzed using either the overall score or the scores at each questionnaire domain.
Results
Based on the inclusion and exclusion criteria adopted in this study, it was necessary to evaluate a large number of patients. For the composition of a sample of 20 patients, a total of 140 patients who volunteered for this study were evaluated clinically and by laboratory tests (Figure 1; Supplementary Table 1). Hypothesis tests or error bars were not included in the figures due to the size of the studied sample. As this was an initial study with only five participants per group, estimation confidence intervals or error bars were not estimated so as not to induce readers to inadequately extrapolate the results. In addition, one of the hypothesis tests was not performed due to the risk of finding false results in type I or even type II error.
The results of phenotypic characterization of the ADSC of each patient presented high viability levels (mean 96.22 ± 1.97) with high concentrations (>97%) for the CD73/CD90 (99.40% ± 0.38), CD73/CD105 (98.48% ± 1.08), and CD90/CD105 (98.55% ± 0.97) markers. A low proportion of hematopoietic stem cell markers was observed by the occurrence (<2%) of cells labeled by CD34 (1.13% ± 0.48), CD45 (1.62% ± 1.08), CD11b (0.07% ± 0.05), CD19 (0.02 ± 0.03), and HLA-DR (0.03 ± 0.04). These results were repeatedly obtained in all cultures of the 10 patients receiving ADSC infusion alone or in co-infusion with the BMMC pool. BMMC showed a mean viability of 97.56% ± 1.22, with a mean mononuclear cell concentration of 33.39% ± 13.85 and a mean cell concentration of 1.16% ± 0.70 for CD34 and CD45 positive markers (Figure 2A; Supplementary Figure 1; Supplementary Table 2). After isolation of the BMMC pool by the automated method, as described in material and methods, a 0.5 mL sample was taken for analysis of different parameters. The results indicated cell viability above 97% and the proportion of mononuclear cells ranged from 10.92% to 53.53% (Supplementary Table 3). Furthermore, all samples from the BMMC pool had negative results for microbiological tests.
As additional criteria for the analysis and validation of ADSC, differentiation tests were performed. Osteogenic, adipogenic and chondrogenic differentiation were obtained (Figure 2B) for all cell culture samples in all groups that received ADSC (alone or with co-infusion), confirming the specificity criteria for validating the mesenchymal nature of cells isolated from adipose tissue of patients in the ADSC-treated groups, as proposed by the International Society for Cellular Therapy – ISCT.48 Again, in addition to these results of validation and characterization of the ADSC, cytogenetic analysis was performed using the G-banding technique as one of the criteria for verifying any possible effect of the genetic instability on the cells kept in cultivation, until the third passage. No numerical or structural chromosomal aberrations were detected in all metaphases analyzed, according to the images of the karyotypes presented in Figure 2C.
Patients did not have any complications during the collection of bone marrow and adipose tissue. Only local discrete pain and hematoma were noted as side effects. ADSC and BMMC infusions, isolated or co-infusion, were not associated with any side effects or complications. During the 12-month follow-up period, patients were evaluated clinically and in the laboratory for different parameters in the first 7 days, and after 1, 3, 6, 9 and 12 months (Supplementary Table 1). During the follow-up period, no noteworthy clinical complications were detected in relation to liver, kidney and blood count, as well as significant changes in biochemical parameters. Clinical data showed no changes in mMRC (in other words, no changes in dyspnea). Inflammatory markers such as IL-6, IL-1 did not show any changes among the groups. There was a decrease in TNF-α levels in the co-infusion group (p = 0.045) after 6 months.
Complaints of the patients were restricted to pulmonary function. Signs and symptoms indicative of COPD exacerbation were eventually observed in all groups (control and treatment).
The BMMC group was the only one that showed an increase in FEV1 over 12 months of follow-up (Figure 3A, Supplementary Table 4). The control and co-infusion groups maintained values similar to baseline data, with a more expressive decrease of FEV1 for the ADSC group. In Figure 3B, which shows the result of FEV1/FVC (%), it can verify that all groups showed a pronounced decrease in FEV/FVC (%) in the period from 0 to 3 months. The BMMC group, however, showed improvement in lung function for this parameter in the interval between 3 and 12 months (Figure 3B, Supplementary Table 5). With regard to TLC (Figure 3C, Supplementary Tables 6 and 13) all groups maintained similar values in relation to baseline data (time 0), with a small increase in TLC for the BMMC group. For RV, the control, BMMC, and co-infusion groups maintained values between 180 and 200%, remaining close to baseline data (Figure 3D, Supplementary Tables 7 and 13). The BMMC group showed a slight increase (190–200) in relation to the baseline value. In the DLCO analysis (Figure 3E, Supplementary Tables 9 and 13) it appears that the BMMC group showed continuous improvement over 12 months of follow-up, which was more expressive in the period from 0 to 3 months. There was also a significant improvement in the co-infusion group over 12 months of follow-up. Regarding the results for pCO2(Figure 3F, Supplementary Table 11) it can verify that the different groups showed little variation relatively to the baseline data, remaining for 12 months of follow-up in the range of 35 to 39 mmHg. The BMMC group showed a decrease below 35 mmHg at the end of 3 months of follow-up, returning to the normal baseline value of 35 mmHg at the end of 12 months (Figure 2F and Supplementary Table 11).
Control group presented an increase in TLC only after the first 3 months of follow-up. RV increased in this group between 6 and 12 months, as well as RV/TLC; there was no difference in the other groups. IC decreased in the control patients in the first 3 months (which showed an increase in hyperinflation when associated with an increase in TLC, RV and TLC/RV). Raw was higher after 6 months of follow-up in the control group and remained stable. These data allowed the inference that the control group showed a worsening of lung capacity due to increased hyperinflation and increased airway resistance in the first few months (Supplementary Tables 6 and 8).
In summary, the control group had increased pulmonary hyperinflation and alveolar exchange. The BMMC group showed increased FEV1, without alteration of TLC and IC, and increased DLCO and VA. The ADSC group showed decreased hyperinflation due to increased IC and VA. In the co-infusion group, there was decreased hyperinflation and TLC, with increased IC and alveolar exchange (Supplementary Tables 4, 6, 9, 11 and 13). The co-infusion group showed more evident results up to 6 months of follow-up. Despite this group showing an apparent increase in Raw, this group had the lowest Raw (151 ± 23%) at the beginning of the study (Figure 3F; Supplementary Table 10).
However, the BMMC group had decreased pCO2 after 6 months that suggested an improvement in gas exchange when associated with increased DLCO and VA. Cardiopulmonary tests showed increased VO2peak in the BMMC group during the follow-up and an improvement in the first 6 months in the co-infusion group (Table 1; Supplementary Tables 9, 11 and 13).
 |
Table 1 Different Pulmonary Function Parameters Over 12 Months in Relation to Baseline Data Before Treatment for the Control, BMMC, ADSC and Co-Infusion Groups |
Regarding the scintigraphy data, there was no variation between the groups and no difference in the regeneration of lung injured areas in any of the treatments data (Supplementary Table 12). Concerning the thorax CT scans, no changes were observed during the follow-up in all groups, eg, no changes in emphysema or nodule growth, although software was not used to analyze the results.
The 6MWT did not differ during the 12-month follow-up in any group, considering the general criteria for significance (distance > 50 meters). The quality of life derived from the SGRQ application decreased 46.7% in the control group throughout the 12-month follow-up, indicating the considerable clinical worsening of COPD status. Analysis of the symptom’s domain taken from the SGRQ confirmed that the ADSC and co-infusion groups showed improved symptoms 3-months post-treatment. The co-infusion group had symptom improvement gradually through the 6- and 9-month visits; a significant increase of 10% over baseline at the 12-month visit. In the SGRQ disease-impact domain, the same pattern was observed in all groups, but for the 12-month visit, the co-infusion group showed a more significant improvement (Figure 4 and Table 1).
 |
Figure 4 Saint George Hospital Respiratory Disease Questionnaire (SGRQ). Abbreviations: BMMC, bone marrow mononuclear cells; ADSC, adipose-derived mesenchymal stromal cells. |
During the 12-month follow-up of the patients, signs and symptoms indicative of COPD exacerbation were eventually observed in all groups, which may be considered as an adverse event (AE), but the exacerbations were more intense and frequent in the control group. It is noteworthy that the co-infusion group had fewer episodes of exacerbation compared to the other groups.
Discussion
In the context of regenerative and translational medicine in lung diseases and, particularly in COPD/emphysema, cell-based therapy is impacted by a complex tissue structure, with many cell types inserted in an extracellular matrix network.16,29,49 In this context, the lung represents a major challenge for tissue engineering and regenerative medicine (TERM). Currently, approximately 21 studies are registered on the ClinicalTrials.gov website50 for “Cell Therapy or Stem Cells/COPD” and, to date, only seven papers have been published reporting results on cell-based clinical trials in COPD.2,23–28 This study presented a cell-based therapy in COPD using, for the first time in the literature, a co-infusion of BMMCs and ADSC.
Despite the limitations of this Phase I clinical study, such as the small sample size of each group, the objective of assessing safety has been fully achieved, especially regarding the safety of the co-infusion of BMMC and ADSC. As the first objective of this clinical trial was to test the safety (Phase I) of the procedure, it should be emphasized that no adverse effects were reported in all groups in the period immediately following infusion or during the follow-up of the patients for 12 months. This finding is consistent and confirms all previous cell-based clinical trials in COPD.2,23–28
In accordance with the characterization standards recommended by the International Society of Cell Therapy (ISCT),48 the cells from 10 patients who received ADSCs presented a high degree of purity, including the presence above 97% of markers characteristic of MSCs in all samples, as observed in the cytometry analysis. The ADSCs showed osteogenic, chondrogenic and adipogenic differentiation.
In addition, the cells were analyzed by the G-banding technique to verify any possible effect of genetic instability on the cells kept in cultivation, until the third passage. No numerical or structural chromosomal aberrations were detected in all metaphases analyzed, according to the karyotype images. Several reports in the literature have shown cytotoxic and genotoxic effects on long-time cell culture of mesenchymal stromal cells.51,52 According to these results, our research group adopted, as a criterion for quality and safety control, the proliferation and infusion of ADSC until the third or, exceptionally, until the fourth passage.
Plethysmography showed that the control group worsened lung function due to increased RV/TLC and Raw and decreased IC during 12-month follow-up. These results indicate a worsening in relation to gas exchange and pulmonary hyperinflation, which was not observed in the other groups. These data suggest a beneficial effect of cell-based therapy in gas exchange and hyperinflation, in the BMMC, ADSC and co-infusion groups. Ruppel et al (2012)53 showed that RV and RV/TLC were more sensitive than TLC to the degree of airway obstruction. Recently, there was an interest in IC as a marker of hyperinflation. Casanova et al (2005)54 used the IC/TLC ratio as an indicator of hyperinflation and related it to survival in COPD patients. The worsening of hyperinflation may also have impacted the quality of life (QLQ SGRQ) of patients in the control group, unlike the BMMC, ADSC and co-infusion groups.
Interestingly, the ADSC group had a more effective IC and VA improvement. VA refers to TLC without anatomical dead space.55 In other words, an increase in VA could imply an increase in pulmonary capacity. In this case, this may be due to decreased lung compliance. These results could justify the quality of life improvement in these patients, although VA alone should not be used as a marker of pulmonary capacity. TLC is the best marker.
The BMMC group showed the best results in this study. This group revealed a slight increase in FEV1, better diffusion capacity in the first 3 months (described as DLCO/VA) and worsening in IC after 3 months. It is important to highlight the better gas exchange in the BMMC group, in addition to the improvement of the diffusion capacity. Moreover, this group had improved oxygen consumption and decreased pCO2 retention, implying that BMMCs could be related to improvement of alveolar gas exchange.
The co-infusion group obtained the best quality of life and an augmentation of pulmonary capacity, as demonstrated by VO2, IC stability and DLCO. Notably, they had the best improvement of symptoms during treatment. Unfortunately, scintigraphy results did not confirm these findings.
In summary, this study showed that control patients had the worst follow-up results compared to treated groups. The BMMC group had the most desired responses in alveolar gas exchange and the co-infusion group showed a better quality of life with increased alveolar gas exchange.
COPD/emphysema is a multifactorial disease resulting from the interaction of environmental and genetic factors; thus, the clinical course of disease may vary depending on the particular conditions of each patient. This study was designed to primarily evaluate the safety and secondarily the efficacy of the procedure, especially the co-infusion of BMMC and ADSC. No adverse effects resulting from the infusion of BMMC, ADSC or the co-infusion of BMMC and ADSC have been detected. Thus, it can be stated that the first endpoint of this Phase I clinical trial has been fully achieved.
Interesting results were also obtained regarding lung function after infusion of BMMC and ADSC. Therefore, it was not possible to confirm in a thorough manner the hypothesis that the co-infusion resulted in an additive or synergistic therapeutic effect in COPD. Additional studies, with a larger number of patients, will be needed to evaluate this hypothesis more accurately. However, it should be emphasized that from the results obtained in this clinical study, new approaches open up for research in cell-based therapies in COPD, resulting from the association of BMMC and ADSC, as well as the association of other mesenchymal stromal/stem cells. In this context, considering the good safety and efficacy results with infusion of UC-MSC27,28 in patients with COPD, this study opens the perspective of clinical trials with concomitant infusion of new cellular associations such as BMMC and UC-MSC or ADSC and UC-MS.
Despite the limited number of clinical trials regarding COPD, there are some encouraging results. In the study reported by Stessuk et al,2 one of the patients maintained a table FEV1 after a 30-month follow-up. Le Thi Bich et al27 reported a clinical trial using allogenic UC-MSC, where the authors report a significantly reduced mMRC score, as well as the number of exacerbations in patients with moderate-to-severe COPD. The clinical protocol conducted by Stolk et al25 shows increased expression of CD31, an endothelial marker, which could indicate the occurrence of angiogenesis, resulting in a protective effect or repair of the lung parenchyma. This hypothesis is coherent and corroborates previous results in vitro and in vivo experimental models of a possible angiogenesis effect that would act in the improvement of lung function through a paracrine mechanism of BM-MSC and/or ADSC.9,13,15 These results expose the interesting perspective ie the association of MSC and factors that stimulate the release of cytokines may represent a new therapeutic approach to improve lung function in patients with COPD. Nevertheless, notwithstanding some advances and promising results, there are many doubts, divergences, and issues to be further investigated and more precisely defined. Among the issues to be better defined are the number of cells, the time of administration (acute or chronic phase), route of administration (intratracheal, intrabronchial or venous), homing and engraftment, source of cells, culture conditions (quality of the cells), and standardization of protocols.13,14,19,21,33,40,56–62 Thus, new studies and well-designed randomized trials with standardized methods must be performed to obtain a dataset that consistently supports moving to Phase III randomized, placebo-controlled clinical trials. In this context, it would be important to establish large national and/or international randomized, multicenter collaborative studies.
Conclusion
In this Phase I clinical trial, we evaluated and described for the first time in the literature the concomitant infusion of BMMC and ADSC for the treatment of COPD. The results obtained reinforce previous studies that cell-based therapy with BMMC and ADSC is a safe procedure. In addition, no adverse events were detected resulting from the concomitant infusion of BMMC and ADSC confirming that it is also a safe procedure. The small number of patients in each group impacted the results and did not allow more consistent conclusions regarding efficacy resulting from the concomitant infusion of BMMC and ADSC and did not allow to validate the hypothesis that guided the development of this clinical trial, according to which the association of BMMC and ADSC could result in an additive or synergistic therapeutic effect. In this regard, additional studies with a larger number of patients are needed before Phase III clinical trials are implemented.
Data Sharing Statement
Data are available on request from the authors. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank the patients and all health professionals at the Hospital Mário Covas (Santo André, Sp, Brazil) and the Technical Team of the Instituto de Ensino e Pesquisa IEP – São Lucas (São Paulo, Brazil), for collaboration in the development of this study. The authors also thank The Chico Anysio Institute for the support and collaboration in recruiting and contacting patients included in this clinical study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was funded by the National Council for Scientific and Technological Development –CNPq – Brazil (Proc. 405108/2013-3 and Proc. 437165/2016-7).
Disclosure
All authors declare no conflicts of interest.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global initiat chronic obstr lung disease; (2020 report); 2020. Available from: http://www.goldcopd.org/.
2. Stessuk T, Ruiz MA, Greco OT, et al. Phase I clinical trial of cell therapy in patients with advanced chronic obstructive pulmonary disease: follow-up of up to 3 years. Rev Bras Hematol Hemoter. 2013;35(5):352–357. doi:10.5581/1516-8484.20130113
3. Labaki WW, Han MLK. Chronic respiratory diseases: a global view. Lancet Respir Med. 2020;8(6):531–533. doi:10.1016/S2213-2600(20)30157-0
4. World Health Organization. The top 10 causes of death; 2021. Available form: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
5. Caramori G, Casolari P, Garofano E, et al. Role of Stem Cells in the Pathogenesis of COPD and Pulmonary Emphysema. Recenti Prog Med. 2012;103(1):31–40. doi:10.1701/1022.11157
6. de Faria CA, de Las Heras Kozma R, Stessuk T, et al. Experimental Basis and New Insights for Cell Therapy in Chronic Obstructive Pulmonary Disease. Stem Cell Rev Rep. 2012;8(4):1236–1244. doi:10.1007/s12015-012-9410-7
7. Ribeiro-Paes JT, Stessuk T, Kozma RLH. Cell Therapy in chronic Obstructiv Pulmonary Disease: state of The Art and Perspectives. In: Chungong K, editor. Chronic Obstructive Pulmonary Disease- Current Concepts and Practice. Tech, Rijeka; 2012:455–474.
8. Longhini-dos-Santos N, Barbosa-Oliveira VA, Kozma RH, et al. Cell Therapy with Bone Marrow Mononuclear Cells in Elastase-Induced pulmonary Emphysema. Stem Cell Rev Rep. 2013;9(2):210–218. doi:10.1007/s12015-012-9419-y
9. Tzouvelekis A, Ntolios P, Bouros D. Stem cell treatment for chronic lung diseases. Respiration. 2013;85:179–192. doi:10.1159/000346525
10. Conese M, Piro D, Carbone A, et al. Hematopoietic and mesenchymal stem cells for the treatment of chronic respiratory diseases: role of plasticity and heterogeneity. Sci World J. 2014;2014:1–11. doi:10.1155/2014/859817
11. Lipsi R, Rogliani P, Calzetta L, et al. The clinical use of regenerative therapy in COPD. Int J COPD. 2014;9:1389–1396. doi:10.2147/COPD.S49519
12. De Francesco F, Ricci G, D’Andrea F, et al. Human Adipose Stem Cells: from Bench to Bedside. Tissue Eng Part B Rev. 2015;21(6):572–584. doi:10.1089/ten.TEB.2014.0608
13. Liu X, Fang Q, Kim H. Preclinical studies of mesenchymal stem cell (MSC) administration in chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. PLoS One. 2016;11(6):e0157099. doi:10.1371/journal.pone.0157099
14. Cheng SL, Lin CH, Yao CL. Mesenchymal Stem Cell Administration in Patients with Chronic Obstructive Pulmonary Disease: state of the Science. Stem Cells Int. 2017;2017:1–14. doi:10.1155/2017/8916570
15. Broekman W, Khedoe PPSJ, Schepers K, et al. Mesenchymal stromal cells: a novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax. 2018;73(6):565–574. doi:10.1136/thoraxjnl-2017-210672
16. Kruk DMLW, Heijink IH, Slebos DJ, et al. Mesenchymal Stromal Cells to Regenerate Emphysema: on the Horizon? Respiration. 2018;96(2):148–158. doi:10.1159/000488149
17. Kokturk N, Yıldırım F, Gülhan PY, et al. Stem cell therapy in chronic obstructive pulmonary disease. How far is it to the clinic? Am J Stem Cells. 2018;7(3):56–71.
18. Sun Z, Li F, Zhou X, et al. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis. 2018;10(2):1084–1098. doi:10.21037/jtd.2018.01.46
19. Naji A, Eitoku M, Favier B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–3348. doi:10.1007/s00018-019-03125-1
20. Behnke J, Kremer S, Shahzad T, et al. MSC Based Therapies - New Perspectives for the Injured Lung. J Clin Med. 2020;9(3):682. doi:10.3390/jcm9030682
21. Garcia-Bernal D, Garcia-Arranz M, Yanez RM, et al. The current status of mesenchymal stromal cells: controversies, unresolved issues and some promising solutions to improve their therapeutic efficacy. Front Cell Dev Biol. 2021;9:650664. doi:10.3389/fcell.2021.650664
22. Hiemstra PS, Wu X, Khedoe PSJ, et al. The role of altered stem cell fusion in airway and alveolar repair and remodelling in COPD. In: Marko X. Nikolic and Brigid L. M. Hogan, editors. Lung Stem Cells in Development, Health and Disease. ERS Monograph. 2021. doi:10.1183/2312508X.erm9121
23. Ribeiro-Paes JT, Bilaqui A, Greco OT, et al. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J COPD. 2011;6:63–71. doi:10.2147/COPD.S15292
24. Weiss DJ, Casaburi R, Flannery R, et al. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–1598. doi:10.1378/chest.12-2094
25. Stolk J, Broekman W, Mauad T, et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM. 2016;109(5):331–336. doi:10.1093/qjmed/hcw001
26. De Oliveira HG, Cruz FF, Antunes MA, et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl Med. 2017;6(3):962–969. doi:10.1002/sctm.160315
27. Le Thi Bich P, Nguyen Thi H, Dang Ngo Chau H, et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res Ther. 2020;11:60. doi:10.1186/s13287-020-1583-4
28. Hoang DM, Nguyen KT, Anh HN, et al. Allogeneic human umbilical cord-derived mesenchymal stem/stromal cells for chronic obstructive pulmonary disease (COPD): study protocol for a matched case–control, phase I/II trial. BMJ Open. 2021;11(5):e045788. doi:10.1136/bmjopen-2020045788
29. Garcia O, Carraro G, Navarro S, et al. Cell-based therapies for lung disease. Br Med Bull. 2012;101(1):147–161. doi:10.1093/bmb/ldr051
30. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45(11):e54. doi:10.1038/emm.2013.94
31. Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. doi:10.1038/mt.2011.211
32. Gu W, Song L, Li XM, et al. Mesenchymal stem cells alleviate airway inflammation and emphysema emphysema in COPD through down-regulation of cyclooxygenase-2 via p38 and ERK MAPK pathways. Sci Rep. 2015;5:8733. doi:10.1038/srep08733
33. Broekman W, Amatngalim GD, de Mooij-eijk Y, et al. TNF-α and IL-1β-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res. 2016;17:3. doi:10.1186/s12931-015-0316-1
34. El-Badrawy MK, Shalabi NM, Mohamed MA, et al. The Effect of Bone Marrow Mononuclear Cells on Lung Regeneration and Apoptosis in a Simple Model of Pulmonary Emphysema. Int J Stem Cells. 2016;9(1):145–151. doi:10.15283/ijsc.2016.9.1.145
35. Hou L, Kim JJ, Woo YJ, et al. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. Am J Physiol Heart Circ Physiol. 2016;310(4):H455–H465. doi:10.1152/ajpheart.00726.2015
36. Savukinas UB, Enes SR, Sjöland AA, et al. Concise Review: the Bystander Effect: mesenchymal Stem Cell-Mediated Lung Repair. Stem Cells. 2016;34(6):1437–1444. doi:10.1002/stem.2357
37. Antoniou KM, Karagiannis K, Tsitoura E, et al. Clinical applications of mesenchymal stem cells in chronic lung diseases. Biomed Rep. 2018;8(4):314–318. doi:10.3892/br.2018.1067
38. Armitaje J, Tan DBA, Troedson R, et al. Mesenchymal stromal cell infusion may provide an alternative immune-based therapeutic option for COPD patients. Eur Respir J. 2018;51:1702369. doi:10.1183/13993003.02369-2017
39. Longhini-dos-Santos N, Barbosa-de-oliveira VA, Stessuk T, et al. Cell Therapy Decreases Inflammation and Improves the Morphology of the Lung Parenchyma in a Murine Model of Cigarette Smoke-Induced Emphysema. Int J New Technol Res. 2018;4:58–65. doi:10.31871/IJNTR.4.1.24
40. Fernández-Francos S, Eiro N, Costa LA, et al. Mesenchymal Stem Cells as a Cornerstone in a Galaxy of Intercellular Signals: basis for a New Era of Medicine. Int J Mol Sci. 2021;22(7):3576. doi:10.3390/ijms22073576
41. Thomas ED, Storb R. Technique for Human Marrow Grafting. Blood. 1970;36(4):507–515. doi:10.1182/blood.V36.4.507.507
42. Aktas M, Radke TF, Strauer BE, et al. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 2008;10(2):203–211. doi:10.1080/14653240701851324
43. Zuk PA, Zhu M, Mizuno H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi:10.1089/107632701300062859
44. Borgonovo T, Vaz IM, Senegaglia AC, et al. Genetic evaluation of mesenchymal stem cells by G-banded karyotyping in a Cell Technology Center. Rev Bras Hematol Hemoter. 2014;36(3):202–207. doi:10.1016/j.bjhh.2014.03.006
45. Pereira CADC, Viegas CAA, Alves RR. Capacidade de difusão do monóxido de carbono. J Pneumol. 2002;28:122–138.
46. Sciurba F, Criner GJ, Lee SM, et al. Six-Minute Walk Distance in Chronic Obstructive Pulmonary Disease: reproducibility and Effect of Walking Course Layout and Length. Am J Respir Crit Care Med. 2003;167(11):1522–1527. doi:10.1164/rccm.200203-1660C
47. Camelier A, Rosa FW, Salim C, et al. Using the Saint George’s Respiratory Questionnaire to evaluate quality of life in patients with chronic obstructive pulmonary disease: validating a new version for use in Brazil. J Bras Penumol. 2006;32(2):114–122. doi:10.1590/s1806-37132006000200006
48. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi:10.1080/14653240600855905
49. Brouwer KM, Hoogenkamp HR, Daamen WF, et al. Regenerative medicine for the respiratory system: distant future or tomorrow’s treatment? Am J Respir Crit Care Med. 2013;187(5):468–475. doi:10.1164/rccm.201208-1558PP
50. ClinicalTrials.gov. website. Available from: https://www.clinicaltrials.gov/ct2/home.
51. Turinetto V, Vitale E, Giachino C. Senescence in Human Mesenchymal Stem Cells: function changes and implications in stem cell - based therapy. Int j Mol Sci. 2016;17(7):1164. doi:10.3390/ijms17071164
52. Myungshin K, Rhee JK, Choi H, et al. Passage-dependent accumulation of somatic mutations in mesenchymal stromal cells during in vitro culture revealed by whole genome sequencing. Sci Rep Nat. 2017;7:14508. doi:10.1038/s41598-017-15155-5
53. Ruppel GL. What is the clinical value of lung volumes? Respir Care. 2012;57(1):26–35. doi:10.4187/respcare.01374
54. Casanova C, Cote C, De Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(6):591–597. doi:10.1164/rccm.200407-867OC
55. Stocks J, Qof Theuanjer PH. Reference values for residual volume, function residual capacity and total lung capacity. ATS Workshop on lung volume measurements. Official Statement of the European Respiratory Society. Eur Respir J. 1995;8(3):492–506. doi:10.1183/09031936.95.08030492
56. Copland IB, Galipeau J. Death and inflammation following somatic cell transplantation. Semin Immunopathol. 2011;33(6):535–550. doi:10.1007/s00281-011-0274-8
57. Oh DK, Kim YS, Oh YM. Lung regeneration therapy for chronic obstructive pulmonary disease. Tuberc Respir Dis. 2017;80(1):1–10. doi:10.4046/trd.2017.80.1.1
58. Varghese J, Griffin M, Mosahebi A, et al. Systematic review of patient factors affecting adipose stem cell viability and function: implications for regenerative therapy. Stem Cell Res Ther. 2017;8(1):45. doi:10.1186/s13287-017-0483-8
59. Martin C, Olmos E, Collignon ML, et al. Revisiting MSC expansion from critical quality attributes to critical culture process parameters. Process Biochem. 2017;59:231–243. doi:10.1016/j.procbio.2016.04.017
60. Bieback K, Kuci S, Schafer R. Production and quality testing of multipotent mesenchymal stromal cell therapeutics for clinical use. Transfusion. 2019;59:2164–2173. doi:10.1111/trf.15252
61. Islam D, Huang Y, Fanelli V, et al. Identification and Modulation of Microenvironment Is Crucial for Effective Mesenchymal Stromal Cell Therapy in Acute Lung Injury. Am J Respir Crit Care Med. 2019;199(10):1214–1224. doi:10.1164/rccm.201802-0356OC
62. Cruz FF, Rocco PRM. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev Respir Med. 2020;14(1):31–39. doi:10.1080/17476348.2020.1679628
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


