Back to Journals » Infection and Drug Resistance » Volume 15
Autologous Free Fascia Lata Can Be Used as Dura Graft in the Salvage Treatment of Recalcitrant Postcraniotomy Intracranial Infection Caused by Multidrug-Resistant Gram-Negative Bacteria
Authors Zeng T, Wang M, Xu Z, Ni M, Gao L
Received 18 July 2022
Accepted for publication 3 September 2022
Published 27 September 2022 Volume 2022:15 Pages 5667—5677
DOI https://doi.org/10.2147/IDR.S381087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Tao Zeng,1,* MingSheng Wang,1,* Zijun Xu,2 Min Ni,3 Liang Gao1
1Department of Neurosurgery, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Department of Radiology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 3Department of Clinical Pharmacy, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Liang Gao, Department of Neurosurgery, Shanghai Tenth People’s Hospital, Shanghai, People’s Republic of China, Email [email protected]
Objective: The multidrug-resistant (MDR) gram-negative bacteria-induced intracranial infections after neurosurgical procedures represent a particular therapeutic challenge. Combining the removal of infected prosthetic meninge plus an appropriate antibiotic administration appears to be the only therapeutic strategy likely to succeed when the infection is complicated by artificial dura mater. This study aimed to assess the efficacy of free fascia lata as a substitute for dura reconstruction in the salvage treatment for such recalcitrant nosocomial infections.
Methods: The retrospective, observational study was conducted at Shanghai Tenth hospital. Patients with definite intracranial infection caused by MDR Gram-Negative bacteria who underwent salvage dura reconstruction using autologous free fascia lata were included in the study. Electronic medical data on clinical characteristics, underlying condition, bacterial culture, antibiotic susceptibilities, perioperative management, surgical techniques, outcome, and follow-up were collected and analyzed.
Results: 19 patients were included in the study cohort. All these patients underwent salvage surgery, including removal of infected artificial dura substitute, achievement of complete dura seal with free fascia lata, and other adjunctive procedures to drain the CSF and infuse sensitive antimicrobial agents. Intraventricular or intrathecal administration of antibiotics, including Colistin (14 case), Tigecycline (1 case), Amikacin (1 case), was employed in 16 patients. The infection was cured in 17 patients. In-hospital death occurred in 3 patients. One died from multiple system/organ failure, 1 died from massive occipital ICH, 1 died from brain stem hemorrhage after ventricular-peritoneal shunt surgery. The patients remained without clinical evidence of recurrence during the follow-up period.
Conclusion: On the basis of a comprehensive approach to achieving prompt sterilization of causative pathogens and an optimal healing environment, free fascia lata can serve as a simpler but effective option for dura reconstruction even in the setting of a severe septic area for patients who otherwise need much more complicated and demanding tissue transfer surgery.
Keywords: surgical site infection, intracranial infection, multidrug-resistant, gram-negative bacteria, fascia lata, duraplasty
Introduction
Although overall mortality of neurosurgical site infections has decreased during the last decades, it remains a life-threatening condition, leading to a poor prognosis with prolonged hospitalization and increased costs, even nowadays when newer agents with a broader antimicrobial spectrum are available worldwide. In recent years, due to misuse and overuse of broad-spectrum antibiotics, intracranial infections caused by multidrug-resistant (MDR) or extensively drug-resistant (XDR) isolates, especially Gram-negative (G-) bacilli, such as Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, are becoming an increasingly common clinical entity, posing a substantial challenge for the management.1 Such surgical site infections (SSIs) are intractable in some patients. The difficulty mainly lies in three aspects: first, the choice and efficacy of antibiotics are limited because of the resistance when the infection is caused by MDR isolates. Second, because of the existence of the brain-blood barrier (BBB), concerns have been raised regarding the adequacy of the penetration of antibiotics into CSF. Third, given that various types of artificial meninges are frequently used in craniotomy, it is much more difficult to eradicate bacteria in the presence of artificial meninges. Such infections cannot be cured adequately with antibiotics alone. Combining the removal of infected prosthetic meninge plus an appropriate antibiotic administration appears to be the only therapeutic strategy likely to succeed in this situation.2 Thus, choosing material suitable for dura repair in the septic area after removing the infected substitute becomes a primary concern. There have been sparse cases reports of dura repair with free fascia lata in the circumstance of intracranial infection raised after neurosurgical surgery.3 But it is unclear whether it can remain resistant to MDR or XDR bacilli-induced infection. Herein, we reported what is, to our knowledge, the largest case series of using free fascia lata to repair the dural defect to treat SSIs caused by MDR/XDR organisms and assess its safety and efficacy in the treatment of these challenging infections.
Patients and Methods
This retrospective study was performed at the Department of Neurosurgery, Shanghai Tenth Hospital, a tertiary referral center for complicated CNS infections in east China. The cohort includes all patients with SSIs caused by MDR bacteria who underwent dura repair using free fascia lata in the debridement surgery. The electronic medical files of all these patients are systematically screened. The study complied with the Declaration of Helsinki and was approved by the relevant institutional review board and the research ethics committee of Shanghai Tenth People’s Hospital (SHYS-IEC-5.0/22K156/P01). All patient records used in this study were anonymized before data assessment. Written informed consent for data collection and publication was obtained from the patients or their immediate relatives.
MDR is defined as non-susceptibility to three or more classes of antibiotics, and extensively drug-resistant (XDR) is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories. By definition, XDR can be categorized as MDR.4 In this study, they are collectively referred to as MDR. A classification based on clinical, radiological evaluation, and laboratory tests was adopted to describe the details of SSIs as follows: 1) scalp or superficial infection, including incision infection; 2) bone flap osteitis (either a surgical diagnosis or a suggestive x-ray); 3) meningitis and ventriculitis; and 4) brain abscess and empyema (either a microorganism cultured from abscess/empyema or an intraoperative diagnosis. The diagnosis of meningitis or ventriculitis is established mainly by the analysis of CSF from the lumbar cistern or ventricle according to the definitions of the Centers for Disease Control and Prevention (CDC) as follows:5 (1) isolation of pathogens from CSF; (2) patient was considered at least one of the following signs with no other recognized cause: fever (> 38 °C), headache, stiff neck, meningeal signs, changing level of consciousness; (3) CSF pleocytosis, elevated protein, and/or decreased glucose level. In this study, the patients with scalp infection and/or bone flap only are excluded.
Electronic records of all patients were carefully reviewed and collected for each patient on standardized forms including age, sex, underlying neurological disease, preceding neurosurgical procedures and type of artificial dura mater implanted, site of surgery (supratentorial or infratentorial), detailed description of SSIs, Glasgow Coma Scale score (GCS) and Sequential Organ Failure Assessment (SOFA) score before the debridement surgery. In the postoperative period, administration of antibiotics (agents, route, and duration), subsequent surgery, CSF drainage, and wound healing were registered. All outcomes were reviewed for both major and minor complications. The therapeutic results were evaluated by using Glasgow Outcome Scale (GOS):6 score 1 = death, score 2 = persistent vegetative state, score 3 = severe disability, score 4 = moderate disability, score 5 = good recovery. Scores of 1–3 were classified as “poor prognosis”, while scores of 4 and 5 were “good prognosis.”
Surgical Procedure
The surgical procedures in the salvage surgery include debridement, reconstruction, and some other adjunctive maneuver. The technique of harvesting the fascia Lata involves making a modified “S” or linear incision on the anterior-lateral upper thigh according to the size of the fascia planned for dura repair. The fascia is sharply incised at the borders, and a blunt scissor is used to elevate the fascia off the underlying musculature. Once harvested, the adipose tissue attaching to the fascia lata is carefully stripped. Next, the infected alien materials, including artificial dura substitute, titanium screw, and bone wax, are thoroughly removed. Inactivated or infected tissue, including intracerebral abscess, subdural and extradural pus, affected bone flap are debrided completely. The entire border of the circumferential dura mater in the bone window is exposed. In places where there dural margin was not exposed sufficiently, the bone edge is expanded with a bone rongeur to ensure the sewage. The trimmed pieces of fascia lata are then carefully sewn to the residual native dura in a watertight fashion by running 3-0 absorbable monocryl. Intraoperative CSF leakage is inspected by repeated irrigation with copious antibiotic solution and a Valsalva maneuver performed by an anesthetist before the dural closure. The skin fistula was resected, and the damaged portion of the incision was debrided. The implanted fascia lata is then covered with a well-vascularized scalp. For the patent wound with moderate scalp defect or ill-being incision, Subgalea dissection facilitates tension-reduced incision closure in those with compromised scalps. When necessary, a pedicled temporalis flap is strived to be transferred to the area to enhance regional blood supply and ensure coverage of the fascial lata.
Some other procedures are needed to facilitate reconstructive surgery and postoperative management. The infected EVD catheter or Ommya reservoir was removed and replaced by a new system for CSF drainage and/or for the intraventricular (IVT) administration of antibiotics, depending upon the surgeon’s preference and the specific clinical scenario. Indwelling a lumbar drainage catheter was an alternative for CSF drainage and a route for intrathecal (ITH) administration of antimicrobial agents. For those with pyogenic ventriculitis, sufficient intraventricular lavage with antibiotics solution to clear suppurative materials was usually required.
Neurocritical Care Management
These critically ill patients were admitted to the neurosurgical intensive care unit (NICU) for postoperative surveillance and management to avoid systemic and neurological complications. The bacterial culture and susceptibility test results were the primary reference for an antibiotic prescription. The ability of the agents to penetrate the BBB was another major concern. The antibiotic regimen was formulated and adjusted with the consultation of clinical pharmacists. For patients with meningitis and ventriculitis, IVT or ITH administration of sensitive antibiotics was needed to eradicate the pathogen promptly. According to the guidelines of IDSA (Infectious Diseases Society of America),7 antibiotic therapy for Gram-negative meningitis should last for no less than three weeks. Meanwhile, the discontinuation of antibiotics could not be considered until consecutive sterile CSF cultures were achieved at least three times. CSF drainage, either via EVD or lumbar catheter, was carefully titrated according to the need for concomitant hydrocephalus and reducing regional tension, which minimizes the possibility of postoperative CSF leakage. Other neurocritical managements focused on the treatment of raised intracranial pressure, seizure management, and continued treatment of the underlying hydrocephalus. In addition, systemic therapies conformed to the general principles of management of critically ill patients. Appropriate nutritional support was a particularly significant concern.
Result
Patient Characteristics
Surgical records of 54 patients involved in duraplasty with free fascia lata from June 2015 to December 2020 were retrospectively reviewed. 35 patients were excluded whose fascia lata was applied in the reconstruction of anterior skull base; out of them, 18 patients for tumoral lesion, 13 for refractory CSF rhinorrhea. 2 patients were excluded because of insufficient bacterial culture result. 2 patients whose causative pathogen being enterococcus were also excluded .19 patients were included in the analysis. The cohort consisted of 13 males and 6 females aged from 23 to 68 years (mean 49.2±11.4). The preceding craniotomy was electively scheduled for a brain tumor in 5 patients, emergently performed for traumatic brain injuries (TBI) in 10 patients, for intracerebral hemorrhage (ICH) in 4 patients. The interval between the debridement surgery and the preceding procedure responsible for infection ranged from 13 days to 301 days. At the moment of debridement surgery, the patients presented with GCS scores ranging from 5 to 15 (8.2±3.2) and SOFA scores ranging from 1 to 8 (mean 4.8±2.3). Incisional leakage, either CSF or purulent discharge, was presented in 17/19 patients, In 2 patients without incision leakage, 1 patient presented with intractable meningitis and ventriculitis unresponsive to various antibiotics, 1 patient presented with repeated relapse of meningitis. All patients’ demographics and clinical characteristics are shown in Table 1.
 |
Table 1 Clinical Data, Treatment and Outcome of 19 Patients |
Microbiology and Antibiogram
The causative pathogen was cultured from purulent discharge in 5 patients and from CSF in 14 patients. Klebsiella pneumoniae (42.1%, 8/19) and Acinetobacter baumannii (42.1%, 8/19) were the most frequent organisms responsible for the infections, followed by Pseudomonas aeruginosa (10.5%, 2/19) and Stenotrophomonas maltophilia (5.3%, 1/19). Regarding the resistance pattern, 15 isolates were defined as MDR strains and 5 isolates as XDR strains. No isolates were found to be resistant to Colistin or Tigecycline. The antimicrobial susceptibility of the implicated pathogens is illustrated in Table 2.
 |
Table 2 Antimicrobial Susceptibility of Gram-Negative Pathogens |
Treatment and Outcome
The infected artificial dura, including 8 pieces of bovine pericardium, 4 pieces of synthetic material, 3 pieces of DuraGen, and 4 pieces of unknown dura substitute was removed in the debridement procedure. The dura defect was repaired with free fascia lata in all the patients. A pedicled temporalis flap was transferred in 3 patients. No patient needs free tissue transfer. Postoperative CSF drainage was employed in 17/19 patients. IVT/ITH antibiotics were administrated in 16/19 patients. Out of them, polymyxin E was infused in 14 patients, and Amikacin and Tigecycline in one patient, respectively. IVT/ITH polymyxin E was administered at a dose of 5–10 mg/day, ranging in duration from 8 days to 27 days. Eight of 14 patients concomitantly received intravenous (iv) colistin. IVH Tigecycline was administrated in one patient at a dose of 10mg/d for consecutive 13 days with a combined iv Tigecycline infusion. In another patient, ITH Amikacin was used at a dose of 20mg/d with a duration of 8 days with concomitant iv administration of Amikacin. When the antimicrobial agent was instilled into the ventricle or the lumbar thecal sac, the drain was clamped for 60 minutes to allow the agent to equilibrate throughout the CSF. The infection was cured in 17 patients.1 patient died from multiple system/organ failure (MSOF) 2 weeks after the debridement operation. 1 patient died from massive ICH 1 week after the debridement. Another in-hospital death occurred in a patient whose infection was cured but died from brain stem hemorrhage 2 weeks after ventricular-peritoneal shunt surgery. 16 patients who survived had a median GOS score of 3.13 (3.1±1.1) at discharge.
Follow-Up
The follow-up period was at least 12 months after surgery or until death for patients who survived less than 12 months. The mean follow-up was 29.5 months (range 13–51 months). 1 patient (patient 18) was readmitted 7 months after discharge because of recurrent ventriculitis whose causative pathogen was again confirmed to be Klebsiella pneumoniae. The ventriculitis was cured after removing the Ommya system and repeated intraventricular infusion of polymyxin E. Five patients accepted scheduled CSF diversion surgery, and 6 patients accepted cranioplasty surgery. The patients remained without clinical evidence of recurrence during the follow-up period. The details of treatment and outcome are shown in Table 1.
Discussion
Nosocomial infections of the central nervous system are a severe complication of neurosurgical procedures worldwide, causing significantly increased morbidity and mortality. CSF Leakage, either from the incision or the CSF drainage system/site, is a definite risk factor for post-neurosurgical infections.8–10 It provides access for bacterial invasion leading to subsequent infection, and in turn, if infective hydrorrhea cerebrospinal from the wound persists, the healing of the incision is almost impossible. Providing the increasing prevalence of MDR Gram-negative bacteria in intensive care units or neurosurgical wards, the invading organism is often MDR isolate, which is usually unresponsive to the commonly used prophylactic agents. Supposing aggressive treatment is not initiated promptly, in that case, the infection will spread throughout the space, resulting in subsequent intricate SSIs, including ventriculomeningitis, encephalitis, brain abscess, subdural empyema, or even sepsis. Our case serials showed that most severely infected patients were complicated with prolonged CSF leakage or suppurative discharge from the incision.
Artificial dura substitutes may play an essential role in the pathogenesis of such recalcitrant infections. Various dura substitutes have been used for dura seals in craniotomy over the past decades.11–15 Compared with autologous fascia, the artificial dura mater is more difficult to achieve watertight closure due to its poor malleability. Moreover, when intracranial pressure increases, the suture gap can easily expand, increasing the chance of CSF leakage, leading to an increased incidence of postoperative CSF leakage and deep wound infections.16 Moreover, given the colonization of MDR bacilli on artificial dura substitute, eradicating the pathogens becomes much more difficult, which is responsible for relapse of infection in some patients. Thus, the successful control of infection and CSF leak might lie in removing infected dura and subsequent reliable dura reconstruction.
According to the general principle of surgical infection treatment, alien off-The-shelf dura substitute is contraindicated. With the impression deeply held in mind that the use of non–vascularized free fascia in the infected area is dangerous, surgeons have widely recognized the need to recruit vascularized tissue for dural seal.17 Various authors describe several techniques. Shimada18 reported using an anterolateral thigh flap with the fascia lata to reconstruct the dura mater. Abuzayed et al19 presented their technique with autologous fascia lata reinforced by an on-site pedicled muscle flap. While in the report of Uemura,20 a rectus muscle free flap was used. The use of the rectus abdominis muscle combined with a vascularized fascial perforator flap has also been reported.21,22 Stephan et al23 choose a latissimus dorsi muscle free flap to reinforce the vascularity of the fascia lata. However, these larger surgeries involving free tissue transfer are both technically demanding, needing for microvascular anastomosis, and physically burdensome for those critically ill patients. Are there other simpler but feasible alternatives? Recently, there have been sparse case reports of using free fascial lata to repair dura defects at the site of infections.24,25 In our case serials, 19 patients with MDR pathogen-induced postcraniotomy SSIs were successfully cured after using free fascial lata to repair dural defects. To the best of our knowledge, this is the largest case series of such infection cured to have been reported.
In various neurosurgical procedures, the fascia lata is a commonly-used substitute for dura repair. As a non-immunogenicity autograft, the host response is negligible, eliciting a minimal inflammatory reaction. With the merits of flexibility and mechanical strength similar to native dura, it can be sutured to the entire circumference of the dural and maintain integrity against elevated intracranial pressure. Unique characteristics contributing to its ability of infection tolerant lie in its ability to provide a histological scaffold for integration with the native dura, facilitating its revascularization and rapid wound healing.26–28 In an animal model, when the implanted free fascial lata is covered by vascularized tissue, there is histological evidence exhibiting fibroblastic proliferation and the formation of connective tissue fibers both on the surface of the implanted fascia and in its connecting site with surrounding residual dura.3 In the subsequent cranioplasty, we observed a continuous oozing that required coagulation hemostasis from the surface of the implanted fascia lata. The phenomenon is also reported by Callovini et al.24 Moreover, in their study, the transplanted fascial lata showed uniformed enhancement in postoperative follow-up CT serial, evidencing the process of vascularization. It is speculated that transplanted free fascia lata can be nourished through the overlapping scalp and the surrounding dura mater, creating the ability for improved resistance to infection, even in MDR bacteria-induced infective circumstances. Based on the above understanding, we emphasize that the graft of fascia lata should be used in the way of suturing, not only for definite watertight closure but also for a rapid healing process with native dura mater. The transplanted free fascia should be covered with well-vascularized tissue ensuring the fascia become viable. In those patients with compromised scalp or scalp defect, or incision ill-being, a piece of pedicled temporalis muscle can be contrived to transfer to the area where the fascia lata cannot be covered with a well-vascularized scalp flap. In our group, we made such an arch rotation of pedicle temporalis muscle in three patients; hence, a much more complicated surgery involving free tissue transfer could be avoided. However, free flaps must be used for reconstruction if the scalp defects are so large.
The accomplishment of debridement and reconstruction procedures was only the first step toward success. A more comprehensive approach was necessitated. The focus of postoperative management is on the rapid eradication of pathogenic bacteria. It depends on the appropriate administration of the antimicrobial agent. However, MDR/XDR strains remain resistant to most commercially available antibiotics. Fortunately, colistin, a revived antibiotic from the 1950s, is the only available antimicrobial agent active against most Gram-negative pathogens and is regarded as the last therapeutic resort for treating meningitis and ventriculitis caused by such pathogens.29 Due to the poor ability to penetrate into CSF, local use of colistin bypasses the blood–CSF barrier, with the advantage of achieving high CSF concentrations without high blood concentrations. Thus, IVT or ITH administration of colistin has been applied as the first-choice treatment for ventriculitis/meningitis caused by Gram-negative MDR /XDR strains. Tigecycline is an alternative antimicrobial agent that can also be administrated via the intraventricular route to treat ventriculitis.30 Procedures including intraoperative intraventricular lavage and continuous drainage of CSF are also crucial for rapid sterilization of CSF.Moreover, continuous CSF drainage can reduce the tension of dura and wound, favoring their healing process. Finally, dedicated critical care for these severely infected patients is mandatory to avoid systemic and neurological complications. All these comprehensive approaches contribute to achieving an optimal healing environment, leading to the cure of infection, improved survival rate, and a favorable overall outcome.
It is reported that complications associated with harvesting fascia lata include an undesirable visible scar along the lateral thigh and the potential for gait disturbance, compromised knee stability due to reduction in quadriceps muscle power, and muscle herniation.31,32 However, no substantial surgical site morbidity for harvesting fascia lata occurred in the cohort. Of course, most of the patients in our group survive with severe neurological deficiency, being bedridden and unconscious. Nevertheless, no functional donor site morbidity was observed in 3 well-recovered patients.
The current study has several limitations, mainly owing to its retrospective nature. It is essential to note that our study included a selected group of patients with variable clinical manifestations. The sample size (n=19) is relatively small. Considering that the specific situation in each patient depends on the pathogenesis and course of the SSIs after the predisposing event, the therapeutic approach cannot be standardized accordingly. Furthermore, we recognize that a relatively abbreviated period for follow-up in some patients is insufficient for such infectious disease because relapse of infection is another issue of concern over a long period.33 Further investigations should be moved forward in the future.
Conclusion
This study aims to evaluate the feasibility and efficacy of free fascia lata as dura graft in the reconstruction of dura for intricate postcraniotomy SSIs caused by MDR gram-negative bacteria. As per the results of our investigation, both mortality and relapse of infection are significantly diminished, suggesting that the free fascia lata can serve as a dura substitute, even in those patients who otherwise need much more complicated and demanding tissue transfer surgery.
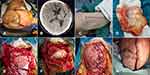
Case 1. A 65-year-old male experienced emergent decompressive craniotomy (DC) for traumatic brain injuries 2 months before his referral. The patient once recovered well. But with the emergency of CSF leakage and later purulent discharge from the incisions, wound dehiscence expands progressively, and the patient’s status deteriorates gradually. An MDR Acinetobacter strain sensitive only to colistin and tigecycline was isolated from the discharge from wound swab specimens in a bacteriological culture. The attempts, including various advance antibiotics and repeated bedside debridement, failed. The patients deteriorated with high fever and decreased consciousness. At admission, a scalp defect measuring 6cm*4cm can be observed with the exposed cerebral cortex, a piece of artificial dura mater, and purulent discharge. The tension of the flap increased moderately. A lumbar catheter was indwelled before the emergent debridement surgery. A large piece of fascia lata was harvested. After controlled CSF drainage, the incision was created along with the previous incision and extended toward the direction of the zygomatic arch to increase the exposure of the temporalis muscle. After the scalp flaps were unveiled, the infected bovine pericardium was removed, and subdural empyema was irrigated with copious polymyxin E solution. The cortex was affected mildly. The trimmed fascia lata graft was sewed to the residual dura closely in a tension-reduced fashion. Part of the temporalis muscle was dissected and used as a pedicled flap transferred to the area of scalp deficiency. The compromised incisional edge was carefully resected. After extensive subgaleal dissection, the scalp incision can be sutured in a tension-reduced manner. The lumbar catheter was kept for about 10 days for continuous CSF drainage. One month after debridement surgery, the scalp incision sealed, and the patient recovered well with GOS score of 4. The process of management is illustrated in Figure 1.
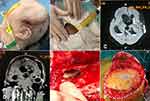
Case 2. A 58-year-old male had emergent DC to remove intracerebral hematoma 2 months before his referral. In the preceding surgery, a piece of suture-free Tissudura was covered over the surface of the cortex. The patient suffered from a high fever 1 week after the surgery. CSF sampled from lumbar acupuncture confirmed meningitis with a strain of MDR Pseudomonas aeruginosa. Three weeks after the craniectomy, the incision broke down with intermittent purulent discharge. Progressive hydrocephalus developed in the patients and the extraventricular drainage (EVD) and Ommya systems had been successively indwelled. Multiple antimicrobial agents, including intravenously and intraventricularly administered polymyxin E, failed to cure the infection. Therefore, the patient was referred. At admission, the patient was in comatose status. An apparent chronic fistula with purulent effusion can be observed. Diffusion Weight imaging of MR exhibited a high signal in the bilateral ventricle, intraparenchymal, and sub-scalp space, evidencing a complex surgical site pyrogenic infection. The causative pathogen cultured from purulent fluid aspired from the sub-scalp cystic fluid collection was confirmed to be MDR Pseudomonas aeruginosa. The infected artificial dura mater, subdural empyema, and intracerebral abscess were removed thoroughly in the debridement. The dura mater was carefully reconstructed with the free fascia lata. The skin fistula was resected. A new ventricular catheter with a long tunnel was indwelled. After a long-term administration of antibiotics, the SSIs in this patient were eventually cured. Major clinical presentations and management are shown in Figure 2.
Acknowledgments
Thank Miss Jiao Li and Meiying Yu for their efforts in manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362(2):146–154. doi:10.1056/NEJMra0804573
2. Kim BN, Peleg AY, Lodise TP, et al. Management of meningitis due to antibiotic-resistant Acinetobacter species. Lancet Infect Dis. 2009;9(4):245–255. doi:10.1016/s1473-3099(09)70055-6
3. Tachibana E, Saito K, Fukuta K, Yoshida J. Evaluation of the healing process after dural reconstruction achieved using a free fascial graft. J Neurosurg. 2002;96(2):280–286. doi:10.3171/jns.2002.96.2.0280
4. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
5. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi:10.1016/j.ajic.2008.03.002
6. Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. doi:10.1016/s0140-6736(75)92830-5
7. Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 infectious diseases society of America’s Clinical Practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi:10.1093/cid/ciw861
8. Kourbeti IS, Jacobs AV, Koslow M, Karabetsos D, Holzman RS. Risk factors associated with postcraniotomy meningitis. Neurosurgery. 2007;60(2):317–25;discussion 325–6. doi:10.1227/01.neu.0000249266.26322.25
9. Beer R, Lackner P, Pfausler B, Schmutzhard E. Nosocomial ventriculitis and meningitis in neurocritical care patients. J Neurol. 2008;255(11):1617–1624. doi:10.1007/s00415-008-0059-8
10. Luque-Paz D, Revest M, Eugène F, et al. Ventriculitis: a severe complication of central nervous system infections. Open Forum Infect Dis. 2021;8(6):ofab216. doi:10.1093/ofid/ofab216
11. Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 2006;104(1 Suppl):16–20. doi:10.3171/ped.2006.104.1.16
12. Hida K, Yamaguchi S, Seki T, et al. Nonsuture dural repair using polyglycolic acid mesh and fibrin glue: clinical application to spinal surgery. Surg Neurol. 2006;65(2):136–42;discussion 142–3. doi:10.1016/j.surneu.2005.07.059
13. Dufrane D, Marchal C, Cornu O, Raftopoulos C, Delloye C. Clinical application of a physically and chemically processed human substitute for dura mater. J Neurosurg. 2003;98(6):1198–1202. doi:10.3171/jns.2003.98.6.1198
14. Sabatino G, Della Pepa GM, Bianchi F, et al. Autologous dural substitutes: a prospective study. Clin Neurol Neurosurg. 2014;116:20–23. doi:10.1016/j.clineuro.2013.11.010
15. Zhang GL, Yang WZ, Jiang YW, Zeng T. Extensive duraplasty with autologous graft in decompressive craniectomy and subsequent early cranioplasty for severe head trauma. Chin J Traumatol. 2010;13(5):259–264.
16. Malliti M, Page P, Gury C, Chomette E, Nataf F, Roux FX. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery. 2004;54(3):599–603;discussion 603–4. doi:10.1227/01.neu.0000108640.45371.1a
17. Mikami T, Minamida Y, Sugino T, Koyanagi I, Yotsuyanagi T, Houkin K. Free flap transfer for the treatment of intractable postcraniotomy subdural empyemas and epidural abscesses. Neurosurgery. 2007;60(2Suppl 1):ONS83-7;discussion ONS87–8. doi:10.1227/01.neu.0000249253.63546.19
18. Shimada K, Ishikura N, Heshiki T, Kawakami S. Treatment for chronic abscess after cranioplasty: reconstruction of dura maters using the anterolateral thigh flap with fascia lata. J Craniofac Surg. 2007;18(6):1305–1308. doi:10.1097/scs.0b013e31811ec238
19. Abuzayed B, Kafadar AM, Oğuzoğlu SA, Canbaz B, Kaynar MY. Duraplasty using autologous fascia lata reenforced by on-site pedicled muscle flap: technical note. J Craniofac Surg. 2009;20(2):435–438. doi:10.1097/scs.0b013e31819b968f
20. Uemura T, Suse T, Yokoyama T, Mitsukawa N, Yoshikawa A, Anegawa S. Staged cranial reconstruction after epidural abscess associated with dural substitute exposure. J Craniofac Surg. 2002;13(3):415–417. doi:10.1097/00001665-200205000-00011
21. Soon Sung K, Hak C. Staged reconstruction of infected dura mater using vascularized rectus abdominis muscle. J Craniofac Surg. 2012;23(6):1741–1743. doi:10.1097/SCS.0b013e31825877ee
22. West CA, Towns G, Bachelor AG, Liddington MI. Reconstruction of skull base and dura using rectus abdominis muscle combined with a vascularised fascial perforator flap. J Plast Reconstr Aesthet Surg. 2006;59(6):631–635. doi:10.1016/j.bjps.2005.06.001
23. Barrientos S, Leif M, Hon HH, Aizenberg M, Wong S. Duraplasty using autologous fascia lata and latissimus dorsi free flap for chronic cerebrospinal fluid leak. J Craniofac Surg. 2019;30(7):e671–e674. doi:10.1097/scs.0000000000005747
24. Callovini GM, Bolognini A, Callovini T, Giordano M, Gazzeri R. Treatment of CSF leakage and infections of dural substitute in decompressive craniectomy using fascia lata implants and related anatomopathological findings. Br J Neurosurg. 2021;35(1):18–21. doi:10.1080/02688697.2020.1735301
25. Nakano T, Yoshikawa K, Kunieda T, et al. Treatment for infection of artificial dura mater using free fascia lata. J Craniofac Surg. 2014;25(4):1252–1255. doi:10.1097/scs.0000000000000929
26. Chang DW, Langstein HN, Gupta A, et al. Reconstructive management of cranial base defects after tumor ablation. Plast Reconstr Surg. 2001;107(6):1346. doi:10.1097/00006534-200105000-00003
27. Gök A, Erkutlu I, Alptekin M, Kanlikama M. Three-layer reconstruction with fascia lata and vascularized pericranium for anterior skull base defects. Acta Neurochirurgica. 2004;146(1):53–57. doi:10.1007/s00701-003-0175-2
28. Zhang F, Zeng T, Gao L, Cui DM, Cao XY. Treatment of traumatic cerebrospinal fluid rhinorrhea via extended extradural anterior skull base approach. Chin J Traumatol. 2021;24:280–285. doi:10.1016/j.cjtee.2021.06.002
29. Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41(6):499–508. doi:10.1016/j.ijantimicag.2013.02.006
30. Lauretti L, D’Alessandris QG, Fantoni M, et al. First reported case of intraventricular tigecycline for meningitis from extremely drug-resistant Acinetobacter baumannii. J Neurosurg. 2017;127(2):370–373. doi:10.3171/2016.6.jns16352
31. Dubiel WT, Wigren A. Functional status of the lower extremity after resection of fascia lata. A clinical and physiological follow-up study in patients with fascia lata heart valve replacement. Acta Orthop Scand. 1974;45(4):599–613. doi:10.3109/17453677408989183
32. Wheatcroft SM, Vardy SJ, Tyers AG. Complications of fascia lata harvesting for ptosis surgery. Br J Ophthalmol. 1997;81(7):581–583. doi:10.1136/bjo.81.7.581
33. Kasiakou SK, Rafailidis PI, Liaropoulos K, Falagas ME. Cure of post-traumatic recurrent multiresistant Gram-negative rod meningitis with intraventricular colistin. J Infect. 2005;50(4):348–352. doi:10.1016/j.jinf.2004.05.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.