Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Autologous adipose transplantation an effective method to treat alopecia after trauma: a case report
Authors Nilforoushzadeh MA, Lotfi E , Heidari-Kharaji M
Received 26 May 2019
Accepted for publication 30 July 2019
Published 3 September 2019 Volume 2019:12 Pages 647—651
DOI https://doi.org/10.2147/CCID.S217203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Mohammad Ali Nilforoushzadeh1,2, Elaheh Lotfi1,2, Maryam Heidari-Kharaji1,2
1Skin and Stem Cell Research Center, Tehran University of Medical Sciences, Tehran, Iran; 2Jordan Dermatology and Hair Transplantation Center, Tehran, Iran
Correspondence: Mohammad Ali Nilforoushzadeh
Skin and Stem Cell Research Center, Tehran University of Medical Sciences, No. 4, Maryam Blind Alley, South Kamranieh Street, Tehran 1937957511, Iran
Tel +98 212 221 5558
Email [email protected]
Abstract: Finding new therapies for male and female pattern hair loss treatment remains as great interest. The autologous fat grafting technique is a new method, and clinical experience was increased over the past 10–15 years. Adipose tissue is a biologically active and important tissue and can help drive the complex hair growth cycle. Surgeons have previously reported that autologous fat not only have a positive affect for reconstructive volume and esthetics after transplant but can also have positive skin and hair changes post-transplantation. In this study, lipoaspiration of adipose was performed by cannula and scalp injection was done. In this case report, the authors report that scalp injection of adipose tissue has a positive effect on a patient with alopecia after trauma. The findings suggest adipose tissue can be a promising alternative method to treating men and women alopecia.
Keywords: alopecia, adipose, trauma, transplantation
Introduction
Alopecia is a common problem, but the development of pharmacological solutions is limited because of the lack of any direct health effects from hair loss. It should be mentioned that the treatment should be effective and has no side effects as well.1 Current therapies are most topically to decrease the risk of systemic side effects, but this can be an inopportune method of application in the cases that the hair loss is minimal, and often reduces the treatment efficacy. Such therapies have little effect on the global problem of alopecia, and patients will often choose the surgical options that have greater costs and risks, but have markedly improved results.1 There are some treatment options for men and women with alopecia such as hair transplant surgery, medical therapy, micropigmentation of the scalp, low-level laser therapy, and hair systems.2–8 These routes are not without deficiencies, so researchers are permanently searching for new and alternative treatments.9–12 One developing area of clinical and scientific focus is the study of the role of adipose tissue (fat), and specifically autologous adipose transplantation, on the hair growth cycle. Nowadays, autologous fat is transplanted mainly for esthetic applications and reconstructive volume effect.13 Autologous fat injection is a potential technique that can be used to promote hair growth. This was discovered incidentally by observing effects after the transplant of autologous fat on hair growth.14 Further than volumization, some surgeons have informed positive skin and hair changes after transplantation.15,16
With this background, we stimulated to ask if one could restore or drive the hair cycle by transplanting adipose to the scalp in the patient with scarring alopecia. In this case report, the authors’ experience and results are reported.
Case
A 28-year-old female single patient, who was graduated from a university, and working as a painter. When she was a child, she was riding a carousel and her hair stuck to the carousel and her scalp was lifted. So she had a scarring alopecia because of trauma. Therefore, the patient applied to our clinic with a severe bad presentation of alopecia. She was medically healthy and took no medications. The patient had a body mass index (BMI) within normal limits and had no comorbidities like high blood pressure or diabetes. Also, the patient had normal thyroid function and menstruation. The patient was first transplanted with autologous fat to the scalp. The patient has given her informed consent for the case details and images to be published.
Procedure
Liposuction surgical technique
On the day of surgery, lipoaspiration and scalp injection of adipose were performed. In either general anesthesia or twilight anesthesia, tumescent fluid (500 mL normal saline with 15 mL of 2% lidocaine [Pasteur institute of Iran] and 7 mL of bicarbonate and 1 mg of epinephrine) was infiltrated. After that fat tissue was harvested with a 60 mL Luer Lock syringe from the thigh by using a 2.4 mm cannula (Figure 1). The volume of adipose that was harvested from the patient was about 60 mL. The lipoaspirate was distributed into three aliquots (20 mL). For the first injection, two aliquots (40 mL) were utilized. The adipose was injected into the scalp of the patient. Other aliquots were frozen for further use. Pictures were obtained before and 3 and 9 months after the procedure to compare the results.
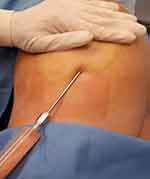 |
Figure 1 Small-volume liposuction. |
Scalp injection
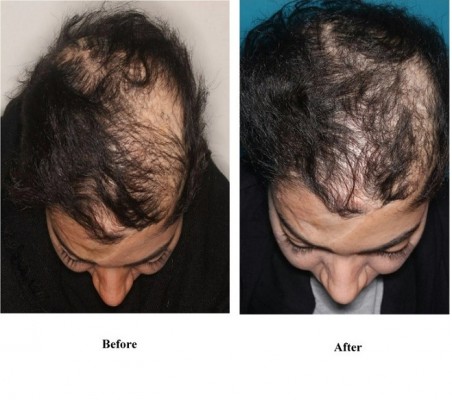
Whereas still under general or twilight anesthesia, the affected area of the scalp of the patient was managed an anesthetic ring block with 1% lidocaine (1:200,000 epinephrine) solution to allow for sufficient and appropriate local anesthesia. When the patient awakens from general anesthesia, she was moved to a standard clinic procedure room. The volume of purified adipose injected into the scalp was 1.0 mL/cm2 scalp (Figure 2). Also, it should be noted that a low level of pain in the patient was reported. After scalp injection, the patient remained in the procedure room for 1 hr to observe and monitor for any complications. Three months after the first injection, the patient was referred to the clinic for the second injection, and the injection was done using the freezed fat tissue according to the previous procedure. The patient agreed to try it again because of the good efficacy. So, 6 months after the second injection, the third injection was performed and lipoaspiration was performed again, and all of the procedures were done as previously described. After the final injection, the patient was followed up and she was very pleased with the results of the transplantation (Figure 3). The percentage of 4 physician’s satisfaction after 3 and 6 months is shown in Table 1. Our results showed that autologous adipose transplantation can start and stimulate the hair growth cycle.
 |
Table 1 The percentage of physician’s satisfaction after 3 and 6 months |
 |
Figure 2 Scalp area injection. |
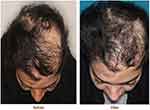 |
Figure 3 The case after 6 months of transplantation. The left picture relates to before and the right picture shows 6 months after transplantation. |
Discussion
While alopecia is a common problem, there is no effective treatment.17 Current therapies consist of repurposed drugs and most of them are used topically to decrease the risk of systemic side effects do not have good effects on hair regrowth. Alopecia treatment can be performed in multiple ways and here have been recent studies investigating therapies designed specifically to regrow hair. Until now, no studies have shown satisfactory results for hair treatment by using adipose tissue. The role of adipocytes in hair cycling was explained in some studies.17,18 It should be noticed that the risk of fat embolism in the fat grafting for the hair lost treatment is low because the amount of the harvest and injection is very low, also by using epinephrine and vasoconstrictors the risk of fat embolism is more reduced.19,20 Most of the previous studies were based on hematopoietically derived plasma or ADSVC conditioned medium (ADSVC-CM).21,22 In a case study by Shin et al, the clinical use of the media of adipose tissue–derived stem cells was investigated in 27 patients with female pattern hair loss by analysis of patient medical records and phototrichographic images.21 They showed hair density and thickness increased from 105.4 to 122.7 hairs per cm2 and 57.5–64.0 mm, respectively. In the study of Fukuoka et al, adipose-derived stem cell–conditioned medium (ADSC-CM) was injected intradermally in 22 patients (11 men and 11 women) every 3–5 weeks for 6 sessions.22 In this study, the 31-gauge needle was used to provide the ADSC-CM. Finasteride was used in 6 of the 11 male patients. Also before and then 7–12 months after the initial treatment, trichograms were taken. The mean increase in the number of hairs in men and women was 29±4.1 and 15.6±4.2, respectively, and no significant difference was detected between the finasteride and no finasteride group and the number of hairs was significantly higher in the treated group compared to placebo. In another study by Fukuoka et al in 2017, they evaluated the effect of the same medium in 21 patients (16 men and 5 women) in the same way.23 It was informed that the number of hairs increased from baseline of 141.2±31.4 in men and 109.8±43.5 in women after 3 months. Also, they reported that hair quality was increased, and a significant difference in increased hair counts between the treatment and placebo was observed (18.4±9.4 vs 6.5±11.7). In astudy by Gentile et al, 23 patients with androgenetic alopecia Norwood 3 to 5 were treated with human follicles isolated stem cells (HFSCs).24 Their results show a 29±5% increase in hair density when compared to a <1% increase in the placebo area 23 weeks after the treatment. This is the first study with HFSCs that indicated a positive therapeutic effect on male androgenic alopecia (AGA). In the study of the Anderi et al, the effect of ADSVCs was evaluated on 20 patients (9 women and 11 men) with hair loss alopecia areata.25 After lipoaspiration, autologous ADSVCs were produced and characterized before cell injection into the scalp of the patient, and hair regeneration was measured by three clinical tests including the hair quality, pull test, and hair density. Their results show the increased hair growth and decreased pull test 3 and 6 months after ADSVC treatment [hair density (85.1±8.7 vs 121.1±12.5 hair/cm2), hair diameter (60.5±1.8 vs 80. 8±2.4 μ), and pull-test values (4.4±0.3 vs 0.8±0.2)]. The application of adipose derived mesenchymal cells was reviewed in other studies.26,27 In this study, the results indicated that the transplantation of autologous adipose tissue is safe and effective and can be used as an effective cell-based therapy for the treatment. Our results show that hair growth was noticeably improved 6 months after the treatment. This can be explained by the injected adipose tissue releasing growth factors and stimulating vascularization, promoting the formation of new capillaries, enhancing the production of hair, and improving the source of blood to the scalp so it makes available a perfect environment for the hair follicles to produce new, and healthy hair. This study is the beginning of many series of studies and more complete clinical work is needed.
Conclusion
In conclusion, treatment using adipose tissue as the easiest reachable source of mesenchymal stem cells seems very effective for alopecia and hair loss and can be a new therapeutic option for hair regeneration. Adipose tissue has a huge potential for hair regeneration by increasing the growth of healthy hair.
Ethical statement
The authors state that the patient has given her informed consent for case details and images to be published.
Acknowledgment
We acknowledge the colleagues and staff of the Jordan Clinic, Tehran University of Medical Sciences, Tehran, Iran.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zarbo A, Shwayder T. Loose anagen hair syndrome. J Pediatr. 2018;199:282–282. e1. doi:10.1016/j.jpeds.2018.03.005
2. Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging. 2007;2(2):189.
3. Ghanaat M. Types of hair loss and treatment options, including the novel low-level light therapy and its proposed mechanism. South Med J. 2010;103(9):917–921. doi:10.1097/SMJ.0b013e3181ebcf71
4. Shapiro J, Kaufman KD. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss).
5. Ross EK, Shapiro J. Management of hair loss. Dermatol Clin. 2005;23(2):227–243. doi:10.1016/j.det.2004.09.008
6. Leavitt M, Perez-Meza D, Rao NA, Barusco M, Kaufman KD, Ziering C. Effects of finasteride (1 mg) on hair transplant. Dermatol Surg. 2005;31(10):1268–1276.
7. Leavitt M, Perez-Meza D, DiBernardo B. Low level laser therapy in hair loss. Laser and non-surgical rejuvenation. Tech Aesthet Plast Surg. 2009;19(2):141–153.
8. Perez-Meza D The use of SVF and PRP for the treatment of Scarring and non-Scarring Alopecias. In:
9. Perez-Meza D, Niedbalski R. Complications in hair restoration surgery. Oral Maxillofac Surg Clin North Am. 2009;21(1):119–148. doi:10.1016/j.coms.2008.10.010
10. Vogel JE. Correction of the cornrow hair transplant and other common problems in surgical hair restoration. Plast Reconstr Surg. 2000;105(4):
11. Mysore V. Finasteride and sexual side effects. Indian Dermatol Online J. 2012;3(1):62. doi:10.4103/2229-5178.93496
12. Magerl M, Paus R, Farjo N, et al. Limitations of human occipital scalp hair follicle organ culture for studying the effects of minoxidil as a hair growth enhancer. Exp Dermatol. 2004;13(10):635–642. doi:10.1111/j.0906-6705.2004.00207.x
13. Zhu M, Zhou Z, Chen Y, et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64(2):222–228. doi:10.1097/SAP.0b013e31819ae05c
14. Festa E, Fretz J, Berry R, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–771. doi:10.1016/j.cell.2011.07.019
15. Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28(1):111–119.
16. Dini M, Mori A, Li AQ. Eyebrow regrowth in patient with atrophic scarring alopecia treated with an autologous fat graft. Dermatol Surg. 2014;40(8):926–928. doi:10.1111/DSU.0000000000000051
17. Kruglikov IL, Zhang Z, Scherer PE. The role of immature and mature adipocytes in hair cycling. Trends Endocrinol Metab. 2018;30(2):93–105.
18. Wollina U, Abdel N, Kruglikov I. Dermal adipose tissue in hair follicle cycling: possible applications in alopecia? Georgian Med News. 2017;265:41–45.
19. Fourme T, Vieillard-Baron A, Loubieres Y, Julie C, Page B, Jardin F. Early fat embolism after liposuction. Anesthesiology. 1998;89(3):782–784. doi:10.1097/00000542-199809000-00031
20. Mofid MM, Teitelbaum S, Suissa D, et al. Report on mortality from gluteal fat grafting: recommendations from the ASERF task force. Aesthet Surg J. 2017;37(7):796–806. doi:10.1093/asj/sjx004
21. Shin H, Ryu HH, Kwon O, Park B-S, Jo SJ. Clinical use of conditioned media of adipose tissue‐derived stem cells in female pattern hair loss: a retrospective case series study. Int J Dermatol. 2015;54(6):730–735. doi:10.1111/ijd.12650
22. Fukuoka H, Suga H. Hair regeneration treatment using adipose-derived stem cell conditioned medium: follow-up with trichograms. Eplasty. 2015;15:65–72.
23. Fukuoka H, Narita K, Suga H. Hair regeneration therapy: application of adipose-derived stem cells. Curr Stem Cell Res Ther. 2017;12(7):531. doi:10.2174/1574888X12666170522114307
24. Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Stem cells from human hair follicles: first mechanical isolation for immediate autologous clinical use in androgenetic alopecia and hair loss. Stem Cell Investig. 2017;4. doi:10.21037/sci
25. Anderi R, Makdissy N, Azar A, Rizk F, Hamade A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res Ther. 2018;9(1):141. doi:10.1186/s13287-018-0889-y
26. Amirkhani, MA., et al., Literature review of adipose-derived mesenchymal cells from history to approaches. Iranian Red Crescent Medical Journal. 2016;19(1):e22940.
27. Nilforoushzadeh, M.A., et al., Engineering the niche for hair regeneration–a critical review. Nanomedicine: Nanotechnology, Biology and Medicine. 2018;15(1):70–85.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
