Back to Journals » Patient Preference and Adherence » Volume 11
Autoinjector preference among patients with multiple sclerosis: results from a national survey
Authors Limmroth V, Reischl J, Mann B, Morosov X , Kokoschka A, Weller I, Schreiner T
Received 22 March 2017
Accepted for publication 24 June 2017
Published 3 August 2017 Volume 2017:11 Pages 1325—1334
DOI https://doi.org/10.2147/PPA.S137741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
V Limmroth,1 J Reischl,2 B Mann,3 X Morosov,2 A Kokoschka,2 I Weller,2 T Schreiner2
1Clinic for Neurology and Palliative Medicine, Municipal Hospital Köln-Merheim, Cologne, 2Bayer Vital GmbH, Leverkusen, 3IFAK Institute GmbH & Co. KG, Taunusstein, Germany
Purpose: Autoinjectors are well-established in supporting multiple sclerosis (MS) therapy. This market survey was aimed at investigating patients’ rating of three devices for subcutaneous interferon beta formulations: the electronic autoinjectors Betaconnect® and RebiSmart™ as well as the mechanical ExtaviPro™ device.
Patients and methods: Organization and conduction of structured face-to-face interviews in five German cities were managed through an independent external market research company. After questionnaire validation (n=15), 85 participants currently either using the Betaconnect (n=39), the RebiSmart (n=36) or the ExtaviPro injector (n=10) were asked 22 questions in the same order. First, patients named their current device in use, watched the corresponding instruction video, and were queried about their device. Second, patients were asked about their opinion of an ideal autoinjector. Third, instruction videos for the two non-used devices were presented and participants could dummy-inject into a pillow. Last, patients evaluated device features and indicated their preferred autoinjector.
Results: Before having been presented the two other autoinjectors not in use, evaluation of patients’ satisfaction with their own device revealed that 82% of the Betaconnect users, 67% of the RebiSmart and 60% of the ExtaviPro users were highly satisfied. All patients desired some improvement of their own device particularly concerning optimization of size and handling. Subsequent to testing and watching instruction videos of all devices, the Betaconnect received the best rating regarding different functions. Finally, participants indicated their preferred autoinjector, provided their own medication was suitable for all three devices: 56.5% of the participants (n=48/85) chose the Betaconnect, 36.5% the RebiSmart (n=31/85), and 5% the ExtaviPro device (n=4/85); 2% did not answer (n=2/85).
Conclusion: In this survey, the Betaconnect device was the preferred autoinjector and may currently best meet patients’ needs. As it was closest to participants’ opinion of an ideal device, the Betaconnect might contribute to treatment adherence. Our results need to be confirmed in further studies.
Keywords: adherence, multiple sclerosis, immunomodulatory therapy, electronic autoinjector, market survey
Introduction
Injectable subcutaneous (s.c.) therapies are well-established first-line multiple sclerosis (MS) treatments and the benefits of autoinjectors in the application of these treatments are widely accepted. As for other therapies, adherence may influence treatment success.1,2 Besides efficacy of a therapeutic strategy, major factors for patient adherence to the prescribed medication and satisfaction with self-administered injections are ease of application as well as acceptable side-effect profiles.2 In addition, MS worsening might induce deteriorations in cognitive and fine-motor skills which interfere with manual injection and subsequently with adherence.2,3
Interferon beta preparations have been available for decades and extensively tested for efficacy and safety. Over time, these agents underwent improvements with respect to injection tolerability as well as handling (e.g., titration at therapy initiation).2,4 The invention of injection devices reduced the need for manual injection5–10 and its use significantly reduced the occurrence of injection site reactions.11 Moreover, their use was found to be a predictor of adherence.12
Autoinjection devices simplify MS therapy and often provide additional features to improve patient comfort and adherence. These features comprise reminder functions – as forgetfulness is a major obstacle for self-administered therapies2,3,13 – simplicity, adjustability of injection speed and depth, optical and acoustic signaling at the beginning/end of the injection process, hidden needle, low-force safety release to ensure the device is positioned on the skin correctly at the time of injection, and an LED-display for visualization of the injection progress.6,8,10,14,15
Several autoinjection devices for application of immunomodulatory drugs are currently available in the MS market and characterized by different handling, features, and design. Among these, the Betaconnect® (Medicom Innovation Partner A/S, Struer, Denmark) and the RebiSmart™ (Ares Trading SA, Coinsins, Switzerland) are the only electronic devices in the MS therapy field, all others are mechanical devices.8,14 Recently in a survey of US patients, the electronic Betaconnect autoinjector has been rated higher than mechanical devices.15
In this market survey, the purpose was to investigate patients’ evaluation of autoinjectors typically used for the application of s.c. interferon beta formulations: the Betaconnect and the RebiSmart electronic autoinjectors as well as the ExtaviPro™ (Owen Mumford Ltd., Oxford, UK) mechanical autoinjection device.
Material and methods
Participants
This market survey was conducted with 100 MS patients from five German cities (Berlin, Frankfurt, Hamburg, Cologne, and Munich) between February and April 2016. The participants were selected via a screening questionnaire containing questions about age, gender, MS diagnosis, medication, and autoinjector use. All participants signed a written informed consent form for this survey. The initial 15 participants served for validation of the questionnaire. After the validation, video instructions were presented to the patients to ensure that every patient got the same information about each device. The main analysis group of patients (n=85) was included in data analysis. Of these participants, 39 currently used the Betaconnect autoinjector, 36 the RebiSmart, and 10 the ExtaviPro device for application of their s.c. interferon beta therapy.
Interviews
Structured face-to-face interviews (40–45 minutes per interview) were organized and conducted through an independent external market research company (IFAK Institut GmbH & CoKG, Taunusstein, Germany) according to international quality standards (e.g., ISO 20252:2012). Participants did not receive any information about the survey initiator and sponsor. Subsequent to the interviews, all participants were compensated for their time with an allowance. The IFAK Institut GmbH & CoKG operates in accordance with all requested Market Research guidelines and follows the Freiwillige Selbstkontrolle für die Arzneimittelindustrie e.V. [Voluntary Self-regulation for the Pharmaceutical Industry] (FSA)-Codex. In case any notifiable adverse events (AEs) are reported of a drug, product or medicine during one or more interviews, IFAK Institut GmbH & CoKG obligates oneself to document these AEs following the client’s standard and forward all information to the client’s safety drug department.
Questionnaire
The questionnaire was developed in close collaboration with Bayer Vital GmbH, Leverkusen, Germany, this information was not communicated to the participants. Questionnaire validation for comprehensibility was conducted with a subgroup of participants (n=15). Data from the validation group were collected in the same way and used for comparisons with the main group (n=85). These data were not included in overall data analysis.
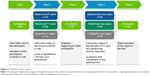
All patients were asked 22 questions in the same order. Depending on the question, answers were given openly, by marking or via a rating scale (1–10, with 10 as best rating). First, participants indicated their autoinjector currently in use, and watched an instruction video – except for the validation group (n=15) where the interviewer presented the patients’ own devices to them instead of video instructions. Then patients answered questions about their satisfaction with the device in use. Second, they were asked for their view of an ideal autoinjector for MS therapy. Third, participants watched instruction videos for the two devices currently not in use (except for the validation group [n=15] where the interviewer presented the two devices currently not in use to the patients instead of video instructions) and had the opportunity to dummy-inject into a pillow. Finally, the patients were asked to compare the devices as well as their functions and to determine the preferred autoinjector, provided their current medication was suitable for all three devices (Figure 1, Table S1).
Statistical analysis
Analyses were primarily of descriptive nature (median, mean, standard deviation). Statistical tests were performed for exploratory purposes and involved Student’s t-test for independent samples.
Results
Demographic characteristics
The majority of the surveyed participants were between 18 and 55 years old (29% 18–35 years, 52% 36–55 years, 18% 56–75 years) and 68% were female. Among participants, 63 had relapsing-remitting MS, 6 had secondary progressive MS, and 16 patients could not specify their MS type. The participants of the validation group were younger, 80% were female, and only from one location (Table 1).
  | Table 1 Demographic characteristics |
Currently, 39 participants used the Betaconnect, 36 the RebiSmart, and 10 the ExtaviPro device. Four of the 85 patients (5%) had earlier experiences with one of the autoinjectors assessed in this survey (Betaconnect [n=2], RebiSmart [n=1], and ExtaviPro [n=1]). There were 14 patients in the main group and 2 patients in the validation group who had used other injection devices not part of this market research.
Satisfaction with autoinjector currently in use
Before presentation of the devices not in use, participants were asked to rate the level of satisfaction with their own device on a scale (1= not satisfied at all, 10= very satisfied). There were 82% of the Betaconnect users who were highly satisfied with their device (8–10 points, mean 8.5), as well as 67% of the RebiSmart (mean 7.9) and 60% of the ExtaviPro users (mean 7.5) (Figure 2). In the validation group, 3 of 7 Betaconnect users, 3 of 5 RebiSmart users, and 1 of 3 ExtaviPro users indicated high levels of satisfaction with their device.
Betaconnect users (n=39) especially appreciated ease of handling (41% of n=39), reminder for next injection (31%), low noise (18%; P<0.05 with respect to RebiSmart users), appealing design (21%) as well as leads patients clearly through the procedure (18%), and pain-free injection (18%). Among RebiSmart users (n=36), ease of handling (36%) and ease of operation (28%) were the most favored features, as well as the design (25%), leads patients clearly through the procedure (22%), and the fact that no syringe is visibly held in the hand (36%; P<0.05 with respect to Betaconnect users and ExtaviPro users). ExtaviPro users (n=10) favored that the device is handy (50%; P<0.05 with respect to Betaconnect and RebiSmart users), has an appealing design, and easy handling (30% each). Inconveniences to the disadvantage of ExtaviPro particularly concerned unpleasant noise (30%; P<0.05 with respect to Betaconnect users), size (20%; P<0.05 with respect to Betaconnect users) as well as unhandiness (20%; P<0.05 with respect to Betaconnect users), and to the disadvantage of RebiSmart, weight (17%; P<0.05 with respect to Betaconnect users) and problems with the needle (17%; P<0.05 with respect to Betaconnect users). There were 18% of the Betaconnect patients who complained about the necessity of high pressure/tension when the device is applied to the skin (multiple answers possible).
Most participants in the main analysis group and in the validation group desired improvements of their own device that concern reduction of weight/noise and enhancement of handling (Table 2).
Proposed features/improvements toward ideal device
As evaluated by the participants, the most important features of an ideal autoinjector comprise general ease of the injection process, uncomplicated preparation of the device before use, possibility of usage without help from others, easy determination of injection start and stop, and ease of pressing button for start of injection (Table 3). The patients of the validation group chose the same characteristics of an ideal autoinjector except for the fact that ease of pressing button for start of injection and intuitive handling of device were more important to them than easy determination of injection start and stop.
Evaluation of the two autoinjectors not in use
After video instruction and dummy testing of the two devices not in use, participants were asked to evaluate device features of the autoinjectors not in use. The Betaconnect autoinjector received the highest valuation from non-users and the values were closest to those of an ideal injection device. Most appreciated features comprised overall easier handling, start/stop signaling, adjustment of injection speed/depth, reminder function, and visual signaling for injection progress (Figure 3, Table 3). In the validation group, the patients evaluated nearly all features of the three devices higher than patients in the main analysis group. But the ease of pressing button for start of the injection (RebiSmart mean 6.8; Betaconnect mean 8.6) and the minimal noise during injection (RebiSmart mean 7.0; Betaconnect mean 7.9) received a lower valuation for RebiSmart and Betaconnect from the patients of the validation group. The rating for the ease of applying enough pressure for starting injection (mean 6.1) and handiness of RebiSmart during injection process (mean 6.0) as well as the reminder function (mean 7.3) and uncomplicated preparation of Betaconnect before use (mean 6.5) were also lower than in the main analysis group.
In addition, participants evaluated inconveniences of the two devices not in use (percentage of non-users; multiple answers possible): Predominantly, participants disliked the weight of the RebiSmart device (43%; P<0.05 with respect to Betaconnect [9% disliked the weight] and ExtaviPro [0% disliked the weight]), lack of sufficient visual/acoustic functions of the ExtaviPro (37%; P<0.05 with respect to Betaconnect and RebiSmart), difficult handling of RebiSmart (33%; P<0.05 with respect to Betaconnect) and ExtaviPro (24%; P<0.05 with respect to Betaconnect), and unhandiness of RebiSmart (24%; P<0.05 with respect to ExtaviPro), and criticized confusing menu guidance of RebiSmart (31%; P<0.05 with respect to Betaconnect and ExtaviPro) as well as unpleasant noise of ExtaviPro (21%; P<0.05 with respect to Betaconnect and RebiSmart). Furthermore, patients disliked complicated assembly (23%) and excessive release force of the ExtaviPro (17%) (each P<0.05 with respect to Betaconnect and RebiSmart) as well as manual needle retraction of the ExtaviPro device (21%; P<0.05 with respect to Betaconnect). Relevant criticism regarding the Betaconnect autoinjector concerned its disposable syringe (20%; P<0.05 with respect to RebiSmart and ExtaviPro). The validation group participants only disliked the weight of RebiSmart (50%; P<0.05 with respect to ExtaviPro) and the unpleasant noise of ExtaviPro (50%; P<0.05 with respect to Betaconnect).
Total evaluation of all three injection devices
After presentation of all devices, participants ranked the injection devices with respect to the preferred autoinjector. On the assumption that their own medication was suitable for all three autoinjectors, 56% of the participants (n=48/85) chose the Betaconnect, 36% the RebiSmart (n=31/85), and 5% the ExtaviPro device (n=4/85); 2% did not answer (n=2/85). Thus, after device presentation, in total 9 Betaconnect non-users in the main analysis group would now prefer switching from RebiSmart (total loss; n=5) and ExtaviPro (total loss; n=6) to this autoinjector (Figure 4), provided their own medication was suitable for the Betaconnect device. A total of 8 patients of the validation group would prefer the Betaconnect, 2 patients the RebiSmart, and 5 patients the ExtaviPro autoinjector.
Discussion
Use of autoinjection devices is well-established in self-administered therapies (e.g., s.c. interferon beta formulations) and might help contribute to improved adherence, which is a hallmark for treatment success.2,12 A variety of surveys has evaluated patient satisfaction with electronic and mechanical autoinjectors as well as manual injection to date.8–10,14–19 In our survey, we queried patient needs and preferences with respect to current electronic (Betaconnect, RebiSmart) and mechanical (ExtaviPro) autoinjectors. These are autoinjectors typically available in Europe for s.c. interferon beta formulations. To our knowledge, a side-by-side evaluation of the available autoinjectors has not been performed before. In this survey, it is the first time that participants received video presentation of all evaluated devices and the possibility to dummy-inject with the two devices not used by the patient to enable objective evaluation of the device in current use and the comparator devices.
Initially, survey participants were largely satisfied with their current device in use – with slightly more satisfied Betaconnect users. These data were received before video presentation and dummy-testing of the two devices not in use. In addition, only 5% of the participants had prior experiences with one of the other two autoinjectors in this market survey. Thus, indicated satisfaction levels were mainly independent of function and features provided by the devices not in use, and participants at this time were not aware of advantages or disadvantages of their device in comparison to the other two devices.
According to our findings, the ideal injection device leads patients through the process with sufficient and clear menu guidance, accompanied by visual and acoustic signaling. Easy assembly of the device, handiness (rendering patients independent from the help of others), adjustable injection settings, and no presence of disturbing noise or movement are important features as well as a hidden needle without necessity of manual retraction. Further details concern the prevention of disposable products, presence of an easily rechargeable battery, and a reminder function for upcoming injections. Some of these aspects have also been rated important in other patient surveys.5,6,8,10,14,15 Besides convenience-associated factors, some features might have direct impact on treatment adherence: since forgetfulness as well as needle-phobia are important obstacles to adherence,3,10 a reminder of upcoming injections and a comfortable injection process (without manual needle retraction) could generally be an advantage.
Among the tested devices, the Betaconnect was rated significantly higher by non-users with respect to most queried features and closest to the participants’ ideal, which is in line with recent survey findings comparing the Betaconnect device with autoinjectors licensed in the US. Appreciated features (including overall easy injection process, handiness, and adjustability of injection) were similar to our data.15 However, a few improvements to the Betaconnect were recommended in our survey (e.g., concerning disposable and visible needle, sensitivity of the contact sensor). RebiSmart and ExtaviPro users mentioned lack of sufficient handiness of their devices. With respect to the mechanical ExtaviPro device, non-users particularly miss those features appreciated regarding the other electronic devices (e.g., sufficient signaling, reminder function).
Considering these findings, it is not surprising that after testing the devices, some participants (n=9) would switch to the Betaconnect, provided their own medication was suitable, and in return, the number of ExtaviPro and RebiSmart users would decline. It is debatable whether these participants were initially already less satisfied with their device in use or if they preferred to switch after discovering the advantages offered by the Betaconnect device. Recent data indicate that patients did not miss certain features in their device before a simulated injection with the Betaconnect autoinjector. Presence of these features (e.g., adjustable injection speed, quietness of injection) were rated high by the same patients.15
A major issue in the treatment of chronic diseases is adherence.20 Treatment support by autoinjectors might help contribute to high medication adherence. A study published in 2011 showed that autoinjectors were baseline predictors of adherence. Patients using an autoinjector at baseline and during the study were more adherent than patients not having used a device.12 Furthermore, the use of the autoinjector is positively associated with quality of life.21
Addressing adherence is not only a matter of the dosage form, but particularly of perceiving patients’ needs in terms of education (necessity of long-term treatment, realistic expectations),2 support (support programs, MS nurse, electronic diary with reminder function),2,21–23 and convenience (ideal autoinjection device). Nowadays it has become more and more usual for patients to track health data on their own and share data with health care providers. The Betaconnect device offers the possibility to transfer the injection data into the myBETAapp® for the patient. This assists patients in self-management of their injection therapy. The patient can share his or her injection data through the app with the health care providers. The RebiSmart also allows data sharing between patient and health care professionals. Mechanical autoinjectors like the ExtaviPro lack the technology to save and share injection-related data.
Despite oral therapies in MS, injectable treatment options like the beta interferons for s.c. administration, have an important value in the MS therapy algorithm and will continue to cover therapeutic needs. S.c. application is not limited to MS therapy, but an application option in many therapeutic areas like oncology. The monoclonal antibodies rituximab and trastuzumab used to be delivered by the intravenous route, but later s.c. formulations were developed to improve patients’ comfort. Recent studies have demonstrated that s.c. injections can shorten administration times, offer resource benefits, and improve patient convenience compared to the intravenous application route. Efficacy and tolerability did not differ from intravenous formulations.24,25
Therefore, with a view to the future, additional innovations of the injection devices might be important to improve patient satisfaction with injection therapy in MS and other therapeutic areas.
Conclusion
Major hallmarks in the management of chronic diseases such as MS are patient convenience and treatment adherence. In the case of self-administered therapies, these issues are particularly addressed by development and advancement of innovative autoinjection devices which meet patients’ needs. In our survey, the Betaconnect device was the most preferred autoinjector and rated closest to an ideal device. Thus, the Betaconnect might contribute to patient convenience and subsequently to improved treatment adherence. These results need to be confirmed in further studies.
Acknowledgments
The survey was funded by Bayer Vital GmbH, the manufacturer of Betaconnect. Medical writing services from Dr Carmen Koch, employee of KW medipoint, were also funded by Bayer Vital GmbH. The results based on the entire cohort (n=100) were presented at the ISPOR 19th Annual European Congress in 2016 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Value in Health.
Author contributions
VL revised the manuscript for intellectual content. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
VL is the Head of Department of Neurology, Cologne General Hospitals, University of Cologne, Cologne, Germany. VL has received speaker’s honoraria, financial research support or consultancy fees from Antisence, Allergan, Almirall, Bayer, Biogen, Bionorica, Genzyme, Novartis, Roche, Sanofi, and Teva. VL received honoraria for a workshop to evaluate the results and to interpret the data of the market research. TS, JR, XM, AK, and IW are salaried employees of Bayer Vital GmbH. BM is a salaried employee of IFAK Institute GmbH & Co. KG, Taunusstein, Germany. The authors report no other conflicts of interest in this work.
References
Bayas A. Improving adherence to injectable disease-modifying drugs in multiple sclerosis. Expert Opin Drug Deliv. 2013;10(3):285–287. | ||
Lugaresi A, Rottoli MR, Patti F. Fostering adherence to injectable disease-modifying therapies in multiple sclerosis. Expert Rev Neurother. 2014;14(9):1029–1042. | ||
Treadaway K, Cutter G, Salter A, et al. Factors that influence adherence with disease-modifying therapy in MS. J Neurol. 2009;256(4):568–576. | ||
Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89(2):225–240. | ||
Bayas A, Japp G, Fulda U, Kallmann BA. Injection devices in MS therapy: survey on neurologists, MS-nurses and patients. Nervenheilkunde. 2010;29:57–62. | ||
D’Arcy C, Thomas D, Stoneman D, Parkes L. Patient assessment of an electronic device for subcutaneous self-injection of interferon beta-1a for multiple sclerosis: an observational study in the UK and Ireland. Patient Prefer Adherence. 2012;6:55–61. | ||
de Sa J, Urbano G, Reis L. Assessment of new application system in Portuguese patients with relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2010;26(9):2237–2242. | ||
Devonshire V, Arbizu T, Borre B, et al. Patient-rated suitability of a novel electronic device for self-injection of subcutaneous interferon beta-1a in relapsing multiple sclerosis: an international, single-arm, multicentre, Phase IIIb study. BMC Neurol. 2010;10:28. | ||
Phillips JT, Fox E, Grainger W, Tuccillo D, Liu S, Deykin A. An open-label, multicenter study to evaluate the safe and effective use of the single-use autoinjector with an Avonex® prefilled syringe in multiple sclerosis subjects. BMC Neurol. 2011;11:126. | ||
Verdun di Cantogno E, Russell S, Snow T. Understanding and meeting injection device needs in multiple sclerosis: a survey of patient attitudes and practices. Patient Prefer Adherence. 2011;5:173–180. | ||
Brochet B, Lemaire G, Beddiaf A, et l’Epicure Study G. Réduction des réactions cutanées aux points d’injection avec deux auto-injecteurs chez des patients ayant une sclérose en plaques rémittente débutant un traitement par interféron bêta-1b : étude randomisée en cross-over. [Reduction of injection site reactions in multiple sclerosis (MS) patients newly started on interferon beta 1b therapy with two different devices]. Rev Neurol (Paris). 2006;162(6–7):735–740. French. | ||
Pozzilli C, Schweikert B, Ecari U, Oentrich W; BetaPlus Study group. Supportive strategies to improve adherence to IFN beta-1b in multiple sclerosis – results of the betaPlus observational cohort study. J Neurol Sci. 2011;307(1–2):120–126. | ||
Bayas A, Ouallet JC, Kallmann B, et al. Adherence to, and effectiveness of, subcutaneous interferon beta-1a administered by RebiSmart® in patients with relapsing multiple sclerosis: results of the 1-year, observational SMART study. Expert Opin Drug Deliv. 2015;12(8):1239–1250. | ||
Weller I, Saake A, Schreiner T, Vogelreuter J, Petroff N. Patient satisfaction with the BETACONNECT™ autoinjector for interferon beta-1b. Patient Prefer Adherence. 2015;9:951–959. | ||
Barone DA, Singer BA, Merkov L, Rametta M, Suarez G. Survey of US patients with multiple sclerosis: comparison of the new electronic interferon beta-1b autoinjector (BETACONNECT™) with mechanical autoinjectors. Neurol Ther. 2016;5(2):155–167. | ||
Ziemssen T, Sylvester L, Rametta M, Ross AP. Patient satisfaction with the new interferon beta-1b autoinjector (BETACONNECT™). Neurol Ther. 2015;4(2):125–136. | ||
Singer B, Wray S, Miller T, et al. Patient-rated ease of use and functional reliability of an electronic autoinjector for self-injection of subcutaneous interferon beta-1a for relapsing multiple sclerosis. Mult Scler Relat Disord. 2012;1(2):87–94. | ||
Hupperts R, Becker V, Friedrich J, et al. Multiple sclerosis patients treated with intramuscular IFN-beta-1a autoinjector in a real-world setting: prospective evaluation of treatment persistence, adherence, quality of life and satisfaction. Expert Opin Drug Deliv. 2015;12(1):15–25. | ||
Thakur K, Manuel L, Tomlinson M. Autoinjectors for administration of interferon beta-1b in multiple sclerosis: patient preferences and the ExtaviPro™ 30G and Betacomfort® devices. Pragmat Obs Res. 2013;4:19–26. | ||
WHO. Adherence to long-term therapies – Evidence for action. Geneva: World Health Organization; 2003. | ||
Pozzilli C, Schweikert B, Ecari U, Oentrich W, Bugge JP. Quality of life and depression in multiple sclerosis patients: longitudinal results of the BetaPlus study. J Neurol. 2012;259(11):2319–2328. | ||
Kohlmann T, Wang C, Lipinski J, et al. The impact of a patient support program for multiple sclerosis on patient satisfaction and subjective health status. J Neurosci Nurs. 2013;45(3):E3–E14. | ||
Zettl UK, Bauer-Steinhusen U, Glaser T, et al. Adherence to long-term interferon beta-1b injection therapy in patients with multiple sclerosis using an electronic diary. Adv Ther. 2016;33(5):834–847. | ||
Assouline S, Buccheri V, Delmer A, et al. Pharmacokinetics and safety of subcutaneous rituximab plus fludarabine and cyclophosphamide for patients with chronic lymphocytic leukaemia. Br J Clin Pharmacol. 2015;80(5):1001–1009. | ||
Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109(6):1556–1561. |
Supplementary material
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.