Back to Journals » Drug Design, Development and Therapy » Volume 13
Aucubin Protects Chondrocytes Against IL-1β-Induced Apoptosis In Vitro And Inhibits Osteoarthritis In Mice Model
Authors Wang B, Jiang Y, Yao Z, Chen P , Yu B, Wang S
Received 27 March 2019
Accepted for publication 30 August 2019
Published 9 October 2019 Volume 2019:13 Pages 3529—3538
DOI https://doi.org/10.2147/DDDT.S210220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Bo-wei Wang,1,* Yi Jiang,1,* Zi-long Yao,1,* Pei-sheng Chen,2 Bin Yu,1 Sheng-nan Wang1
1Department of Orthopaedics and Traumatology, Nanfang Hospital, Southern Medical University, Guangzhou 510515, People’s Republic of China; 2Department of Orthopaedics, Fuzhou Second Hospital Affiliated to Xiamen University, Fuzhou, Fujian 350007, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Yu; Sheng-nan Wang
Department of Orthopaedics and Traumatology, Nanfang Hospital, Southern Medical University, No.1838, North Guangzhou Avenue, North, Baiyun District, Guangzhou 510515, Guangdong Province, People’s Republic of China
Tel +86 20 6164 1741
Email [email protected]; [email protected]
Objective: Chondrocyte apoptosis has also been strongly correlated with the severity of cartilage damage and matrix depletion in an osteoarthritis (OA) joint. Therefore, pharmacological inhibitors of apoptosis may provide a novel treatment option for patients with OA. Aucubin, a natural compound isolated from Eucommia ulmoides, has been proved to possess antioxidative and anti-apoptotic properties. However, anti-osteoarthritis effect of aucubin in animal model and anti-apoptotic response of aucubin in OA chondrocytes remain unclear. This study aimed to determine whether aucubin could slow progression of OA in a mouse model and inhibit the IL-1β-induced chondrocyte apoptosis.
Methods: OA severity and articular cartilage degradation were evaluated by Safranin-O staining, Hematoxylin-eosin (H&E) staining, and Osteoarthritis Research Society International (OARSI) standards. Chondrocyte viability was observed by Cell Counting Kit-8 (CCK8) and live/dead cells assay; the apoptotic rate of chondrocytes was evaluated by flow cytometry (FCM) with Annexin V-FITC/PI kit. Mediators of apoptosis were tested by Western blot of Bax, caspase-3, caspase-9, and Bcl-2 expression. The intracellular levels of Reactive oxygen species (ROS) were assessed by the probe of 2,7-Dichlorofluorescin diacetate (DCFH-DA).
Results: The articular cartilage in the limb with destabilization of the medial meniscus (DMM) exhibited early OA-like manifestations characterized by proteoglycan loss, cartilage fibrillation, and erosion, with lower OARSI score. Oral administration of aucubin remarkably attenuated the loss of proteoglycan and the articular cartilage erosion and decreased the OARSI scores underwent DMM surgery. Aucubin treatment significantly reverses IL-1β-induced cytotoxicity and attenuated the IL-1β-induced chondrocyte apoptosis. In addition, aucubin can significantly inhibit mediators of apoptosis in rat primary chondrocytes. Furthermore, aucubin remarkably attenuated the IL-1β-induced intracellular ROS production.
Conclusion: Our findings suggest that aucubin has a protective effect on articular cartilage and slowing progression of OA in a mouse model. This protective effect may result from inhibiting chondrocyte apoptosis and excessive ROS production.
Keywords: aucubin, osteoarthritis, apoptosis, ROS, IL-1β
Introduction
Osteoarthritis (OA) is an aging-associated progressive degenerative disease characterized by articular cartilage damage and elevated chondrocyte mortality. OA is a leading cause of disability in the world. In the United States, over 22.7 million people reported arthritis-attributable activity limitations.1 To date, the pharmacologic treatments for OA are analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs), which only provide pain relief but do not exert a clear clinical effect on OA disease in prevention or modification. It has been generally accepted that OA is a multi-factorial disease that requires the development of better targeted therapeutic agents. Numerous studies have identified that chondrocyte apoptosis is positively correlated with the severity of OA.2–4 Apoptosis has also been supposed to be strongly associated with articular cartilage destruction and matrix degradation in humans.5 Reactive oxygen species (ROS) are thought to be a crucial factor in mediating chondrocyte apoptosis.6 Excessive accumulation of ROS promotes chondrocyte apoptosis as a result of mitochondrial dysfunction.7,8 Therefore, pharmacologic inhibitors of apoptosis and ROS might provide a novel treatment option and modification for patients with OA.
Aucubin is a naturally occurring compound discovered in various plants including leaves of Aucuba japonica9 and Eucommia ulmoides.10 It has been shown to possess several pharmacological properties, including the anti-inflammatory,11 chondroprotective,12 antioxidative,13 and anti-apoptotic properties.14,15 Our previous study reported that aucubin remarkably suppressed IL-1β induced inflammation and cartilage matrix degradation, including matrix metalloproteinases (MMPs), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and the production of nitric oxide (NO) through the inhibition of NF-κB signaling pathways.11 One study proved aucubin may enhance the anticatabolic and anti-inflammatory effects of hyaluronic acid (HA) on OA chondrocytes.12 Young et al reported aucubin can reduce ROS production, caspase-3 activity, and cell apoptosis and has protective effects in an osteoarthritic chondrocyte model induced by H2O2 and mechanical stimulus.16 To date, no study has reported the protective effect of aucubin in animal model of OA. Moreover, the anti-apoptotic effect of aucubin on chondrocytes has not been completely clarified. In the present work, we evaluated the therapeutic effect of aucubin in a mouse model of OA. We also investigated whether aucubin inhibits the apoptosis and ROS production in IL-1β-stimulated chondrocytes.
Materials And Methods
Animal Models
Destabilization of the medial meniscus (DMM) model was established in 10–12 weeks old C57BL/6 male mice purchased from the Animal Center of Southern Medical University (Guangzhou, China). Mice were randomly grouped as follows: sham; DMM model; DMM model + aucubin (50mg/kg/d). After the mice were anesthetized, microsurgery was performed to transect the medial meniscotibial ligament (MMTL), which contributed to the DMM model. In the sham group, microsurgery was only performed to visualize the MMTL, but the MMTL was not transected. Immediately after surgery, mice from the DMM model and sham group were administered of normal saline (NS), while the DMM model + aucubin group received aucubin (50 mg/kg) dissolved in NS by gavage. The mice were executed using an overdose injection of pentobarbital sodium at the end of the 8th week. The animal study proposal was approved by the Animal Care Committee of Southern Medical University. All mouse experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of People’s Republic of China.
Specimen Preparation And Histological Evaluation
Tibia and femur tissues were separated and fixed in paraformaldehyde, decalcified in EDTA after washed with water, then dehydrated in graded alcohols. Finally, specimens were embedded in paraffin and cut into serial sections. Each glass slides stained with H&E or Safranin-O for general histological evaluation. The OARSI scoring system was used to evaluate the severity of OA by estimating the highest observed score from the medial femoral condyle and the medial tibial plateau according to a previous study.17
Reagents
Aucubin was purchased from Yuanmu Industrial Corporation (Shanghai, China). CCK-8 and Annexin V-FITC/PI Apoptosis kit were obtained from Bestbio (Shanghai, China). A Live and Dead Cell staining kit was purchased from Mountain View (CA, USA). A Reactive Oxygen Species Assay kit was purchased from Beyotime Biotechnology (Jiangsu, China). The primary antibodies used in this study were as follows: mouse monoclonal anti-Bcl-2 and monoclonal anti-Bax from Santa Cruz Biotechnology, Inc. (CA, USA), mouse monoclonal anti-cleaved caspase-3 and mouse monoclonal anti-cleaved caspase-9 from Abcam Biotechnology (MA, US). Recombinant Rat IL-1beta was obtained from PeproTech (NJ, USA).
Cell Isolation And Culture
The methods for cell isolation and culture have been previously described.11 Briefly, 4-week-old SD rats were sacrificed for collection of cartilage from both knees and femoral heads under sterile conditions. Then, we cut the rat cartilage into small pieces, after washing five times with PBS. The specimens were digested in 0.25% trypsin-EDTA solution for 30 mins and DMEM (contains collagenase type II) for 2 hrs at 37°C. The released chondrocytes were seeded in 25 cm2 cell flasks. Cells were passaged at a ratio of 1:3, when they reached 80–90% confluency.
Cell Viability Assay
The CCK8 assay was used to determine cell viability following IL-1β and aucubin treatment, as detailed in our previous study.11 Rat chondrocytes (1 × 104/well) were incubated in 96-well plates for 24 and 48 hrs, respectively. After appropriate treatment according to the experimental grouping, cells were incubated with CCK-8 solution (10 μL) for 2 hrs at room temperature. The level of the dye formed was measured with a microplate reader.
Live And Dead Cells Assay
The Live/Dead Viability Assay kit was used to observe apoptotic cell death. Calcein AM and ethidium homodimer-1 were identified as markers for live and dead cells, respectively, provided a two-color fluorescence. Living cells were defined as green fluorescence while dead cells were identified in red fluorescence. Chondrocytes were seeded and incubated with various stimulations for 24 hrs. Fluorochromes were then added and incubated in washed chondrocytes for 15 mins. Fluorescence images were visualized under a confocal fluorescent microscope (Olympus FluoView FV1000, Melville, NY, US).
Apoptosis Evaluation
The rates of chondrocytesapoptosis were determined using flow cytometry (FCM) analysis with an Annexin V-FITC/PI Apoptosis kit. Chondrocytes were cultured in 6-well plate with various treatments for 24 hrs, and then re-suspended in 500 μL binding buffer and stained with 5 μL of FITC Annexin V and 10 μl of propidium iodide (PI), which were added for 15 mins at room temperature in the dark. After incubation, the samples were tested using a flow cytometer (Moflo XDP, USA). Early apoptotic chondrocytes were detected with Annexin V positive and PI negative cells, and late apoptotic chondrocytes were stained with both annexin V and PI positive cells. The results are represented as the percentage of apoptotic cells.
Western Blot
The method for Western blot analysis was according to our previous study.11 Whole-cell proteins were homogenized in ice-cold incubation with lysis buffer after washed with PBS solution for three times. Then, cell debris was cleared by centrifugation for 10 mins at 12,000 rpm under 4°C. Protein concentration level was determined by BCA kit. Equivalent amounts of extracted proteins (20 μg) were separated by SDS-PAGE (10% gels), and then transferred onto PVDF membranes. The membranes were pre-incubated in 5% blocking buffer for 30 mins at room temperature, after which the blots were incubated overnight at 4°C with primary antibodies: Bcl-2 (1:200, Santa Cruz), Bax (1:200, Santa Cruz), Cleaved Caspase-3 (1:500, Abcam), and Cleaved Caspase-9 (1:500, Abcam). Subsequently, the membranes were incubated with the secondary antibody (1:15,000) for 2 hrs before washed 3 times with TBST. The blots were quantified using LI-COR Odyssey (Lincoln, Nebraska, USA).
Measurement Of ROS
The intracellular levels of ROS in chondrocytes were assessed by the probe of DCFH-DA, a ROS detection kit, according to the manufacturer’s optimized instructions. To determine the ROS levels, washed chondrocytes were incubated with diluted DCFH-DA (10 µM) for 20 mins in the dark. After washing with PBS, the chondrocytes were imaged under a confocal laser scanning microscope (Olympus FluoView FV1000, NY, USA). To quantitate ROS levels, chondrocytes were cultured with different stimulations in 96-well plates, fluorescence was measured with a spectra Max M5 (Molecular devices, CA, USA).
Statistical Analysis
All results were analyzed using Prism (GraphPad, Diego, USA), presented as the mean ± SEM. For multiple comparisons, a two-tailed t-test with Tukey post hoc tests was used to analyze differences depending on the experiment design. A P < 0.05 was considered statistically significant.
Results
Aucubin Prevents Progression Of OA In DMM Mice
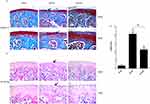
To determine the efficacy of aucubin on severity and progression of OA, the proteoglycan and structural degeneration of the articular cartilage was evaluated under microscopy by Safranin O-fast green staining, H&E staining, and OARSI. Aucubin (50 mg/kg) was administered by oral gavage to mice once daily for 8 weeks post-operation. Safranin O staining showed 8 weeks after DMM mice representing early OA-like manifestations including loss of proteoglycan content, cartilage fibrillation, and erosion. While aucubin-treated DMM model mice (50 mg/kg) showed retention of proteoglycan, less cartilage fibrillation, and less cartilage erosion, compared to those in vehicle-treated mice (Figure 1A). H&E staining demonstrated that hyaline cartilage (HC) thickness is declined, the chondrocytes of articular cartilage disordered lined and tend to hypertrophic in the DMM model group (Figure 1B). However, oral administration of aucubin remarkably inhibits hyaline cartilage (HC) thickness declined, attenuates chondrocyte disordering and hypertrophy in mouse knee joint after DMM surgery. The OARSI scores were improved in aucubin-treated DMM mice compared with those in DMM controls (P < 0.05; Figure 1C).
Aucubin Reverses IL-1β-Induced Cytotoxicity
The cytotoxicity of IL-1β and protection of aucubin on chondrocytes were determined by CCK8 assay at 24 hrs and 48 hrs, respectively. As shown in Figure 2, IL-1β-induced cytotoxicity in chondrocytes and treatment with IL-1β (10 ng/mL) for 24 hrs and 48 hrs significantly decreased cell viability (P<0.05). However, aucubin markedly improved cell viability in IL-1β-stimulated chondrocytes in a dose-dependent manner, and 50μM of aucubin had the significant protective effect at 24-hr and 48-hr time point (P<0.05). All of these findings indicated that IL-1β induced cytotoxicity in chondrocytes, whereas aucubin has a potential protective effect on chondrocytes.
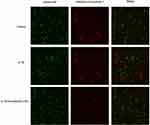
The fluorescence microscopy images in live/dead assay were used to visualize the live and dead chondrocytes. As shown in Figure 3, we found that there are obvious features of death or necrosis in chondrocytes exposed to IL-1β, as evidenced by a greater extent of red fluorescence (dead cells), compared with control ones. Additionally, aucubin (50 μM) remarkably decreases IL-1β-induced chondrocyte death, as shown by a greater extent of green fluorescence (live cells) and decreased red fluorescence (dead cells). Consistent with CCK8 assay, aucubin could reverse cytotoxic effects on IL-1β-induced chondrocytes.
Aucubin Prevents IL-1β-Induced Chondrocyte Apoptosis
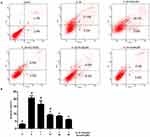
We assessed the prevention of aucubin in different concentrations (1, 10, 20, and 50 μM) on IL-1β (10 ng/mL)-induced apoptosis. The rate of early chondrocyte apoptosis is represented in the Q4 region, and the rate of late chondrocyts apoptosis is represented in the Q2 region as shown in Figure 4. After incubation with IL-1β (10ng/mL) for 24 h, the rate of chondrocyte apoptosis was significantly increased compared with control group. While pretreatment with aucubin for 2 hrs, the rates of chondrocyte apoptosis decreased relative to the IL-1β stimulation group, 10 μM, 20 μM, and 50 μM of aucubin showed the significant protection effects (Figure 4B). The flow cytometry analysis demonstrated that aucubin potently prevented IL-1β-induced apoptosis and had a potential anti-apoptotic effect on chondrocytes.
Aucubin Blocks IL-1β-Induced Chondrocyte Apoptosis By Regulating Mediator Of Apoptosis
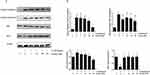
To determine whether aucubin can modulate the expression of anti-apoptotic or pro-apoptotic proteins, we examined IL-1β-stimulated rat chondrocytes with or without pre-treatment of aucubin by Western blot. Consistent with prior studies, Western blot analysis showed that expression of Bax, cleaved caspase-3, and cleaved caspase-9 increased but that of Bcl-2 decreased in IL-1β-stimulated chondrocytes compared to control. Furthermore, aucubin remarkably inhibited IL-1β-induced Bax, cleaved caspase-3, and cleaved caspase-9 overexpression, but enhanced Bcl-2 expression in a dose-dependent manner at 24-hr time point (Figure 5). These collective data indicated that aucubin might have an anti-apoptotic effect on IL-1β-stimulated chondrocytes via suppressing Bax, cleaved caspase-9, and cleaved caspase-3 expression, promoting Bcl-2 expression.
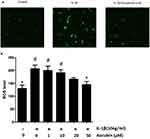
Aucubin Decreases ROS Production In Chondrocytes
To identify the effect of aucubin on IL-1β-induced ROS in chondrocytes, the intracellular ROS image visualized by confocal microscopy and ROS levels were recorded by spectrophotometer. A number of studies have confirmed that IL-1β induces the generation of ROS in chondrocytes. After treatment with IL-1β for 30 mins, ROS level was assessed using a DCFH-DA probe and quantified using a fluorescence microplate reader or imaged by fluorescence microscopy and spectrophotometer. Consistent with previous studies,16 IL-1β increased ROS generation in chondrocytes, and ROS levels were increased significantly compared with the control group (Figure 6). In contrast, aucubin (50 μM) significantly attenuated the ROS generation after stimulation of IL-1β in chondrocytes (Figure 6B). These results indicated that IL-1β could markedly increase the intracellular ROS generation and aucubin could attenuate those increasing.
Discussion
OA is currently treated with drugs or surgery, but no satisfactory efficacy has been achieved. Chondrocyte apoptosis is significantly increased in OA patients, but cell survival is significantly decreased.18 Therefore, it is an interest to find a way to inhibit chondrocyte apoptosis and increase cell survival for a more efficient therapy in OA. Aucubin is a compound extracted from numerous plants, including Eucommia ulmoides and Castilleja tenuiflora. As noted, more attention was paid on anti-apoptotic effect of aucubin in herbal therapy, although there are numerous biological activities on it.19 These findings inspired us to investigate whether aucubin has a protective effect in animal model and how it functions on chondrocyte viability.
The present work first demonstrated that intragastric administration of aucubin delays the progression of OA in a DMM animal model. This finding was shown by increased proteoglycan, reduced cartilage destruction, and decreased OARSI scores following aucubin treatment in DMM mice when compared with those in DMM control. Taken together, the study confirmed protective function of aucubin in an OA mouse model and also suggested that aucubin is a potential therapy for OA.
Furthermore, we demonstrated whether aucubin inhibits apoptosis to protect articular cartilage chondrocytes from OA. It has been generally accepted that chondrocyte apoptosis displays a key role in pathogenesis of OA due to inducing articular cartilage degeneration.20 IL-1β is an important pro-inflammatory cytokine have been shown to induce chondrocyte apoptosis in vivo and in vitro settings,21,22 resulted in cartilage matrix degradation and joint inflammation, thereby contributing to OA progression.8,23 Thus, our study uses IL-1β to mimic an in vitro model of OA. Our results showed that IL-1β significantly decreased chondrocyte viability, which was consistent with previous studies.11,12 Additionally, a live/dead kit assay was conducted to demonstrate the influence of aucubin on IL-1β-induced chondrocyte viability and visualize the live and dead chondrocytes. Previously, apoptosis inhibition properties of aucubin via Annexin-V/PI flow cytometry on chondrocytes evaluated by Young et al16 demonstrated that in the aucubin with 100 μM pretreated group, the percentages of chondrocytes in both early and late apoptosis states were significantly reduced compared to the H2O2 stimulated group. Our study further confirmed pre-treatment aucubin in various concentration (10 μM, 20 μM, and 50 μM) could remarkably reduce the cell apoptosis in IL-1β-stimulated chondrocytes. All of these results suggest that aucubin has a chondroprotective effect on OA chondrocytes by inhibiting apoptosis in IL-1β-cultured chondrocytes. However, this requires further investigation regarding the action of aucubin in regulating chondrocyte apoptosis.
It is well known that members of the Bcl-2 family not only modulate the chondrocyte survival and apoptosis but also trigger caspases cascade activation.24 The Bcl-2 family is divided into pro-apoptotic protein Bax which accelerates apoptosis25 and anti-apoptotic protein Bcl-2 which inhibits apoptosis. It has been identified that Bcl-2 level in OA cartilage is lower than those in healthy cartilage, which can promote survival of chondrocytes.26 Bax is responsible for activation of the mitochondrial pathway of apoptosis and caspase, as well as initiated cell death. The central events in apoptosis are activation of a family of cysteine proteases called caspases which lead to cellular disruption.27 Caspase-9 is a vital cysteine protease enzyme that activates pro-caspase-3 (the executioner caspases) and mediates pathway of apoptosis.23 Caspase-3, a key executioner caspase can cleave the death substrates, eventually lead to apoptosis.28 Therefore, inhibition of caspase-3 activation may be the key to decreasing chondrocyte apoptosis. Western blot was used to investigate these mediators of apoptosis and how apoptosis signaling proceeds. Xue et al reported that aucubin not only inhibited lower Bcl-2 expression, high Bax expression but also modulated caspase-3 activation in H2O2-induced PC12 cells.15 Similarly, we found that the expressions of Bax were increased and the expressions of Bcl-2 were decreased in IL-1β-stimulated chondrocytes, and these effects were attenuated by aucubin. One recent study reported that aucubin could reduce the caspase-3 activity induced by H2O2.16 In accordance with previous studies, our work indicated that aucubin reduced the expressions of caspase-9 and caspase-3 in IL-1β-induced chondrocytes. Findings from the present study further suggested that aucubin exerts anti-apoptotic effects on chondrocytes via regulating those key mediators, including Bcl-2, Bax, caspase-9, and caspase-3 (Figure 7).
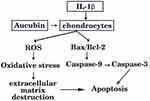 |
Figure 7 Schematic diagram showing apoptotic and ROS signaling pathway induced by IL-1β in articular chondrocytes. |
ROS has been proven to be a part of regulative mechanisms of apoptosis. Accumulation of ROS can trigger oxidative stress and is implicated in cell dysfunction and apoptosis.29 In cartilage, ROS displays a vital intracellular second messenger to maintain cartilage function by regulating cartilage homeostasis and chondrocyte differentiation.30 With respect to chondrocyte-related etiology and pathological circumstances, excessive accumulation of ROS is associated with extracellular matrix destruction, ultimately leading to cell death and cartilage degradation.31,32 Young and co-workers16 speculated that 10–100 μM of aucubin reduce ROS production on dose-dependently after at least 1 hr of pretreatment in H2O2 stimulated chondrocytes. In agreement with Liu’s study,33 we demonstrated that IL-1β induced a very rapid ROS generation in chondrocytes and pretreatment with aucubin significantly suppressed ROS production. These data imply that aucubin exerts chondroprotective effects on IL-1β-induced chondrocytes via attenuating the production of ROS (Figure 7).
In summary, we have verified that aucubin has significant efficacy in slowing the progression of OA in DMM mice model. This evidence has been investigated in vitro based on the anti-apoptotic effect of aucubin in IL-1β-stimulated chondrocytes. Aucubin possibly exerts its efficacy by inhibiting apoptosis and excessive ROS production.
Acknowledgments
This work was supported by Natural Science Foundation of Guangdong Province of China (Grant No.: 2018A030313806), Natural Science Foundation of Guangdong Province of China (Grant No.: 2017A030310110) and Science and Technology Planning Project of Guangdong Province of China (Grant No.: 2015A020212022).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Centers for Disease C, Prevention. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation–United States, 2010–2012. MMWR. 2013;62(44):869–873.
2. Mistry D, Oue Y, Chambers MG, Kayser MV, Mason RM. Chondrocyte death during murine osteoarthritis. Osteoarthritis Cartilage. 2004;12(2):131–141.
3. Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15(1):27–34. doi:10.1016/j.joca.2006.06.012
4. Sharif M, Whitehouse A, Sharman P, Perry M, Adams M. Increased apoptosis in human osteoarthritic cartilage corresponds to reduced cell density and expression of caspase-3. Arthritis Rheum. 2004;50(2):507–515. doi:10.1002/art.20020
5. Musumeci G, Loreto C, Carnazza ML, Strehin I, Elisseeff J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol Histopathol. 2011;26(10):1265–1278. doi:10.14670/HH-26.1265
6. Carlo MD
7. Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74(4):324–329. doi:10.1016/j.jbspin.2007.02.002
8. Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8(2):333–345. doi:10.2174/138945007779940025
9. Chang LM, Yun HS, Kim YS, Ahn JW. Aucubin: potential antidote for alpha-amanitin poisoning. J Toxicol Clin Toxicol. 1984;22(1):77–85. doi:10.3109/00099308409035083
10. Li Y, Sato T, Metori K, Koike K, Che QM, Takahashi S. The promoting effects of geniposidic acid and aucubin in Eucommia ulmoides Oliver leaves on collagen synthesis. Biol Pharm Bull. 1998;21(12):1306–1310.
11. Wang SN, Xie GP, Qin CH, et al. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-kappaB signaling pathway in rat articular chondrocytes. Int Immunopharmacol. 2015;24(2):408–415. doi:10.1016/j.intimp.2014.12.029
12. Huang TL, Yang SH, Chen YR, Liao JY, Tang Y, Yang KC. The therapeutic effect of aucubin-supplemented hyaluronic acid on interleukin-1beta-stimulated human articular chondrocytes. Phytomedicine. 2019;53:1–8. doi:10.1016/j.phymed.2018.09.233
13. Jin L, Xue HY, Jin LJ, Li SY, Xu YP. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur J Pharmacol. 2008;582(1–3):162–167. doi:10.1016/j.ejphar.2007.12.011
14. Kim YM, Sim UC, Shin Y, Kim Kwon Y. Aucubin promotes neurite outgrowth in neural stem cells and axonal regeneration in sciatic nerves. Exp Neurobiol. 2014;23(3):238–245. doi:10.5607/en.2014.23.3.238
15. Xue HY, Niu DY, Gao GZ, Lin QY, Jin LJ, Xu YP. Aucubin modulates Bcl-2 family proteins expression and inhibits caspases cascade in H₂O₂-induced PC12 cells. Mol Biol Rep. 2011;38(5):3561–3567. doi:10.1007/s11033-010-0466-7
16. Young IC, Chuang ST, Hsu CH, et al. Protective effects of aucubin on osteoarthritic chondrocyte model induced by hydrogen peroxide and mechanical stimulus. BMC Complement Altern Med. 2017;17(1):91.
17. Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–S23. doi:10.1016/j.joca.2009.11.014
18. Huang H, Quan YY, Wang XP, Chen TS. Gold nanoparticles of diameter 13 nm induce apoptosis in rabbit articular chondrocytes. Nanoscale Res Lett. 2016;11(1):249.
19. Zhu Z, Xie Q, Huang Y, Zhang S, Chen Y. Aucubin suppresses titanium particlesmediated apoptosis of MC3T3E1 cells and facilitates osteogenesis by affecting the BMP2/Smads/RunX2 signaling pathway. Mol Med Rep. 2018;18(3):2561–2570. doi:10.3892/mmr.2018.9286
20. Charlier E, Relic B, Deroyer C, et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17(12):2146. doi:10.3390/ijms17122146
21. Shakibaei M, John T, Seifarth C, Mobasheri A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann N Y Acad Sci. 2007;1095:554–563. doi:10.1196/annals.1397.060
22. Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1 beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res Ther. 2008;26(12):1643–1648. doi:10.1002/jor.20683
23. Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16(11):26035–26054. doi:10.3390/ijms161125943
24. Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem. 2002;277(13):11345–11351. doi:10.1074/jbc.M109893200
25. van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16(2):203–213. doi:10.1038/sj.cr.7310028
26. Kim HA, Lee YJ, Seong SC, Choe KW, Song YW. Apoptotic chondrocyte death in human osteoarthritis. J Rheumatol. 2000;27(2):455–462.
27. Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27(48):6194–6206. doi:10.1038/onc.2008.297
28. Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001;19(5):785–796. doi:10.1016/S0736-0266(00)00078-4
29. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049.
30. Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–591. doi:10.1016/j.bbadis.2016.01.003
31. Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13(8):643–654.
32. Grishko VI, Ho R, Wilson GL, Pearsall A. Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthritis Cartilage. 2009;17(1):107–113.
33. Liu M, Zhong S, Kong R, et al. Paeonol alleviates interleukin-1beta-induced inflammatory responses in chondrocytes during osteoarthritis. Biomed Pharmacother. 2017;95:914–921.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.