Back to Journals » International Medical Case Reports Journal » Volume 9
Atypical oculopalatal tremor as the presentation of vertebral artery dolichoectasia
Authors Vanikieti K, Cheecharoen P, Jindahra P, Lueangaram S, Padungkiatsagul T
Received 27 March 2016
Accepted for publication 18 June 2016
Published 6 September 2016 Volume 2016:9 Pages 273—277
DOI https://doi.org/10.2147/IMCRJ.S109357
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Supplementary video of Videonystagmography
Views: 2519
Kavin Vanikieti,1 Piyaphon Cheecharoen,2 Panitha Jindahra,3 Sirin Lueangaram,4 Tanyatuth Padungkiatsagul1
1Department of Ophthalmology, 2Department of Radiology, 3Department of Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 4Department of Ophthalmology, Queen Sirikit National Institute of Child Health, Bangkok, Thailand
Abstract: Vertebrobasilar dolichoectasia (VBD) is a rare dilative arteriopathy defined as elongation or widening of the intracranial vertebral and/or basilar arteries. The prevalence ranges from 0.06% to 5.8%. The majority of VBDs are asymptomatic. Downbeat nystagmus has been reported as a component of an infrequent ocular movement disorder in VBD. Nevertheless, oculopalatal tremor (OPT), delayed sequelae of a brainstem lesion, has never been demonstrated in VBD cases. Synchronized rhythmic involuntary contractions of the soft palate with an ocular pendular nystagmus, predominantly vertical pendular nystagmus, are the hallmark presentation. Our case demonstrated a 50-year-old female who presented with 3-month history of oscillopsia. Examination showed binocular conjugate torsional jerk nystagmus simultaneous with a contraction of the soft palate, defined as an atypical OPT, resulting from dolichoectatic left vertebral artery compressing on medulla, demonstrated by magnetic resonance imaging. Bilateral conjugate torsional jerk nystagmus simultaneous with palatal tremor, considering as an atypical OPT, should be included as one of the symptomatic presentations of vertebral artery dolichoectasia in spite of its rarity.
Keywords: vertebral artery dolichoectasia, oculopalatal tremor, torsional nystagmus
Introduction
Vertebrobasilar dolichoectasia (VBD) is a rare dilative arteriopathy defined as elongation or widening of the intracranial vertebral and/or basilar arteries. The prevalence ranges from 0.06% to 5.8%.1,2 The majority of VBDs are asymptomatic,2 but it may present with symptoms due to: 1) compression of brainstem, 2) direct compression of cranial nerve, 3) ischemia of vertebrobasilar territory, 4) rupture of vessel, and 5) hydrocephalus.3–6 Downbeat nystagmus has been reported as a component of an infrequent ocular movement disorder in VBD.7–11 Nevertheless, oculopalatal tremor (OPT), rare, delayed sequelae of a brainstem lesion, has never been demonstrated in VBD cases. Vascular injury either infarction or hemorrhage is the most common etiology of OPT.12 Synchronized rhythmic involuntary contractions of the soft palate with an ocular pendular nystagmus, predominantly vertical pendular nystagmus, are the hallmark presentation.13 It is proposed that the muscles involved are derived from branchial arches; this rare complication often involves extraocular muscles and the muscles of soft palate and pharynx.13,14
Our case demonstrated binocular conjugate torsional jerk nystagmus simultaneous with contraction of the soft palate, defined as an atypical OPT, resulting from VBD.
Case report
A 50-year-old female presented with 3-month history of oscillopsia. This was accompanied with gait disturbance. Her prior medical history was significant for right hemiparesis in the previous year. She denied any history of chronic alcoholic exposure or lithium intoxication.
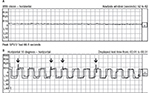
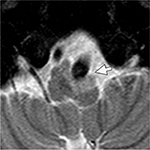
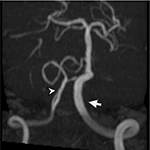
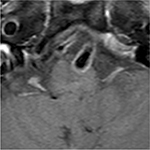
On ocular examination, visual acuity was 20/20 in both eyes. Anterior segment and fundus examination were unremarkable. Binocular conjugate counterclockwise torsional jerk nystagmus without vertical or horizontal component was demonstrated by videonystagmography (Video S1) (Figure 1A). The ocular nystagmus and palatal movements were simultaneous. The amplitude and frequency of nystagmus were similar in all gazes. Null point was absent. Ocular alignment was orthotropic. Extraocular muscles movements, saccadic velocity, and smooth pursuit eye movements were within normal limit except horizontal saccades that were hypermetric to the right (Figure 1B). Neurological examination showed right hemiparesis with hyperreflexia. No facial palsy was noted. Cerebellar examination, including rapid alternating movements and finger-to-nose test, was impaired on the right side. Moreover, hypermetric saccades to the right and staggering movements with a wide-based gait were observed. Magnetic resonance imaging (MRI) of the brain revealed dolichoectatic left vertebral artery, exerting pressure effect to the left medulla (Figure 2). Increased diffusion on the apparent diffusion coefficient (ADC) image without restricted diffusion on diffusion-weighted imaging (DWI) at the left medulla was also depicted (Figures 3 and 4). Supratentorial cerebral hemispheres appeared unremarkable. Magnetic resonance angiogram (MRA) of the posterior circulation revealed dolichoectatic left vertebral artery with redundancy to the right. Hypoplasia of the right vertebral artery was also noted (Figure 5). A diagnosis of dolichoectatic left vertebral artery compressing left anterior medulla was made.
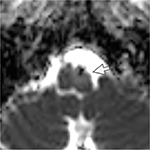  | Figure 3 MRI of the brain. ADC image shows increased diffusion at the left medulla (arrow). Abbreviations: ADC, apparent diffusion coefficient; MRI, magnetic resonance imaging. |
Subsequently, craniotomy with microvascular decompression (MVD) was performed. Intraoperative finding confirmed the diagnosis. Her symptoms and examination remained unchanged over 2 months of follow-up. Ethical approval for this study was obtained from the Institutional Review Board of Faculty of Medicine Ramathibodi Hospital, Mahidol University. Written informed consent regarding study and images publication was obtained from the patient. The study adhered to the tenets of the Declaration of Helsinki.
Discussion
OPT is rare, delayed sequelae of a brainstem lesion. OPT usually presents with an asymptomatic palatal or pharyngeal tremor with a symptomatic nystagmus, predominantly vertical pendular nystagmus. Oscillopsia is always disturbing for the patients. Some patients may experience an abnormal sound described as an “ear-click” resulting from pharyngeal tremor, but not other symptoms in oropharyngeal area. Oscillations are commonly synchronized at a rate of 2–3 Hz.13,14
Guillain and Mollaret reported that OPT resulted from a lesion along Guillain and Mollaret’s triangle, also known as Dentato-rubro-olivary pathway.15 More recent evidence has shown red nucleus not to play an active part in Guillain and Mollaret’s triangle and that it is a lesion of the central tegmental tract that leads to OPT by interrupting the pathway from the contralateral cerebellar dentate nucleus to inferior olivary nucleus (ION).16,17 We agreed that this mechanism would lead to OPT in our case. Unlike typical OPT in which ocular component of OPT is predominantly vertical pendular nystagmus,13 our case presented with a binocular conjugate torsional jerk nystagmus. Furthermore, most cases of VBD are asymptomatic.2 However, dolichoectatic vessel may produce complications, such as compression of brainstem or cranial nerve, ischemia of vertebrobasilar territory, and rupture of vessel. Right hemiparesis in our case was explained as a result of compression or infarction at left anterior medulla, above pyramidal decussation, by left dolichoectatic vertebral artery. A disruption of central tegmental tract also produced atypical OPT in this case. In rare cases, downbeat nystagmus is associated with compression of caudal brainstem by dolichoectatic vessel.7–11 None of the prior reports documented torsional nystagmus or palatal tremor. To our knowledge, this is the first case report of binocular torsional jerk nystagmus simultaneous with palatal tremor, considering atypical OPT, as a rare presentation of VBD.
MRI and MRA brain demonstrated dolichoectatic left vertebral artery, exerting a pressure effect to the left medulla. A hypersignal T2 change with increased diffusion on the ADC image without restricted diffusion on DWI at the compressed left medulla was depicted (Figures 2–4). No gadolinium enhancement was noted (Figure 6). Supratentorial cerebral hemispheres appeared unremarkable. MRA of the posterior circulation revealed dolichoectatic left vertebral artery with redundancy to the right (Figure 5). Our findings from neuroimaging were most likely to be a gliosis of compressed medulla related to pulsatility. The extent of compressed lesions at left medulla involving central tegmental tract and corticospinal tract could explain clinical presentation of atypical OPT and right hemiparesis, respectively. Cerebellar signs, including hypermetric saccades to the right, impaired rapid alternating movements, and finger-to-nose test on the right, resulted from disruption of left central tegmental tract from VBD. Nevertheless, chronic infarction cannot be differentiated from VBD per se as the etiology of OPT in our patient given that increased signal in ADC image (Figure 3) and absence of restricted diffusion in DWI (Figure 4) may result from either chronic infarction or gliosis. Inferior olivary nucleus hypertrophy (IONH) could not be clearly demonstrated from the study. Potential explanations include marked distortion of left ventral medulla anatomy due to compression by left vertebral artery. We are unable to distinguish IONH from an area of hypersignal T2 change of compressed medulla. Another explanation is the timing of the neuroimaging studies. According to Goyal,18 hypersignal T2 change develops within the first month after disease onset, and the hyperintensity persists for many years and may be permanent. Hypertrophy of the ION, on the other hand, typically appears 10 to 18 months after the insult but may be seen as early as 6 months and disappears by 4 years. In our case, IONH is probably still too early to develop since the duration of OPT is only 3 months. In some rare patients, OPT may be manifested without any evidence of IONH.12
The clinical course in vertebral artery compression of medulla can present with transient symptoms or permanent deficits, and motor with vestibular or cerebellar disturbances are the most common manifestations. The best treatment is still controversial.19 Literature review showed that MVD surgery might provide only temporary symptoms relief.19 As in our case, on 2 months post-MVD, the patient still experienced oscillopsia correlated with persistence torsional nystagmus with simultaneous palatal tremor and right cerebellar signs. Despite the unfavorable outcome in relieving symptoms, our goal of treatment was to prevent further damage of the brainstem resulting from compression of vertebral artery dolichoectasia.
In conclusion, binocular conjugate torsional jerk nystagmus simultaneous with palatal tremor, considering as atypical OPT, should be included as one of the symptomatic presentations of vertebral artery dolichoectasia in spite of its rarity.
Acknowledgments
The authors would like to thank Suwimol Ruencharoen, MA, Department of Communication Sciences and Disorders, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
Disclosure
The authors report no conflicts of interest in this work.
References
Dziewasa R, Freund M, Ludemann P, Muller M, Ritter M, Droste DW, Stögbauer F. Treatment options in vertebrobasilar dolichoectasia – case report and review of the literature. Eur Neurol. 2003;49(4):245–247. | ||
Resta M, Gentile MA, Di Cuonzo F, Vinjau E, Brindicci D, Carella A. Clinical-angiographic correlations in 132 patients with megadolichovertebrobasilar anomaly. Neuroradiology. 1984;26(3):213–216. | ||
D’Andrea F, Maiuri F, Gangemi M, Iaconetta G. Megadolichobasilar anomaly. Clinical and diagnostic considerations on 30 cases. Acta Neurol (Napoli). 1992;14(4–6):611–619. | ||
Ekbom K, Greitz T, Kugelberg E. Hydrocephalus due to ectasia of the basilar artery. J Neurol Sci. 1969;8(3):465–477. | ||
Milandre L, Bonnefoi B, Pestre P, Pellissier JF, Grisoli F, Khalil R. Vertebrobasilar arterial dolichoectasia. Complications and prognosis. Rev Neurol (Paris). 1991;147(11):714–722. | ||
Passero S, Filosomi G. Posterior circulation infarcts in patients with vertebrobasilar dolichoectasia. Stroke. 1998;29(3):653–659. | ||
Himi T, Kataura A, Tokuda S, Sumi Y, Kamiyama K, Shitamichi M. Downbeat nystagmus with compression of the medulla oblongata by the dolichoectatic vertebral arteries. Am J Otol. 1995;16(3):377–381. | ||
Jacobson DM, Corbett JJ. Downbeat nystagmus associated with dolichoectasia of the vertebrobasilar artery. Arch Neurol. 1989;46(9):1005–1008. | ||
Kobayashi T, Ogawa A, Kameyama M, Uenohara H, Yoshimoto T. Chiari malformation with compression of the medulla oblongata by the vertebral arteries. J Neurosurg. 1992;77(2):307–309. | ||
Lee AG. Downbeat nystagmus associated with caudal brainstem compression by the vertebral artery. J Neuroophthalmol. 2001;21(3):219–220. | ||
Moon KH, Lee SA, Ahn JS, Kwun BD. Downbeat nystagmus associated with brainstem compression by vertebral artery. J Korean Neurosurg Soc. 2007;41(3):190–192. | ||
Jang L, Borruat FX. Oculopalatal tremor: variations on a theme by Guillain and Mollaret. Eur Neurol. 2014;72(3–4):144–149. | ||
Kim JS, Moon SY, Choi KD, Kim JH, Sharpe JA. Patterns of ocular oscillation in oculopalatal termor: imaging correlations. Neurology. 2007;68(14):1128–1135. | ||
Borruat FX. Oculopalatal tremor: current concepts and new observations. Curr Opin Neurol. 2013;26(1):67–73. | ||
Guillain G, Mollaret P. Deux cas de myoclonies synchrones et rythmées vélo-pharyngo- laryngo-oculo-diaphragmatiques. Le problème anatomique et physiologique de ce syndrome. Rev Neurol (Paris). 1931;2:545–566. | ||
Matsuo F, Ajax ET. Palatal myoclonus and denervation supersensitivity in the central nervous system. Ann Neurol. 1979;5(1):72–78. | ||
Leigh RJ, Zee DS. The Neurology of Eye Movements. Oxford: Oxford University Press; 2006. | ||
Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: meta-analysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol. 2000;21(6):1073–1077. | ||
Savitz SI, Ronthal M, Caplan LR. Vertebral artery compression of medulla. Arch Neurol. 2006;63(2):234–241. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.