Back to Journals » OncoTargets and Therapy » Volume 13
Atypical Mature T-Cell Neoplasms: The Relevance of the Role of Flow Cytometry
Authors Statuto T , D'Auria F, Del Vecchio L, Mansueto GR, Villani O, Lalinga AV, Possidente L, Nozza F, Vona G, Rago L, Storto G, Gasparini VR, Zambello R, D'Arena G , Valvano L
Received 28 April 2020
Accepted for publication 23 June 2020
Published 3 August 2020 Volume 2020:13 Pages 7605—7614
DOI https://doi.org/10.2147/OTT.S258512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Teodora Statuto,1,* Fiorella D’Auria,2,* Luigi Del Vecchio3,4,†, Giovanna Rosaria Mansueto,5 Oreste Villani,5 Anna Vittoria Lalinga6,†, Luciana Possidente,6 Filomena Nozza,1 Gabriella Vona,1 Luciana Rago,7 Giovanni Storto,8 Vanessa Rebecca Gasparini,9 Renato Zambello,10 Giovanni D’Arena,5,* Luciana Valvano1,*
1Laboratory of Clinical Research and Advanced Diagnostics, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy; 2Unit of Clinical Pathology, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy; 3CEINGE Biotecnologie Avanzate S.c.a.r.l, Federico II University, Naples, Italy; 4Department of Molecular Medicine and Medical Biotechnology (DMMBM), Federico II University, Naples, Italy; 5Hematology Department of Basilicata, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy; 6Pathology Unit, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy; 7Radiotherapy Unit, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy; 8Department of Nuclear Medicine, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy; 9Department of Medicine, University of Padova - Veneto Institute of Molecular Medicine, VIMM, Padova, PD, Italy; 10Hematology and Clinical Immunology, Department of Medicine, Padua School of Medicine, Padova, PD, Italy
†Anna Vittoria Lalinga passed away on January 26, 2020 and Luigi Del Vecchio passed away on August 16, 2018
*These authors contributed equally to this work
Correspondence: Luciana Valvano
Laboratory of Clinical Research and Advanced Diagnostics, Centro Di Riferimento Oncologico Della Basilicata (IRCCS-CROB), Rionero in Vulture, Pz, Italy
Tel +39 0972 726395
Fax +39 0972 723509
Email [email protected]
Abstract: Lymphoproliferative disorders are a heterogeneous group of malignant clonal proliferations of lymphocytes whose diagnosis remains challenging, despite diagnostic criteria are now well established, due to their heterogeneity in clinical presentation and immunophenotypic profile. Lymphoid T-cell disorders are more rarely seen than B-cell entities and more difficult to diagnose for the absence of a specific immunophenotypic signature. Flow cytometry is a useful tool in diagnosing T-cell lymphoproliferative disorders since it is not only able to better characterize T-cell neoplasms but also to resolve some very complicated cases, in particular those in which a small size population of neoplastic cells is available for the analysis. Here, we report three patients with mature T-cell neoplasms with atypical clinical and biological features in which analysis of peripheral blood and bone marrow specimens by means of multicolor flow cytometry was very useful to identify and characterize three rare T-cell lymphoproliferative disorders, such as angioimmunoblastic T-cell lymphoma, peripheral T-cell lymphoma not otherwise specified and T-cell prolymphocytic leukemia. The aim of this case series report is not only to describe three rare cases of lymphoproliferative neoplasms but also to raise awareness that a fast, highly sensitive, and reproducible procedure, such as flow cytometry immunophenotyping, can have a determinant diagnostic role in these patients.
Keywords: flow cytometry, immunophenotype, angioimmunoblastic T-cell lymphoma, peripheral T-cell lymphoma not otherwise specified, T-cell prolymphocytic leukemia, diagnosis
Introduction
T-cell lymphomas account approximately for 10–15% of lymphoid malignancies.1 According to the most recent World Health Organization (WHO) classification of lymphoid neoplasms, angioimmunoblastic T-cell lymphoma (AITL), peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) and T-cell prolymphocytic leukemia (T-PLL) are categorized as mature T-cell neoplasms.2,3 Common clinical manifestations of T-cell neoplasms include systemic lymphadenopathy, hepatosplenomegaly, fever, anemia, skin rash and serous effusions.
In particular, AITL is a distinct clinical-pathological form of PTCL, accounting for 15–20% of PTCL and 1–2% of all non-Hodgkin lymphoma (NHL) cases and is associated with poor prognosis and a 5-year survival rate of 30–36%.4–6 The most common and heterogeneous subtype of PTCL is PTCL-NOS, accounting for up to 35% of PTCL cases in Europe and North America. Prognosis is poor, with a 5-year overall survival (OS) of 20–30%.7 T-PLL is an uncommon and aggressive neoplasm, representing 2% of mature lymphoid leukemias. It derives from clonal proliferation of post-thymic T-cells and is associated with a poor prognosis and short survival.8
However, despite diagnostic criteria of these lymphoid matures disorders are now well established, heterogeneity in clinical presentation and immunophenotypic profile often challenges our diagnostic ability. Here we present three cases of mature T-cell neoplasms with clinical and pathological atypical features in which flow cytometry (FCM) allowed a correct diagnosis.
Patients and Methods
Peripheral blood (PB) samples and bone marrow (BM) aspirates from three patients with suspected lymphoproliferative disorders were processed for FCM immunophenotyping using a stain-lyse-no wash technique and a comprehensive seven-color antibody-panel (FITC/PE/PerCP-Cy5-5/PE-Cy7/APC/APC-H7/V500 fluorescent conjugates) on a BD FACSCantoTM II. Our T-cell neoplasms diagnosis panel included monoclonal antibodies against surface (s) and cytoplasmic (cy) antigens: CD3, CD16, CD56, CD45, CD20, CD19, CD8, CD4, CD7, CD2, CD5, TCRαβ, TCRγδ, CD57, CD10, CD34, TdT, HLA-DR and CD1a. Analysis of T-cell clonality was performed using the IOTest Beta Mark TCR Vβ Kit. The B-cell neoplasms diagnostic panel included monoclonal antibodies against CD19, CD5, CD45, CD20, CD23, CD43, CD79b, CD200, FMC7, CD22, sIgkappa, sIglambda. Antibodies were purchased from BD Biosciences (BD), Miltenyi Biotec (MB) or Beckman Coulter (BC). The immunophenotypic analyses were performed using FACSDiva (BD) and Kaluza (BC) Analysis Softwares. To check CD45 negativity in case no. 2, different clones and fluorochromes were tested: 1) BD PerCP Mouse Anti-Human CD45 (2D1 clone), 2) BD V500 Mouse Anti-Human CD45 (HI30 clone), 3) BD APC-H7 Mouse Anti-Human CD45RA (HI100 clone), 4) MB CD45RO-APC human (UCHL1 clone), 5) BC monoclonal CD45-APC-Alexa Fluor 750 (J33 clone). Intracellular staining was performed using the BD IntraSure Kit, according to the manufacturer’s protocol. For CD3, two different clones and fluorochromes were used: CD3 FITC (SK7 clone, BD) and CD3 APC (UCHT1 clone, BD). In our assays, we included viable cells in the analysis and excluded any debris, dead cells and clumps or doublets using forward and side scatter (very low) parameters. The pathological populations were established based on the evidence of monoclonality or of an aberrant B or T immunophenotype. The quantitative determination of the TCR-Vβ repertoire of human T lymphocytes was assessed using IOTest® Beta Mark TCR V Kit (BC), including CD3-FITC and CD45-PerCP in each tube for gating aberrant T-cell populations. The FC TCR-Vβ results were considered to be positive for clonality if: (a) a single TCR-Vβ was expressed by at least 50% of a gated T cell subset; or (b) a TCR-Vβ was expressed at a frequency 10 times above its normal limit, based on the manufacturer’s reported ranges for total T cells or T cell subsets; or (c) at least 70% of gated T cells failed to react to any of the TCR-Vβ antibodies, presumably due to expression of a TCR-Vβ not recognized by the antibody panel. The last set of cases was defined as “non-reactive”.9 Morphological features were evaluated by hematoxylin and eosin (H&E)-stained BM aspirates, lymph nodes (LNs) and sections of the skin lesions. Fluorescent In Situ Hybridization (FISH) was performed according to the instructions of the manufacturer on fixed nuclei by using the commercially available panel Vysis CLL FISH Probe Kit (Abbott Molecular) to detect deletion of the LSI TP53, LSI ATM and LSI D13S319 probe targets. A selective Polymerase chain reaction (PCR) was used to analyze exon 16 of STAT5b and exon 21 of STAT3, as previously reported.10–12 The analysis of the immunoglobulin heavy variable (IGHV) sequences was performed by Next Generation Sequencing (NGS) technique, carried out according to the IMGT-V-Quest, Arrest/AssignSubsets criteria.13
Clinical and laboratory features, immunohistochemical results at diagnosis and FCM immunophenotyping data at diagnosis on BM aspirates of the three patients are summarized in Tables 1, 2 and 3
 |
Table 1 Clinical and Laboratory Features of the Three Cases at Diagnosis |
 |
Table 2 Immunohistochemical Results at Diagnosis |
 |
Table 3 FC Immunophenotype Results at Diagnosis on BMA Samples |
Case 1
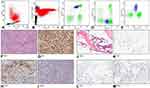
A 77-year-old man with multiple enlarged axillary and inguinal LNs was admitted at our Hospital in March 2016. Despite a normal leukocyte count, without atypical morphological findings, a routine FCM immunophenotyping analysis of PB lymphocytes revealed the presence of a small (2% of CD45+ cells) and abnormal population of CD4+ CD8− T-cells with decreased CD3 expression (CD3+ dim) (Figure 1A–D). This population also showed the positive expression of the T-cells markers CD7, CD2 and CD5 and the negative expression for the 24 rearrangements of TCRVβ repertoire tested. Furthermore, this T-cell population also expressed CD10 (Figure 1E). A biopsy of a left axillary LN established the diagnosis of AITL. The LN displayed a prominent proliferation of high endothelial venules, an increase of dendritic cells and a diffuse infiltration of a mixed population of eosinophils, neutrophils, plasma cells, with medium- to small-sized atypical lymphoid cells (Figure 1F) positive for CD3, CD4, CD5 (Figure 1G), CD10 (Figure 1H) and CD7 (focal), and negative for CD20 (Figure 1I), CD79a and CD30. Proliferative index, assessed by Ki67 staining, was 65–70%. Abnormal cells were mixed to several reactive CD8+ T lymphocytes. Whole-body positron emission computed tomography (PET-CT) scan evidenced generalized pathological lymphadenopathy above and below the diaphragm (highest SUV: 5.4).
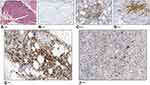
In April 2016, FCM analysis performed on both BM and PB confirmed the presence of CD3+dim CD4+CD5+CD8−CD10+ T-cells accounting for 3% of CD45+ cells in PB and 0.3% of all nucleated cells in BM. Notably, morphologic evaluation of BM smears and bone marrow biopsy (BMB) was not able to detect any BM infiltration (Figure 1J–M). From April 2016 to September 2016, the patient underwent chemotherapy with COMP (cyclophosphamide, vincristine, liposomal doxorubicin and prednisone) regimen for a total of six courses. In September 2016, PET-CT scan was negative, and the patient was considered in complete remission. In February 2017, the patient was still in good general conditions, with a completely negative total body CT scan. However, 6 months later (July 2017), the patient developed paresthesia of the hands and PET-CT scan showed some areas of doubtful abnormal uptake, including axillary and mediastinal LNs (SUV up to 2.4). In November 2017, the patient reported high fever and CT scan showed a clear progressive disease at multiple sites, including bilateral LNs of cervical, intra-retroperitoneal and inguinal regions, axillary fossa and mediastinum. Splenomegaly was also detected. FCM analysis performed on BM to monitor AITL cells, confirmed the presence of a very small pathological T-cell population (0.36% of all nucleated cells) with the same immunophenotype seen at diagnosis, while FSC (Forward Scatter), SSC (Side Scatter) and CD45 FC parameters did not apparently detect abnormal CD3 negative lymphoid cells, so that a more extensive study of B cell markers (CD20, CD19, sIg kappa, sIg lambda) was not performed. Unexpectedly, BMB showed a diffuse, interstitial infiltration of “immunoblastic-like” lymphoid cells (Figure 2A), negative for CD5 (Figure 2B), but positive for CD20 (Figure 2C), CD30 (Figure 2D) and CD79a, mixed to several small reactive T lymphocytes and rare mature plasma cells. The conclusion was a diagnosis of DLBCL. The LN biopsy of the initial AITL-diagnosis and the BMB involved by DLBCL were tested by immunohistochemistry for Epstein-Barr Virus (EBV) (by using DAKO Monoclonal Mouse Anti-Epstein-Barr Virus, LMP, CS.1–4 clone): both samples resulted positive (Figure 2E and F). The patient died in December 2017 for a progressive multiorgan failure.
Case 2
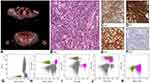
In May 2018 a 60-year-old man with a history of diabetes mellitus, arterial hypertension, and skin lesions on head and inguinal regions treated for 8 months with clobetasol was admitted at our hospital because of the appearance of new skin lesions. PET-CT scan (Figure 3A) demonstrated a faint hyperaccumulation of the tracer in correspondence of the voluminous subcutaneous thickenings in the left parietal, right dorsal and homolateral lumbar regions and an absence of uptake in a nodulariform thickening in the subcutaneous tissue of the right scapular region and in the numerous LNs in bilateral inguinal and axillary regions and in Barety’s space. Physical examination showed cutaneous plaques on the scalp (3 cm, purple with a central encrusted region), on the right gluteus (8x8 cm, red-violet), on the right shoulder blade (5 x 8 cm, pink) and in the right inguinal region (3 x 2.5 cm, reddish), splenomegaly and LN enlargements. Cutaneous biopsies of backside and scalp lesions showed infiltration, diffuse and subtotal, of small and medium-sized lymphoid elements, with pleomorphic nuclei, extended to the superficial and deep reticular dermis, in the absence of epidermotropism (Figure 3B). These cells were found to be CD3+, CD4+, CD5+, CD8− (Figure 3C–F), CD56−, CD7−, CD57−, CD30−, CD20− with Ki67 cell proliferation index about 20%. In addition, some small B-cell lymphocytes (CD20+) were interspersed. The conclusive diagnosis was PTCL-NOS. BM examination did not show a cytologically relevant lymphomatous infiltration while FC immunophenotyping (Figure 3G–K) documented a CD3+ T-cell population (13%) more than half (51.5%) of which were abnormal T-cells displaying the following phenotype: CD4+, CD2+, CD7+dim, CD5+, TCRαβ+, CD45−, CD45RA−, CD45RO−, CD8−, CD57−, CD10−, CD16−, CD56−, TdT−, TCRγδ− and “non-reactive” for the 24 rearrangements of TCRVβ tested. The patient received six cycles of CHOEP (Cyclophosphamide–Doxorubicin–Vincristine–Etoposide–Prednisone) chemotherapy regimen. Afterwards, the patient underwent radiation treatment on constant PET localizations. The last FCM follow-up performed on BM (in May 2019) showed a minimal residual disease (MRD) of 3.1% on all nucleated cells. Currently, the patient had a relapse.
Case 3
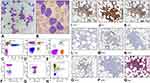
A 79-year-old male was admitted at our Hospital in June 2018, due to an increased number of circulating white blood cells (WBC) (38 x 103/µL; lymphocytes 84%, neutrophils 16%). The patient had a previous history of ischemic heart disease and presented with bilateral neck, axillary and inguinal small (<1–2 cm) superficial LNs. BM aspirate showed a widespread lymphoid infiltration, predominantly small “mature” lymphocytes with very rare nucleoli, representing about half of the total cells (Figure 4A and B). CT scan also showed multiple abdominal LNs. FCM immunophenotyping of both PB and BM aspirates showed the presence (50% and 60%, respectively, on all nucleated cells) of sCD3-, cyCD3+, CD8-, CD2+, CD7+, CD5+, CD4+, CD56-, CD57-, CD16-, HLA-DR-, CD34-, CD1a-, TdT-, CD10-, TCRαβ- and TCRγδ- pathological T cells; the 75% of these cells expressed CD45 on their membrane. Notably, these T-cells were mixed with a small population of clonal B lymphocytes (3,1% of leukocytes in PB, 2.2% of all nucleated cells in BM), that expressed CD19, CD5, CD20 (dim), CD23, CD43, CD200, sIgkappa (dim), with a D’Arena Score of 4 (Figure 4C–H).14 Trephine BM biopsy showed two nodular aggregates of small and sometimes medium-sized lymphoid elements with predominantly T phenotype (CD57-, CD8-, CD5+, CD3+, CD4+, CD56-), mixed with rare CD20+, CD79α+ small B lymphocytes (Figure 4I–Q). FISH analysis, performed on unpurified nuclei, documented ATM and 13q14.3 deletions in 50% of cells, while 36% only displayed ATM deletion. A second FISH performed on purified CD19+ cells showed only the deletion of chromosome 13q14.3. IGHV sequences showed an unmutated status. Molecular analysis highlighted no mutations for both the exon 16 of the STAT5b gene and the exon 21 of the STAT3 gene. A final diagnosis of T-PLL (likely “small cell variant”, according to WHO classification)3 with concomitant Monoclonal B-cell lymphocytosis (MBL) was made. Despite the treatment with bendamustine and CHOP (cyclophosphamide, vincristine, doxorubicin and prednisone) regimen the patient showed a progressive increase of WBC up to 500 x 103/µL, with an increase of pathological T population (90% of total nucleated cells) and disappearance of the clonal MBL B-cells. The patient died during pentostatin treatment.
Discussion
Multiparameter FCM is a fast, highly sensitive, and reproducible procedure to evaluate the immunophenotype of T lymphocytes,15 allowing the identification and characterization of T-cell neoplasms. Among them, AITL, PTCL-NOS and T-PLL must be considered despite very rarely seen. Typical immunophenotype of AITL is CD3+, CD4+, CD8− and, frequently CD10+. However, several authors believe that the only presence of a small number of CD4+CD10+ T-cells, in the absence of other phenotypic aberrancies, should not be considered a sign of the presence of neoplastic cells and correlation with all available morphologic and immunophenotypic data is necessary to make a definitive diagnosis.16–19 In this setting, Loghavi et al20 reported a case of AITL involving LNs and PB without CD10 expression on T-cells. We have described here an unusual case of CD3+ dim CD4+ CD8− CD5+ CD10+ AITL, with a limited infiltration of BM, detected by FCM on BM (and PB), but not by BMB, using conventional IHC. Another interesting aspect of this case was the sequential development of DLBCL after the treatment of AITL with COMP regimen.3 DLBCL is the most frequent type of NHL, representing about 30–40% of all cases21 and cases of DLBCL arising after the initial diagnosis of AITL have been already reported.2–6 Interestingly, however, very few EBV positive cases are described in the literature22–24 and both LN biopsy of the initial AITL-diagnosis and the BMB involved by DLBCL resulted positive for EBV, as assessed by IHC. PTCL-NOS is characterized by an aberrant T-cell phenotype, with frequent loss of CD5 and CD7. A CD4+/CD8− phenotype predominates in nodal cases. CD4+/CD8+ or CD4−/CD8− phenotypes are sometimes seen as CD8, CD56 and cytotoxic granule expression.25 An analysis of prognostic factors in a group of 82 patients found that a favorable outcome was associated with a CD3+CD4+CD8− phenotype and with localized skin lesions.26 PTCL-NOS usually lacks CD10 expression and proliferation rate is usually high with Ki-67 exceeding 80% associated with a worse prognosis.27 The expression of CD30 has been reported in about 32–58% of PTCL-NOS cases with variable prognosis.28,29
CD45 is expressed on nucleated hematopoietic cells in healthy individuals with variable intensity. According to the cell type, the two major human isoforms (CD45RA and CD45RO) are expressed differently on T-cells.30–33 Different hematopoietic neoplasms with the loss of CD45 surface expression have been reported in the literature.30,34-37
The European Group for the Immunological Characterization of Leukemias (EGIL) has established guidelines for the characterization of acute leukemias based on marker expression. T lineage acute lymphoblastic leukemia (ALL) is defined by the cytoplasmic or membrane expression of CD3. Expression of the T-cell antigens CD2 and CD7 is not sufficient to define a case as T-ALL. Four subgroups from pro-T-ALL (or T-I) to mature T-ALL (or T-IV) have been also described according to the degree of thymic differentiation. Within T-ALL, two subgroups were defined according to the membrane expression of TCR α/β chains and TCR γ/δ in association with CD3. Mature-T-ALL is defined by the expression of membrane CD3 and the negative expression of CD1a. Most cases of T-ALL are TdT+, HLA-DR−, CD34−, but these markers are not considered for diagnosis or disease classification. The expression of TdT is considered important for the diagnosis of ALL, to distinguish them from mature lymphoid malignancies; however, TdT as well as other hematopoietic cell precursor associated markers, such as CD34 and class II HLA-DR, do not account for disease classification purposes.38 In the current case, pathological T-cells expressed CD3, but were negative for CD45. The expression of TCRαβ, as surface transmembrane receptor, the lack of TdT and morphological (predominantly small “mature” lymphocytes with very rare nucleoli and two nodular aggregates of small and sometimes medium-sized lymphoid elements) and histological analyses excluded the possibility that pathological cells could be leukemic blasts, confirming infiltration of lymphoid elements with T phenotype. The “non-reactive” TCR Vβ FCM phenotype further demonstrated the presence of a pathological population, characterized by the loss of CD45. T-PLL is a rare T-cell neoplasm with a post-thymic (TdT−, CD1a−), mature (CD2+, CD5+, CD7+, CD16− and CD56−) T-cell immunophenotype, with variable CD4 and CD8 expression (generally CD4+/CD8−). It is mostly a CD45+ cyCD3+ T-cell leukemia, but in 5% to 10% of cases, either of these markers may be negative, while simultaneous loss of both is extremely rare.39 CD3 is an important T cell marker. Its presence at all stages of T cell development makes it an ideal T cell marker for both the detection of normal and neoplastic T cells and a useful immunohistochemical marker for T cells in tissue sections. CD3 can be found both on the cell membrane and/or in the cytoplasm of T cell neoplasms.40
In the current case, pathological T-cells were negative for surface CD3 and only the 76% of them expressed the CD45 antigen. Furthermore, they were also accompanied by a small population of B lymphocytes with chronic lymphocytic leukemia (CLL) immunophenotype.
MBL is defined as the presence of a clonal B-cell population in the peripheral blood with fewer than 5 × 109/L B-cells and no other signs of a lymphoproliferative disorder (eg, lymphadenopathy, organomegaly, cytopenia, or extramedullary involvement).41 The majority of cases of MBL have the immunophenotype of CLL.42 The use of alemtuzumab (anti-CD52 antibody) in symptomatic T-PLL patients has improved OS over the traditional alkylating agents or purine analog-based chemotherapy. For patients who fail to attain a complete remission with alemtuzumab, the addition of pentostatin should be considered.43 A retrospective study in 15 patients showed that bendamustine is a valuable treatment option for T-PLL, confirming the good overall response rate (ORR) of single-agent bendamustine in mature T cell neoplasms.44 In the present case, considering the ischemic heart disease of the patient and the cardiotoxic side effects of alemtuzumab,45 the patient initially received three cycles of bendamustine. Cases no. 2 and 3 demonstrated the FCM ability to analyze multiple markers simultaneously on populations with anomalous immunophenotype (eg, the lack of CD45 and CD3 on surface), which, if not evidenced, could lead to an incorrect immunophenotypic diagnosis and monitoring of neoplasm. Our data confirm that there are situations where FCM may play a determinant role, providing objective and quantitative results, even on very small populations within few hours. Furthermore, this advanced technique results in superior to IHC in the cases where the pathological cellular population is characterized by the concurrent expression of multiple aberrant antigens. Currently, the high sensitivity and the multiparametric feature of FCM analysis make it a useful tool for diagnosis and residual disease monitoring of T-cell neoplasms.
Informed Consent
Written informed consents were obtained from the patients for their anonymized data to be published in this article.
Ethics Approval
Ethical approval to report these cases was obtained from Comitato Etico Unico Regionale per la Basilicata (20140040750).
Acknowledgments
We would like to thank Dr. Luigi Del Vecchio and Dr. Anna Vittoria Lalinga, who, although no longer with us, continue to inspire us with the example and the dedication demonstrated over the course of their careers.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Armitage JO. The aggressive peripheral T-cell lymphomas: 2017. Am J Hematol. 2017;92(7):706–715. doi:10.1002/ajh.24791
2. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
3. Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (Revised 4th Edition). Lyon: IARC; 2017.
4. Rüdiger T, Weisenburger DD, Anderson JR, et al. Non-Hodgkin’s Lymphoma Classification Project. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 2002;13(1):140–149. doi:10.1093/annonc/mdf033
5. Alizadeh AA, Advani RH. Evaluation and management of angioimmunoblastic T-cell lymphoma: a review of current approaches and future strategies. Clin Adv Hematol Oncol. 2008;6(12):899–909.
6. Mourad N, Mounier N, Brière J, et al. Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111(9):4463–4470. doi:10.1182/blood-2007-08-105759
7. Oluwasanjo A, Kartan S, Johnson W, et al. Peripheral T-Cell Lymphoma, not Otherwise Specified (PTCL-NOS). Cancer Treat Res. 2019;176:83–98.
8. Chen X, Cherian S. Immunophenotypic Characterization of T-Cell Prolymphocytic Leukemia. Am J Clin Pathol. 2013;140(5):727–735. doi:10.1309/AJCPG71KYOXTKLQW
9. Feng B, Jorgensen JL, Hu Y, Medeiros LJ, Wang SA. TCR-Vbeta flow cytometric analysis of peripheral blood for assessing clonality and disease burden in patients with T cell large granular lymphocyte leukaemia. J Clin Pathol. 2010;63(2):141–146. doi:10.1136/jcp.2009.069336
10. Teramo A, Barilà G, Calabretto G, et al. STAT3 mutation impacts biological and clinical features of T-LGL leukemia. Oncotarget. 2017;8(37):61876–61889. doi:10.18632/oncotarget.18711
11. Koskela HL, Eldfors S, Ellonen P, et al. STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905–1913. doi:10.1056/NEJMoa1114885
12. Rajala HL, Porkka K, Maciejewski JP, Loughran TP, Mustjoki S. Uncovering the pathogenesis of large granular lymphocytic leukemia-novel STAT3 and STAT5b mutations. Ann Med. 2014;46(3):114–122. doi:10.3109/07853890.2014.882105
13. Bystry V, Agathangelidis A, Bikos V, et al. European Research Initiative on CLL. ARResT/AssignSubsets: a novel application for robust subclassification of chronic lymphocytic leukemia based on B cell receptor IG stereotypy. Bioinformatics. 2015;31(23):3844–3846. doi:10.1093/bioinformatics/btv456
14. D’Arena G, Vitale C, Rossi G, et al. CD200 included in a 4-marker modified Matutes score provides optimal sensitivity and specificity for the diagnosis of chronic lymphocytic leukaemia. Hematol Oncol. 2018;36(3):543–546. doi:10.1002/hon.2510
15. Gorczyca W, Weisberger J, Liu Z, et al. An approach to diagnosis of T-cell lymphoproliferative disorders by flow cytometry. Cytometry. 2002;50(3):177–190. doi:10.1002/cyto.10003
16. Iannitto E, Ferreri AJ, Minardi V, Tripodo C, Kreipe HH. Angioimmunoblastic T-cell lymphoma. Crit Rev Oncol Hematol. 2008;68(3):264–271. doi:10.1016/j.critrevonc.2008.06.012
17. Attygalle AD, Diss TC, Munson P, Isaacson PG, Du MQ, Dogan A. CD10 expression in extranodal dissemination of angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2004;28(1):54–61. doi:10.1097/00000478-200401000-00005
18. Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129(9):1095–1102. doi:10.1182/blood-2016-09-692541
19. Stacchini A, Demurtas A, Aliberti S, et al. The usefulness of flow cytometric CD10 detection in the differential diagnosis of peripheral T-cell lymphomas. Am J Clin Pathol. 2007;128(5):854–864. doi:10.1309/MC7QRGPTV0LRR98X
20. Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57(12):2804–2812. doi:10.3109/10428194.2016.1170827
21. Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74–87. doi:10.1016/j.pathol.2017.09.006
22. Huang J, Zhang PH, Gao YH, Qiu LG. Sequential development of diffuse large B-cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. Diagn Cytopathol. 2012;40(4):346–351. doi:10.1002/dc.21641
23. Skugor ND, Perić Z, Vrhovac R, Radić-Kristo D, Kardum-Skelin I, Jaksić B. Diffuse large B-cell lymphoma in patient after treatment of angioimmunoblastic T-cell lymphoma. Coll Antropol. 2010;34(1):241–245.
24. Zhou Y, Rosenblum MK, Dogan A, Jungbluth AA, Chiu A. Cerebellar EBV-associated diffuse large B cell lymphoma following angioimmunoblastic T cell lymphoma. J Hematop. 2015;8(4):235–241. doi:10.1007/s12308-015-0241-8
25. Savage KJ, Ferreri AJM, Zinzani PL, Pileri SA. Peripheral T-cell lymphoma—not otherwise specified. Crit Rev Oncol Hematol. 2011;79(3):321–329. doi:10.1016/j.critrevonc.2010.07.007
26. Bekkenk MW, Vermeer MH, Jansen PM, et al. Peripheral T-cell lymphomas unspecified presenting in the skin: analysis of prognostic factors in a group of 82 patients. Blood. 2003;102(6):2213–2219. doi:10.1182/blood-2002-07-1960
27. Went P, Agostinelli C, Gallamini A, et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24(16):2472–2479. doi:10.1200/JCO.2005.03.6327
28. Sabattini E, Pizzi M, Tabanelli V, et al. CD30 expression in peripheral T-cell lymphomas. Haematologica. 2013;98(8):e81–2. doi:10.3324/haematol.2013.084913
29. Maura F, Dodero A, Carniti C, Bolli N. Biology of peripheral T cell lymphoma, not otherwise specified: is something finally happening? Pathogenesis. 2016;3(1):9–18. doi:10.1016/j.pathog.2016.02.002
30. Dupéré-Minier G, Desharnais P, Bernier J. Involvement of tyrosine phosphatase CD45 in apoptosis. Apoptosis. 2010;15(1):1–13. doi:10.1007/s10495-009-0413-z
31. Ortolani C. Flow Cytometry of Hematological Malignancies. Chichester, West Sussex: John Wiley & Sons; 2011:311.
32. Boehrer S, Hinz T, Schui D, et al. T-large granular lymphocyte leukaemia with natural killer cell-like cytotoxicity and expression of two different alpha- and beta-T-cell receptor chains. Br J Haematol. 2001;112(1):201–203. doi:10.1046/j.1365-2141.2001.02559.x
33. Richards SJ, Short M, Steed AJ, Scott CS. A biclonal large granular lymphocyte (LGL)/NK-associated (NKa) disorder of CD4+ and CD8+ lymphocyte subpopulations characterized by the simultaneous presence of distinct TCR rearrangements. Br J Haematol. 1994;88(3):629–632. doi:10.1111/j.1365-2141.1994.tb05087.x
34. Ozdemirli M, Mankin HJ, Aisenberg AC, Harris NL. Hodgkin’s disease presenting as a solitary bone tumor. A report of four cases and review of the literature. Cancer. 1996;77(1):79–88. doi:10.1002/(SICI)1097-0142(19960101)77:1<79::AID-CNCR14>3.0.CO;2-5
35. Ratei R, Sperling C, Karawajew L, et al. Immunophenotype and clinical characteristics of CD45-negative and CD45-positive childhood acute lymphoblastic leukemia. Ann Hematol. 1998;77(3):107–114. doi:10.1007/s002770050424
36. Kumar S, Rajkumar SV, Kimlinger T, Greipp PR, Witzig TE. CD45 expression by bone marrow plasma cells in multiple myeloma: clinical and biological correlations. Leukemia. 2005;19(8):1466–1470. doi:10.1038/sj.leu.2403823
37. Wang F, Xu D, Cui W. Leukocyte common antigen (CD45) negative follicular lymphoma, a rare immunophenotypic presentation. Clin Chim Acta. 2015;442:46–48. doi:10.1016/j.cca.2014.12.033
38. Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia. 1995;9(10):1783–1786.
39. Thakral B, Wang SA. T-cell prolymphocytic leukemia negative for surface CD3 and CD45. Blood. 2018;132(1):111. doi:10.1182/blood-2018-03-840611
40. Jamal S, Picker LJ, Aquino DB, McKenna RW, Dawson DB, Kroft SH. Immunophenotypic analysis of peripheral T-cell neoplasms. A multiparameter flow cytometric approach. Am J Clin Pathol. 2001;116(4):512–526. doi:10.1309/QF6N-VAQW-N74H-4JE2
41. Marti GE, Rawstrom AC, Ghia P, et al. International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br J Haematol. 2005;130(3):325–332. doi:10.1111/j.1365-2141.2005.05550.x
42. Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015;126(4):454–462. doi:10.1182/blood-2015-02-585059
43. Kaseb H, Madan A, Hozayen S. Cancer, T Cell Prolymphocytic Leukemia.StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020–2019 Jun 26.
44. Herbaux C, Genet P, Bouabdallah K, et al. Bendamustine is effective in T-cell prolymphocytic leukaemia. Br J Haematol. 2015;168(6):916–919. doi:10.1111/bjh.13175
45. Lenihan DJ, Alencar AJ, Yang D, Kurzrock R, Keating MJ, Duvic M. Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sézary syndrome. Blood. 2004;104(3):655–658. doi:10.1182/blood-2003-07-2345
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.