Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Attenuation of the macrophage inflammatory activity by TiO2 nanotubes via inhibition of MAPK and NF-κB pathways
Authors Neacsu P, Mazare A, Schmuki P, Cimpean A
Received 8 July 2015
Accepted for publication 6 September 2015
Published 12 October 2015 Volume 2015:10(1) Pages 6455—6467
DOI https://doi.org/10.2147/IJN.S92019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Patricia Neacsu,1 Anca Mazare,2 Patrik Schmuki,2 Anisoara Cimpean1
1Department of Biochemistry and Molecular Biology, University of Bucharest, Bucharest, Romania; 2Department of Materials Science, University of Erlangen-Nuremberg, Erlangen, Germany
Abstract: Biomaterial implantation in a living tissue triggers the activation of macrophages in inflammatory events, promoting the transcription of pro-inflammatory mediator genes. The initiation of macrophage inflammatory processes is mainly regulated by signaling proteins of mitogen-activated protein kinase (MAPK) and by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways. We have previously shown that titania nanotubes modified Ti surfaces (Ti/TiO2) mitigate the immune response, compared with flat Ti surfaces; however, little is known regarding the underlying mechanism. Therefore, the aim of this study is to investigate the mechanism(s) by which this nanotopography attenuates the inflammatory activity of macrophages. Thus, we analyzed the effects of TiO2 nanotubes on the activation of MAPK and NF-κB signaling pathways in standard and lipopolysaccharide-evoked conditions. Results showed that the Ti/TiO2 significantly reduce the expression levels of the phosphorylated forms of p38, ERK1/2, c-Jun NH2-terminal kinase (JNK), IKKβ, and IkB-α. Furthermore, a significant reduction in the p65 nuclear accumulation on the nanotubular surface was remarked. Following, by using specific MAPK inhibitors, we observed that lipopolysaccharide-induced production of monocyte chemotactic protein-1 and nitric oxide was significantly inhibited on the Ti/TiO2 surface via p38 and ERK1/2, but not via JNK. However, the selective inhibitor for JNK signaling pathway (SP600125) was effective in reducing tumor necrosis factor alpha release as well as monocyte chemotactic protein-1 and nitric oxide production. Altogether, these data suggest that titania nanotubes can attenuate the macrophage inflammatory response via suppression of MAPK and NF-κB pathways providing a potential mechanism for their anti-inflammatory activity.
Keywords: titania nanotubes, macrophage, mitogen-activated protein kinases, NF-κB, inflammatory mediators
Introduction
The influence of surface topography in modulating macrophage activity is well known, and it takes place through the activation of multiple intracellular signaling pathways involved in the foreign body reaction, which in turn, will lead to the expression of inflammatory cytokine genes.1–3 Among the existing signaling pathways, mitogen-activated protein kinase (MAPK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways are key regulators of the inflammatory processes.4,5 These pathways are known to be involved in the pro-inflammatory mediator expression induced by lipopolysaccharide (LPS), having a critical role in the regulation of cellular growth and differentiation as well as in controlling the cell behavior toward cytokines and stress stimuli.6 LPS-evoked signaling leads to the activation of these signal transduction pathways, in either a myeloid differentiation factor 88-dependent or -independent manner.7 The MAPK families are Ser and Thr protein kinases, which are activated in response to various extracellular stimuli and regulate essential cellular events including macrophage-mediated inflammatory responses.5 The activation through phosphorylation of three major MAPKs (p38 MAPK; ERK, extracellular signal-regulated kinase; and JNK, c-Jun NH2-terminal kinase) has been shown to promote inflammatory gene expression in LPS-induced macrophages, thus controlling different steps in the pro-inflammatory cytokine production process.8,9 p38 MAPK is a key player in the inflammatory responses and is involved in stress-induced gene expression.5,10,11 In addition, p38 is rapidly activated after LPS stimulation and was suggested to have an important role in controlling the expression of tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (iNOS).12 Similarly, ERK and JNK kinases are also known as stress-activated proteins and are involved in LPS-induced macrophage responses, increasing the production of pro-inflammatory cytokines and iNOS.12–15 The activation of the MAPK pathway is crucial for the subsequent activation of LPS-induced NF-κB signaling complex.16,17 NF-κB is able to bind to the specific promoter regions (that regulate the transcription of inflammatory genes) and for this reason it is a key regulator of inflammation.18 The NF-κB transcription factor consists of p65 and p50 subunits, and is normally located (when in resting state) in the cytoplasm, bound to the inhibitory proteins IkBs (IkB-α, IkB-β, and IkB-ε).19,20 The exposure of macrophages to pro-inflammatory stimuli causes a rapid phosphorylation of the IkB protein at two critical serine residues (Ser 32 and Ser 36) by IkB kinase (IKK).21,22 This results in IkB protein ubiquitination followed by proteasome-mediated degradation, and then NF-κB translocates to the nucleus to promote the transcription of inflammation-associated target genes.23–25 Furthermore, NF-κB is important for LPS-induced inflammation as it regulates a plethora of inflammatory genes (including TNF-α, IL-1, and iNOS) by binding to transcription-regulatory elements in a nucleotide sequence-specific manner.4,26–28
Studies investigating the surface-dependent inflammatory responses have shown that nanostructured surfaces are able to mitigate the activation of macrophages by reducing the secretion of the pro-inflammatory mediators.29–34 Moreover, surface nanostructuring of Ti (or Ti alloys) can be easily performed by electrochemical anodization, resulting in nanotubular structures directly coated on the Ti substrate.35 For TiO2 nanotubes, the activation of macrophages was shown to depend on the nanotopography, that is the diameter of the nanotubes is the key morphological parameter influencing inflammatory responses (from diameters ranging from 30 to 100 nm, the 70 nm showed the weakest inflammatory response).30 Our group has recently reported that TiO2 nanotubes exhibited a significant reduction in pro-inflammatory activity of LPS-stimulated RAW 264.7 macrophages with respect to cytokine/chemokine gene expression and protein secretion, and nitric oxide (NO) release, as compared with titanium flat surface.33 This attenuation effect of titania nanotubes was sustainable in long-term culture, as remarked by the decrease in the percentage of macrophage fusion into multinucleated cells at 7 days postseeding, as compared with commercial pure titanium (cpTi). More than that, under LPS-treatment, these cells adopted the morphology of foreign body giant cells (a hallmark histological feature of the chronic inflammation and foreign body response) only on the cpTi surface.
With these data as background, we further aimed to identify the molecular targets for TiO2 nanotubular surfaces and to correlate the inhibition of specific signaling proteins with the inhibitory action on the release of pro-inflammatory mediators (eg, TNF-α, monocyte chemotactic protein-1 [MCP-1] and NO). To the best of our knowledge, this is the first study that investigates the immunomodulatory properties of titania nanotubes by assessing the activation of the involved signaling pathways. RAW 264.7 murine macrophage cell line was used as cellular model and can be activated with LPS to simulate infection and inflammatory conditions through binding to Toll-like receptor 4. Toll-like receptor 4 stimulation activates the downstream MAPK and NF-κB pathways that are essential in cytokine and chemokine production.36 Therefore, the early activation of MAPKs and NF-κB pathways was investigated as to identify the mechanism(s) underlying the inhibitory effect of TiO2 nanotubes on the macrophage inflammatory activity (for comparative purposes, flat Ti surfaces were used as control samples, in both the standard and pro-inflammatory culture conditions).
Finally, one has to point out that optimization of the immunomodulatory potential of implants using defined surface topographies is important for implant osseointegration, and it is also beneficial for the biomaterials design and their eventual clinical use.
Materials and methods
Samples preparation and characterization
TiO2 nanotubes were prepared by electrochemical anodization of Ti foils (Advent, 0.1 mm thickness, purity >99.6%) as previously described.33 Briefly, the nanotubes are grown in a two-electrode configuration (sample – anode, Pt grid – cathode) in a Glycerol:H2O (60:40 vol%) +0.5 wt% NH4F electrolyte at 20 V for 2 hours (using a sweep ramp of 0.1 V s−1).33 Before anodization, the Ti foils were cleaned by ultrasonication (acetone, ethanol, and water – for 5 minutes each). The morphology and properties of the samples were investigated using scanning electron microscopy (Hitachi FE-SEM S4800), atomic force microscopy (A.P.E. Research, Trieste, Italy), and contact angle (CA) measurements (Contact Angle Meter – CAM 100, KSV Instruments Ltd, Helsinki, Finland). Ti foils cleaned by ultrasonication are used as control samples (cpTi). Before cell culture experiments, samples were cleaned by soaking in 70% ethanol and sterile-filtered Milli-Q water for 30 minutes each, and then air-dried and exposed to ultraviolet light for 30 minutes on each side, in a sterile culture hood.
Cell culture
The murine macrophage line, RAW 264.7 – purchased from American Type Culture Collection (ATCC, Manassas, VA, USA, TIB-71™), was maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 100 U·mL−1 penicillin and 100 μg·mL−1 streptomycin. Macrophages were incubated at 37°C in the presence of 5% CO2-humidified air and were routinely passaged using a cell scraper and replated in tissue culture flasks at a ratio of 1:6 when they reached ~80% confluence. None of the cell cultures undergone contact inhibition that could produce changes in MAPK or NF-κB signaling. Cells at passages 5–10 were used for the experiments. RAW 264.7 cells were plated in triplicate onto the Ti/TiO2 and cpTi surfaces for specific time periods and at different initial cell densities. Thus, to investigate the protein phosphorylation status of the specific inflammatory signaling molecules, cells were seeded at a population density of 5×104 cells·cm−2, maintained in culture for 24 hours and then were treated with 1 μg·mL−1 LPS for 10 and 30 minutes, respectively. For indirect immunofluorescent labeling, macrophages were seeded at 1.5×104 cells·cm−2 and left to adhere for 24 hours. To study NF-κB translocation, the cells were stimulated with 1 μg·mL−1 LPS for 10 and 30 minutes. To assess the level of cytoplasmic IkB-α phosphorylation, the RAW 264.7 macrophages were treated with the same stimulatory concentration of LPS for 5, 10, and 30 minutes.
Cell lysate preparation
LPS-stimulated and non-stimulated macrophages were washed once with ice-cold phosphate buffered saline (PBS). After removing PBS, the cells were lysed in lysis buffer (20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM disodium ethylenediaminetetraacetate, 1 mM ethylene glycol tetraacetic acid, 1% Triton, 20 mM sodium pyrophosphate, 25 mM sodium fluoride, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg·mL−1 leupeptin, 1 mM phenylmethylsulfonyl fluoride) by incubation on ice for 2 minutes. This lysate solution was stored at -80°C in single-use aliquots until required. The Bio-Rad protein assay, based on the method of Bradford, was performed to determine the protein concentrations in the cell lysates. The optical density was evaluated at 595 nm using a microplate reader (Appliskan, Thermo Scientific, Waltham, MA, USA).
ELISA determination of phosphorylated p38, ERK1/2, and JNK
To determine the intracellular level of phosphorylated p38, ERK1/2, and JNK, we used a DuosetIC enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions (R&D Systems, Inc., Minneapolis, MN, USA). An immobilized capture antibody specific for p38, ERK1/2 or JNK binds both phosphorylated and unphosphorylated proteins. After incubation with cell lysates followed by washing away unbound material, a biotinylated detection antibody is used to detect only phosphorylated forms of MAPKs, utilizing a standard Streptavidin – horseradish peroxidase (HRP) format. The optical density was recorded at 450 nm using a microplate reader (Thermo Scientific Appliskan).
ELISA assay of phospho-IKKβ
The endogenous level of phosphorylated IKKβ was detected using a solid phase sandwich ELISA kit, according to the manufacturer’s instructions (PathScan Phospho-IKKβ [Ser177/181] Sandwich ELISA Kit, Cell Signaling Technology, Inc., Danvers, MA, USA). A phospho-IKKβ rabbit monoclonal antibody has been coated onto the microwells. After incubation with cell lysates, phospho-IKKβ (Ser177/181) protein is captured by the coated antibody. Following extensive washing, an IKKβ mouse detection monoclonal antibody is added to detect the captured IKKβ protein. Anti-mouse IgG, HRP-linked antibody is then used to recognize the bound antibody. HRP substrate, 3,3′,5,5′-tetramethylbenzidine, is added to develop color. The optical density was recorded at 450 nm using a microplate reader (Appliskan, Thermo Scientific). The magnitude of the absorbance for the developed color is proportional to the quantity of phospho-IKKβ (Ser177/181).
Immunofluorescence staining
RAW 264.7 cells were seeded for 24 hours, onto each sample, to detect the phosphorylated IkB-α protein and NF-κB p65 subunit by immunofluorescence microscopy. After stimulation with LPS for specified times, the cells were fixed with 4% paraformaldehyde in PBS for 15 minutes and permeabilized and blocked with a solution containing 0.1% Triton X-100 and 2% bovine serum albumin for 30 minutes, at room temperature. After washing with PBS, polyclonal antibodies against NF-κB-p65 or phospho-IkB-α were applied for 2 hours followed by 20 minutes incubation with Alexa Fluor 488- and Alexa Fluor 546-conjugated specific secondary antibodies, respectively. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, 2 μg·mL−1), and the fluorescence was visualized using an inverted fluorescence microscope Olympus IX71. Representative microscopic fields were captured using Cell F software. To quantify NF-κB nuclear translocation, the cells with or without p65 colocalization with the nucleus were counted, using the ImageJ analysis software. The percentage of cells exhibiting nuclear localization of p65 was calculated and graphically represented.
Assessment of TNF-α, MCP-1, and NO production in the presence of specific MAPK inhibitors
To determine if inhibition of p38, ERK1/2, or JNK contributes to the attenuation of the macrophage inflammatory response to titania nanotubes, RAW 264.7 cells were grown in contact with the tested substrates for 18 hours, and then were exposed to specific inhibitors: SB202190 for p38, U0126 for ERK1/2, and SP600125 for JNK (Sigma–Aldrich Co., St Louis, MO, USA) for 1 hour. Then, the macrophages were additionally incubated for 24 hours under standard (−LPS) and pro-inflammatory (1 μg·mL−1 LPS) culture conditions. The concentration chosen for MAPK inhibitors (50 μM) was selected considering the smallest inhibitor concentration able to induce statistically significant results, without considerably affecting the cell viability. Cell culture media were collected and stored at -80°C before measurements of TNF-α, MCP-1, and NO production – these measurements were performed as previously described.33
Statistical analysis
The data values are presented as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance with Bonferroni’s multiple comparison tests. A probability of P<0.05 was considered significant.
Results
Synthesis and characterization of titania nanotubes
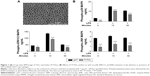
The morphological and chemical characterization of the nanotubular surface used in the present work was previously described.33 Briefly, the morphology of TiO2 nanotubes consists of ~78 nm diameter, ~1 μm length, and ~18 nm tube spacing (the top morphology is shown in Figure 1A). In addition, the nanotubular surface exhibits a higher roughness and hydrophilicity than the flat surface of cpTi, that is, the nanotubular samples have an average roughness of 104.1 nm and CA of 22.6°, while cpTi has an average roughness of 28.1 nm and a less hydrophilic surface with a CA of 75.4°.33
Effects of titania nanotubes on p38, ERK1/2, and JNK activation in RAW 264.7 cells
To elucidate whether the inhibition of the secretion of inflammatory mediators by the surface of Ti/TiO2 is mediated through the activation of MAPK signaling pathways, we evaluated several key MAPK signaling molecules (ie, p38, ERK1/2, and JNK) in both standard and LPS-stimulated culture conditions, by ELISA studies. Figure 1B indicates that the LPS treatment induced a strong phosphorylation of p38 and ERK1/2 that peaks at 10 minutes and decreases for the 30 minutes incubation times. In the case of JNK, only a weak phosphorylation after the LPS treatment was observed at 10 minutes and a slight decrease at 30 minutes. Moreover, the exposure of RAW 264.7 macrophages to Ti/TiO2 substrates significantly suppressed the phosphorylation of all studied MAPKs at both time points as compared with cpTi (P<0.001). More importantly, the nanotopography-dependent inhibition effect was also observed in the absence of the pro-inflammatory stimulus with a significance of P<0.001 for p38 and JNK and P<0.05 for ERK1/2 (Figure 1B). These results indicate that the attenuation of macrophage activation by titania nanotubes might be exerted, at least in part, by blocking the activation of the MAPK signal transduction pathway.
Surface-dependent phosphorylation of IKKβ and IkB-α in RAW 264.7 macrophages
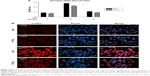
Following, we investigated the inhibitory effect of the nanotubular surface on NF-κB activation by assessing the phosphorylation level of IKKβ protein and the expression level of phospho-IkB-α (p-IkB-α), by ELISA studies and immunofluorescence analysis, respectively. As depicted in Figure 2A, the stimulation of cells with LPS resulted in a rapid phosphorylation of IKKβ on Ser 177/181, reaching a maximum intensity level after 10 minutes and decreasing almost to the basal level at the 30 minutes time point. Comparing with the reference material (cpTi), it is evident that the contact between RAW 264.7 cells and Ti/TiO2 surface leads to a significant inhibition of the LPS-induced phosphorylation of IKKβ at both tested poststimulation time points, that is, 10 and 30 minutes (P<0.001), as well as in the absence of the pro-inflammatory stimulus (untreated macrophages, P<0.01). Further, IkB-α phosphorylation was studied by immunofluorescence staining to investigate its involvement in the inhibitory effect of TiO2 nanotubes on pro-inflammatory activation of macrophages. The cytosolic expression of p-IkB-α was observed even at 5-minute poststimulation, and was visible up to 30 minutes (data not shown). Within 10 minutes of LPS stimulation, a high level of phosphorylated IkB-α was remarked onto the surface of cpTi, while the nanotubular surface maintained a low expression level of p-IkB-α in the cytoplasm but with a slight increase as compared with untreated cultures (Figure 2B). The suppression of IKKβ and IkB-α phosphorylation by surface nanotopography is an essential event that is associated with the subsequent inhibition of NF-κB translocation into the nucleus.
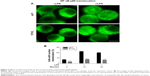
TiO2 nanotubes inhibit NF-κB activation and p65 nuclear translocation in RAW 264.7 cells
The activation of IKK phosphorylates the inhibitory IkB-α protein, leading to its degradation and the subsequent release of the NF-κB complex, which then translocates into the nucleus as to activate the transcription factors of the pro-inflammatory genes. To examine the NF-κB nuclear translocation, we performed immunocytochemistry studies on the p65 subunit of NF-κB. The fluorescent images show that for untreated macrophages, p65 was sequestered in the cytoplasm, whereas for LPS-stimulated macrophages p65 appeared in both the cytosol and the nuclear zone (Figure 3A). Furthermore, as shown in Figure 3B, LPS-induced a rapid nuclear accumulation of p65, peaking at 10 minutes and slowly decreasing in time. The percentage of cells displaying a NF-κB shift to the nucleus was significantly higher (P<0.001) on the flat Ti surface as compared with Ti/TiO2, for non-stimulated and LPS-stimulated (10 minutes and 30 minutes) macrophages. Moreover, a slight increase in the NF-κB nuclear accumulation upon LPS-stimulation was remarked on the nanotubular surface, while the cpTi surface elicited a marked enhancement of NF-κB nuclear translocation. Consequently, Ti/TiO2 nanotubes inhibited the nuclear translocation of the NF-κB p65 subunit, after the subunits were dissociated from IkB-α.
Involvement of MAPK activation in synthesis of TNF-α, MCP-1, and NO by murine RAW 264.7 cells
To further determine whether the inhibition of MAPKs contributes to the attenuation of macrophage inflammatory activity by TiO2 nanotubes, we have investigated the effect of specific MAPK inhibitors on the secretion of TNF-α, MCP-1, and NO by RAW 264.7 macrophages. A pretreatment of the cells with specific inhibitors (ie, SB202190 – p38 inhibitor, U0126 – ERK inhibitor, and SP600125 – JNK inhibitor) led to a decrease in the release of all analyzed pro-inflammatory mediators, both in the presence and absence of LPS (Figures 4 and 5). As shown in Figure 4, pretreatment with the ERK1/2-specific inhibitor (U0126) led to a dramatically diminished cytokine and chemokine expression in both culture conditions. Interestingly, under pro-inflammatory culture conditions (+LPS), a significant reduction in the release of TNF-α and MCP-1 on the surface of Ti/TiO2 was observed when RAW 264.7 cells were treated with the p38 (SB202190) and ERK (U0126) inhibitors. The only exception is for the JNK inhibitor (SP600125), which was ineffective in blocking the LPS-induced MCP-1 release. The above results suggest that ERK1/2 and p38 MAPK may constitute critical components in nanotopography-dependent regulation of TNF-α and MCP-1 release by macrophages. On the contrary, inhibition of the JNK signaling pathway contributes to the inhibitory action of titania nanotubes on LPS-induced TNF-α release, but not on MCP-1.
Similarly, we investigated the inhibitory mechanism of the Ti/TiO2 surface on LPS-stimulated NO production by RAW 264.1 macrophages. As shown in Figure 5, all three MAPK inhibitors lead to an inhibition of the LPS-induced nitrite accumulation and titania nanotubes attenuated NO production. Significant differences between the two surfaces were determined in the presence of U0126 and SB202190, suggesting that this nanoarchitecture inhibits NO production by blocking (at least partially) the ERK1/2 and p38 signal transduction pathways.
It is worth mentioning that, at the concentrations used, the MAPK inhibitors did not significantly affect cell viability, which was assessed by MTT assay (results not shown).
Discussion
Our previous study demonstrated that TiO2 nanotube layers exhibited significantly decreased inflammatory activity of macrophages, as compared with flat cpTi surface.33 However, the possible mechanism(s) underlying such cell behavior has not been explored yet. Hence, to demonstrate the molecular mechanism(s) that could explain the attenuation of macrophage inflammatory activity by titania nanotubes, we used the RAW 264.7 cell line that can be stimulated with LPS to mimic a state of infection and inflammation. The TiO2 nanotubular structures have a specific morphology with a mean diameter of ~78 nm and tube spacing of ~18 nm. The morphology of the selected TiO2 nanotubes is close in dimensions to the one (70 nm diameter) reported to exhibit the best advantage in terms of diameter size by producing the weakest macrophage inflammatory response30 and by achieving the most favorable level of success for in vivo osseointegration.37 There are few studies investigating the signaling pathways directly involved in the macrophage response to alterations on the surface of the material. Recently, Waterfield et al38 evaluated the early activation of the NF-κB pathway in RAW 264.7 macrophages in response to Ti surfaces with different roughness, for example, mechanically polished (PO), coarse and blasted (CB), acid etched (AE), and sandblasted and acid etched (SLA) Ti surfaces. The authors observed that PO and coarse and blasted surfaces exhibited the highest level of activation, followed by AE and SLA surfaces; thus stating that the activation of NF-κB pathway is topography-dependent. Moreover, Ghrebi et al3 demonstrated that these surface topographies can differently activate the components of the ERK1/2 signaling pathway. Namely, the PO surface induced an increase of ERK activation over time, while ERK1/2 phosphorylation decreased on SLA and AE surfaces. Likewise, nuclear translocation of pERK1/2 tended to increase in time on PO surface, but decreased on SLA and AE surfaces. These studies3,38 suggest that the surface topography interferes with macrophage-signaling pathways, subsequently affecting the cellular functions.
To explore whether the Ti/TiO2 surface has an effect on the activation of p38, ERK1/2 and JNK signaling molecules, macrophages were grown onto the studied biomaterials (in the presence or absence of LPS) and the time-dependent phosphorylation of these proteins was assessed by the ELISA technique. There are ample literature data indicating that LPS is a potent inductor able to trigger the activation of MAPKs and NF-κB signaling pathways.9,25,27,28,39–44 Our results confirm this statement, that is, the early activation of p38, ERK1/2, and JNK occurred quickly in LPS-treated macrophages. The phosphorylation level peaked at 10 minutes after LPS stimulation and the intensity appeared to decrease until 30 minutes. Moreover, Ti/TiO2 surface induced a significant reduction in the phosphorylated states of ERK1/2, p38, and JNK over time, as compared with cpTi. These findings clearly suggest that TiO2 nanotubes exert their protective effects against inflammatory responses partly by blocking the MAPK signaling pathways.
Next, we demonstrated that the nanotubular surface inhibits the activation of NF-κB and its subsequent translocation to the nucleus. As already presented, NF-κB is sequestered in the cytosol (by the inhibitor of NF-kappa B [IkB-α] protein)45 and its activation requires the activation of the IKK complex.46 The activation of IKKβ is associated with IkB-α phosphorylation and its subsequent degradation, followed by the migration of p65 subunit to the nucleus.23,24,36,47 We have shown that the Ti/TiO2 surface significantly diminished the NF-κB activation by inhibiting the IKKβ phosphorylation and suppressing the formation of phosphorylated IkB-α, thus leading to the reduction of p65 nuclear translocation. Therefore, the inhibition of IKKβ can constitute a possible mechanism of action by which titania nanotubes inhibit the NF-κB signaling pathway. Moreover, the inhibition of this pathway has been recently linked to an increase in the hydrophilic character of the cellular substrate.48 More precisely, immunofluorescence and Western blotting studies highlighted a lower expression of nuclear NF-κB-p65 in macrophages cultured on hydrophilic Ti-H2O2 surface than on hydrophobic Ti-polished surfaces. Consequently, we cannot rule out that the inhibitory effects of Ti/TiO2 on the NF-κB signal transduction pathway can also be partly due to the hydrophilicity of this surface (Ti/TiO2 have a hydrophilic CA of 22.6°).33 Furthermore, several reports indicated the involvement of MAPKs in the regulation of NF-κB activation through phosphorylation of IkB-α.7,16,49–51 Thus, TiO2 nanotubes might partially contribute to the suppression of NF-κB activation by reducing the amounts of phosphorylated MAPKs.
In addition, many independent studies have shown that the MAPK pathways are involved in the upregulation of LPS-induced pro-inflammatory mediators.7,13–15,17,26,51–56 To establish the level of implication of these signaling pathways in reducing the macrophage activation by nanotubular structure, the effect of selective MAPK inhibitors on the secretion of TNF-α, MCP-1, and NO was investigated. Upon treatment of RAW 264.7 cells with LPS and the ERK1/2 and p38 inhibitors, the production of all three analyzed proinflammatory mediators was significantly reduced, while the JNK inhibitor (SP600125) significantly suppressed only the TNF-α production. These results indicate that titania nanotubes exert protective effects against inflammation, through their downregulating effects on the production of MCP-1, NO, and TNF-α, by regulating ERK/p38 and ERK/p38/JNK pathways, respectively. Furthermore, the immuno-suppressive effects of TiO2 nanotubes might be associated with the inactivation of NF-κB signaling pathway as a result of the decrease in IKKβ and IkB-α phosphorylation and subsequent inhibition of the NF-κB-p65 nuclear translocation (Figure 6). Moreover, some studies indicate that the NF-κB transcription factor plays an important part in the regulation of genes encoding the pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors, and inflammation-associated enzymes.9,57–60
The present molecular modeling study suggests that the suppressive effects of Ti/TiO2 on RAW 264.7 activation depend on two signaling pathways that are closely involved in the inflammatory processes (Figure 6). Specifically, it was demonstrated that such nanotubular surfaces lead to the attenuation of macrophage inflammatory responses through the simultaneous inactivation of the p38/ERK/JNK MAPK and NF-κB pathways.
Conclusion
To elucidate the possible mechanism(s) underlying the surface-dependent mitigation of the macrophage inflammatory response by TiO2 nanotubes, the level of activation of MAPK and NF-κB signaling pathways by these modified surfaces was investigated, as compared with flat cpTi surface. It was observed that TiO2 nanotubes suppressed the LPS-induced phosphorylation of MAPKs (ERK1/2, p38, and JNK), IKKβ, and IkB-α, and inhibited the nuclear translocation of NF-κB-p65 as well. Together with the reduced level of inflammatory mediators secreted into the culture media (such as MCP-1, TNF-α, and NO) in the presence of selective MAPK inhibitors, we can conclude that the attenuation of the macrophage inflammatory response by TiO2 nanotubes may depend primarily on the suppression of MAPK (ERK1/2, p38, JNK) phosphorylation and NF-κB activation. Consequently, TiO2 nanotube-modified surfaces are potential candidates for biomaterials with protective effects against inflammatory responses.
Acknowledgment
This work was supported by the Romanian Ministry of National Education, CNCS-UEFISCDI through project PNII-ID-PCE 188/2011.
Disclosure
The authors report no conflicts of interest in this work.
References
Bota PC, Collie AM, Puolakkainen P, et al. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A. 2010;95(2):649–657. | ||
Chen S, Jones JA, Xu Y, Low H-Y, Anderson JM, Leong KW. Characterization of topographical effects on macrophage behavior in a foreign body response model. Biomaterials. 2010;31(13):3479–3491. | ||
Ghrebi S, Hamilton DW, Douglas Waterfield J, Brunette DM. The effect of surface topography on cell shape and early ERK1/2 signaling in macrophages; linkage with FAK and Src. J Biomed Mater Res A. 2013;101(7):2118–2128. | ||
Lawrence T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Prospect Biol. 2009;1(6):a001651. | ||
Yang Y, Kim SC, Yu T, et al. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:352–371. | ||
Soromou LW, Zhang Z, Li R, et al. Regulation of inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 murine macrophage by 7-O-Methyl-naringenin. Molecules. 2012;17(3):3574–3585. | ||
Chan ED, Riches DW. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 (mapk) in a mouse macrophage cell line. Am J Physiol Cell Physiol. 2001;280(3):C441–C450. | ||
Hommes DW. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut. 2003;52(1): 144–151. | ||
Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther. 2001;296(1):181–187. | ||
Raingeaud J, Gupta S, Rogers JS, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270(13):7420–7426. | ||
Lee JC, Young PR. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59(2):152–157. | ||
Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18(5):1633–1641. | ||
Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-γ. J Infect Dis. 1999;179(4):939–944. | ||
Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103(7):1071–1083. | ||
Uto T, Fujii M, Hou DX. 6-(Methylsulfinyl)hexylisothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem Pharmacol. 2005;70(8):1211–1221. | ||
Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression: the role of TATA-binding protein (TBP). J Biol Chem. 1999;274(43):30858–30863. | ||
Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta. 2005;1754(1–2):253–262. | ||
Jang KJ, Kim HK, Han MH, et al. Anti-inflammatory effects of saponins derived from the roots of Platycodon grandiflorus inlipopolysaccharide-stimulated BV2 microglial cells. Int J Mol Med. 2013;31(6):1357–1366. | ||
Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. | ||
Bergqvist S, Ghosh G, Komives EA. The IkBa/NF-κB complex has two hot spots, one at either end of the interface. Protein Sci. 2008;17(12):2051–2058. | ||
Rajapakse N, Kim MM, Mendis E, Kim SK. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via downregulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology. 2008;123(3):348–357. | ||
Lee KH, Chow YL, Sharmili V. BDMC33, a curcumin derivative suppresses inflammatory responses in macrophage-like cellular system: role of inhibition in NF-κB and MAPK signaling pathways. Int J Mol Sci. 2012;13(3):2985–3008. | ||
Baldwin AS Jr. The NF-κB and IKB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. | ||
Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. | ||
Abarikwu SO. Kolaviron, a natural flavonoid from the seeds of Garcinia kola, reduces LPS-induced inflammation in macrophages by combined inhibition of IL-6 secretion, and inflammatory transcription factors, ERK1/2, NF-κB, p38, Akt, p-c-JUN and JNK. Biochim Biophys Acta. 2014;1840(7):2373–2381. | ||
Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipopolysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999:97(1):124–129. | ||
Kim YH, Lee SH, Lee JY, Choi SW, Park JW, Kwon TK. Triptolide inhibits murine-inducible nitric oxide synthase expression by down-regulating lipopolysaccharide-induced activity of nuclear factor-kappa B and c-Jun NH2-terminal kinase. Eur J Pharmacol. 2004;494(1):1–9. | ||
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. | ||
Ainslie KM, Tao SL, Popat KC, et al. In vitro inflammatory response of nanostructured titania, silicon oxide, and polycaprolactone. J Biomed Mater Res. 2009;91(3):647–655. | ||
Chamberlain ML, Brammer SK, Johnston WG, Chien S, Jin S. Macrophage inflammatory response to TiO2 nanotube surfaces. J Biomater Nanobiotechnol. 2011;2(3):293–300. | ||
Rajyalakshmi A, Ercan B, Balasubramanian K, Webster JT. Reduced adhesion of macrophages on anodized titanium with select nanotube surface features. Int J Nanomed. 2011;6:1765–1771. | ||
Smith BS, Capellato P, Kelley S, Gonzalez-Juarrero M, Popat KC. Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomater Sci. 2013;1:322–332. | ||
Neacsu P, Mazare A, Cimpean A, et al. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int J Biochem Cell Biol. 2014;55:187–195. | ||
Ion R, Stoian AB, Dumitriu C, et al. Nanochannels formed on TiZr alloy improve biological response. Acta Biomater. 2015;24:370–377. | ||
Lee K, Mazare A, Schmuki P, One dimensional titanum dioxide nanomaterials: nanotubes. Chem Rev. 2014;114(19):9385–9454. | ||
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. | ||
Wang N, Li H, Lü W, et al. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of impants in minipigs. Biomaterials. 2011;32:6900–6911. | ||
Waterfield JD, Ali TA, Nahid F, Kusano K, Brunette DM. The effect of surface topography on early NFkB signaling in macrophages. J Mater Res A. 2010;95A(3):837–847. | ||
Weinstein SL, Sanghera JS, Lemke K, DeFranco AL, Pelech SL. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267(21):14955–14962. | ||
Feng GJ, Goodridge HS, Harnett MM, et al. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol. 1999;163(12):6403–6412. | ||
Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br J Clin Pharmacol. 2002;136(7):1058–1064. | ||
Sung MJ, Davaatseren M, Kim W, et al. Vitisin A suppresses LPS-induced NO production by inhibiting ERK, p38, and NF-kappaB activation in RAW 264.7 cells. Int Immunopharmacol. 2009;9(3):319–323. | ||
Budai MM, Varga A, Milesz S, Tozsér J, Benko S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol Immunol. 2013;56(4):471–479. | ||
Kim KN, Ko YJ, Yang HM, et al. Anti-inflammatory effect of essential oil and its constituents from fingered citron (Citrus medica L. var. sarcodactylis) through blocking JNK, ERK and NF-κB signaling pathways in LPS-activated RAW 264.7 cells. Food Chem Toxicol. 2013;57:126–131. | ||
Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkB kinase. Cell. 1997;90(2):373–383. | ||
Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012;4(3):a006049. | ||
Achoui M, Appleton D, Abdulla MA, Awang K, Mohd MA, Mustafa MR. In vitro and in vivo antiinflammatory activity of 17-OAcetylacuminolide through the inhibition of cytokines, NF-κB translocation and IKKβ activity. PLoS One. 2010;5(12):e15105. | ||
Dai X, Wei Y, Zhang X, et al. Attenuating immune response of macrophage by enhancing hydrophilicity of Ti surface. J Nanomater. 2015;2015:ID712810. | ||
Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134(5):1055–1065. | ||
Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. 2006;542(1–3):1–7. | ||
Kim DH, Lee JH, Park S, Oh, et al. 6-Acetonyl-5,6-dihydrosanguinarine (ADS) from Chelidonium majus L. triggers proinflammatory cytokine production via ROS–JNK/ERK–NF-κB signalling pathway. Food Chem Toxicol. 2013;58:273–279. | ||
Chan ED, Riches DW. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochem Biophys Res Commun. 1998;253(3):790–796. | ||
Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxin-induced nitric oxide production and differentially modulates cytokine expression. Pharmacol Res. 2004;49(5):433–439. | ||
Tsao LT, Tsai PS, Lin RH, Huang LJ, Kuo SC, Wang JP. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by phenolic (3E)-4-(2-hydroxyphenyl)but-3-en-2-one in RAW 264.7 macrophages. Biochem Pharmacol. 2005;70(4):618–626. | ||
Zhou HY, Shin EM, Guo LY, et al. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-kappaB, JNK and p38 MAPK inactivation. Eur J Pharmacol. 2008;586(1–3):340–349. | ||
Yoon WJ, Heo SJ, Han SC, et al. Anti-inflammatory effect of sargachromanol G isolated from Sargassum siliquastrum in RAW 264.7 cells. Arch Pharm Res. 2012;35(8):1421–1430. | ||
Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. | ||
Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]N activity. Annu Rev Immunol. 2000;18:621–663. | ||
Choi HJ, Eun JS, Park YR, et al. Ikarisoside A inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated RAW 264.7 cells via p38 kinase and nuclear factor-kappaB signaling pathways. Eur J Pharmacol. 2008;601(1–3):171–178. | ||
Yuan F, Chen J, Sun P, Guan S, Xu J. Wedelolactone inhibits LPS-induced pro-inflammation via NF-kappaB pathway in RAW 264.7 cells. J Biomed Sci. 2013;20:84. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





 ) indicate the inhibition by Ti/TiO2 surfaces. The oval dashed line illustrates the degradation of the phosphorylated IkB-alpha. Yellow “P” symbol denotes the phosphorylated biomolecules.
) indicate the inhibition by Ti/TiO2 surfaces. The oval dashed line illustrates the degradation of the phosphorylated IkB-alpha. Yellow “P” symbol denotes the phosphorylated biomolecules.