Back to Journals » International Medical Case Reports Journal » Volume 12
Atrial myxoma presenting as a non-ST-segment elevation myocardial infarction
Authors Lalla S, Kawall J , Seecheran R, Ramadhin D , Seecheran V , Persad S , Seecheran NA
Received 3 March 2019
Accepted for publication 21 May 2019
Published 19 June 2019 Volume 2019:12 Pages 179—183
DOI https://doi.org/10.2147/IMCRJ.S207448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Sasha Lalla,1 Jessica Kawall,2 Rajeev Seecheran,2 Divya Ramadhin,1 Valmiki Seecheran,3 Sangeeta Persad,3 Naveen Anand Seecheran2
1Cardiology Unit, Advanced Cardiovascular Institute, Port of Spain, Trinidad and Tobago; 2Department of Clinical Medical Sciences, University of the West Indies, St. Augustine, Trinidad and Tobago; 3Department of Medicine, North Central Regional Health Authority, Mt. Hope, Trinidad and Tobago
Abstract: Cardiac myxomas are the most common benign tumors of the heart. We describe the rare phenomenon of myxomatous embolization, resulting in a non-ST-elevation myocardial infarction treated successfully with surgical excision. The routine early use of both transthoracic and transesophageal echocardiography is pivotal in selecting an optimal management strategy for these patients.
Keywords: atrial, myxoma, embolization, myocardial infarction
Introduction
Myxomas are the most common intra-cardiac tumors, representing 50% of primary cardiac tumors. They are commonly found in the left atrium (85%), right atrium (10%) and ventricles (5%).1,2 Most originate from the inter-atrial septum in close association with the fossa ovalis.3,4
They have a female preponderance, usually presenting in the fourth to seventh decades with most cases being sporadic. In the case of familial myxomas, patients often display genetic abnormalities with two or more of the following phenotypic presentations; cardiac and skin myxomas, cutaneous lentiginosis, endocrine hyperplasia, testicular tumors, and myxoid breast fibroadenomas. These comprise the “Carney Complex” or “myxoma syndrome,” a rare autosomal dominant condition.5
Symptoms are generally variable, relating to the tumor position, size, and mobility. The classic triad of 1) intracardiac obstruction, 2) systemic embolization, and 3) constitutional signs encompass a spectrum of presentations.6 Obstructive symptoms in (55–95%) patients present with progressive cardiac failure (dyspnea, orthopnea or paroxysmal nocturnal dyspnea) or more dramatically with syncope and sudden death from myxomatous obstruction of the mitral valve or coronary artery embolization.7 We describe the rare phenomenon of myxomatous embolization, resulting in a non-ST-elevation myocardial infarction treated successfully with surgical excision.
Case report
A 62-year-old female with a medical history of controlled hypertension on an angiotensin-converting enzyme inhibitor presented to the cardiovascular center with abrupt, typical angina associated with pre-syncope and palpitations. Her vital signs and physical examination were unremarkable. A 12-lead electrocardiogram revealed sinus tachycardia with a right bundle branch block. Pertinent diagnostic laboratory investigations included an elevated erythrocyte sedimentation rate 48 mm/hr (normal 0–30 mm/hr), d-dimer 2757 ng/dL (normal≤500 ng/mL), pro-brain natriuretic peptide 858 pg/mL (normal≤300 pg/mL), cardiac biomarkers, CK-MB 25U/L (normal 0–20 U/L), troponin I 1.36 ng/mL (normal 0.0–0.15 ng/dL). The patient’s complete blood count, comprehensive metabolic panel, fasting lipid panel and HbA1c were within normal limits, and she was subsequently initiated on non-ST-segment myocardial infarction (NSTEMI) pharmacotherapies including aspirin, clopidogrel, enoxaparin, and high-intensity rosuvastatin.
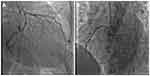
Initial transthoracic and subsequent transesophageal echocardiography demonstrated a large 42 mmx40 mm mass arising from the left atrium and adherent to the inter-atrial septum (see Figure 1A and B). There was preserved left ventricular systolic function with an estimated ejection fraction of 65% and no regional wall motion abnormalities. The left atrial dimensions were normal, however, there was prolapse of the myxoma, obstructing both the mitral valve orifice during atrial systole and the left ventricular outflow tract (see Figure 1C and D). Diagnostic coronary angiography demonstrated mild, non-obstructive coronary artery disease with thrombolysis in myocardial infarction (TIMI) 3 antegrade flow (see Figure 2A and B). Urgent open-heart surgery was performed the following day, and gross pathology of the specimen excised revealed a large polypoidal mass. The mass suspected to be a myxoma was excised by resecting the pedicle implanted on the interatrial septum (Figure 3). Histopathological examination of the specimen confirmed the diagnosis of myxoma with papillary fragments of tumor composed of fibro-myxoid hyalinized cores with elongated stellate shaped myxoma cells with abundant eosinophilic cytoplasm. The patient’s postoperative course was uneventful, and she was safely discharged on optimal medical therapy with routine follow-up appointments.
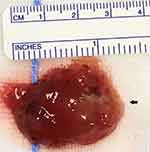 | Figure 3 Resected gross specimen. Histopathology examination of the specimen confirmed the diagnosis of myxoma. |
Discussion
Our patient did not display any of the aforementioned familial myxoma-associated phenotypes such as Carney complex. There was a possible element of intracardiac obstruction resulting in mild heart failure symptomatology and angina from suspected embolization without overt constitutional symptoms. She did not report trepopnea preceding her admission, nor was there any evident “tumor plop” auscultated during the physical examination.8,9 A case series of 112 myxoma patients describes 65% of patients as having cardiac auscultatory abnormalities with only 15% displaying the characteristic “tumor plop.” Left atrial hypertrophy on electrocardiogram accounted for the most frequent electrocardiographic abnormality in 35% of patients, whereas in our patient a sinus tachycardia with a right bundle branch block was present.6
She atypically presented with an NSTEMI and mild clinical heart failure. The development of acute heart failure, due to inadequate coaptation of the mitral valves with obstruction of the left ventricular outflow tract in diastole depends on the myxoma’s size and position. Some authors have hypothesized the release of interleukin-6 and -8 by the atrial myxoma may provoke myocardial tissue inflammation and global left ventricular dysfunction.10,11 These cytokine mechanistic effects promote the adhesion of neutrophils; and with the attachment of myxoma cells to the coronary endothelium, may be responsible for triggering myocardial ischemia and infarction at the cellular level.12,13 Distal embolization of tumor fragments in the coronary vasculature may also be implicated in the pathogenesis of myxoma-associated myocardial infarction. With respect to the patient, there were no visible tumor fragments, nor “slow-flow” observed during coronary angiography, which suggests or alludes to the suspected embolization being a transient phenomenon.
Systemic embolization (10–45%) has a predilection for the central nervous system with resultant cerebrovascular events; however, there are many reports of embolization to multiple arterial beds, with hepatic and renal involvement. Catastrophic total occlusion of the abdominal aorta has also been reported.14,15 Non-specific constitutional signs such as fatigue, fevers, weight loss, myalgias, and arthralgias can often resemble autoimmune disease due to cytokine secretion by the tumor.12
Histopathological examination of the specimen confirmed the diagnosis of myxoma with papillary fragments of tumor composed of fibro-myxoid hyalinized cores with elongated stellate shaped myxoma cells with abundant eosinophilic cytoplasm.16
Echocardiography is the diagnostic method of choice in securing an early diagnosis in these patients, with the transthoracic and transesophageal modalities providing 95 and 100% sensitivities respectively. Both methods are non-invasive, reliable and reproducible with coronary angiography being reserved for patients over age forty to further evaluate coexistent coronary artery disease preoperatively, or to evaluate for ischemia in myxomatous acute coronary syndromes.2
Coronary angiography has played a role in demonstrating neovascularization of these vascular tumors with literature describing an associated “tumor blush.”17,18 Our patient, however did not display this angiographic image, which is typically ascribed to late or delayed contrast opacification of the vascularized tumor. The incidence of myocardial infarction due to coronary embolization from atrial myxoma is only 0.06%.19 This low incidence of embolization may be explained by the angulation and juxtaposition of the coronary ostia in relation to aortic blood flow with the protection of the ostia by aortic valve cusps, which open during systole.20 Myxomatous embolization is often accompanied by cardiac biomarker elevations making it an uncommon differential in the diagnosis of myocardial infarction. Panos et al described angiographic findings with the majority affecting the right coronary artery (~50%), although embolization to left anterior descending (~20%) and circumflex (~10%) arteries have been reported.20 El Zaharani et al reported normal coronary arteries (~55%) in a series of 17 patients from 2003 to 2014 suggesting spontaneous recanalization after myxomatous embolization may account for the findings of normal coronary arteries, which was clinically consistent in the patient’s scenario.21–27
Surgical excision offers a successful outcome with low operative mortality. The recurrence rate post-surgical excision is relatively low at 5%, but long-term follow-up with echocardiographic imaging is recommended.28,29 Familial cases or Carney Complex carries a recurrence rate of 10–25% versus 1–5% for sporadic cases.28
Transthoracic echocardiography prior to angiography proved to be instrumental in this clinical scenario, as it revealed an atrial myxoma as the etiology of the myocardial infarction which ultimately required definitive surgical management. It is possible that a non-invasive, pharmacotherapy approach would have been instituted or even percutaneous coronary intervention of a suspected false “culprit” lesion, for example, stenting an intermediate or borderline stenosis (if present) without initially identifying the underlying diagnosis of atrial myxoma.
Conclusion
We describe a non-ST-segment elevation myocardial infarction resulting from myxomatous embolization into the coronary arteries in a female with successful surgical resection via open heart surgery. The importance of early transthoracic echocardiography before coronary angiography in clinching the diagnosis cannot be understated.
Compliance with ethics guidelines and standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethics statement
The patient has provided written, informed consent to have the details of her case published. Institutional approval was not required for publication.
Data Sharing Statement
All available data can be obtained by contacting the corresponding author.
Key Clinical Message
The clinician should be cognizant that a non-ST-elevation myocardial infarction can be precipitated by embolic phenomenon of an atrial myxoma, and echocardiographic imaging before coronary angiography is integral in establishing the diagnosis.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ladich E, Virmani R. Tumors of the cardiovascular system. Cardiovasc Pathol. 2016;735–772. doi:10.1016/b978-0-12-420219-1.00019-7
2. Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. Elsevier; 11 2018.
3. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38:261–262.
4. Swartz MF, Lutz CJ, Chandan VS, Landas S, Fink GW. Atrial myxomas: pathologic types, tumor location, and presenting symptoms. J Card Surg. 2006;21:435–440. doi:10.1111/j.1540-8191.2006.00265.x
5. Casey M, Mah C, Merliss AD, et al. Identification of a novel genetic locus for familial cardiac myxomas and carney complex. Circulation. 1998;98:2560–2566. doi:10.1161/01.CIR.98.23.2560
6. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. Medicine. 2001;80:159–172. doi:10.1097/00005792-200105000-00002
7. Arcenas RF, Ali MI. Left atrial myxoma: a rare nonatherosclerotic cause of acute myocardial infarction. Case Rep Cardiol. 2013;2013:1–4. doi:10.1155/2013/407935
8. Gul M, Sahan E, Sen F, Avci S, Tufekcioglu O. Trepopnea in a patient with right ventricular myxoma. Herz. 2013;39:880–881. doi:10.1007/s00059-013-3916-x
9. Kolluru A, Desai D, Cohen GI. The etiology of atrial myxoma tumor plop. J Am Coll Cardiol. 2011;57:e371. doi:10.1016/j.jacc.2010.09.085
10. Chiariello GA, Bruno P, Colizzi C, et al. Acute heart failure related to a large left atrial myxoma. Baylor Univ Med Center Proc. 2018;31:331–333. doi:10.1080/08998280.2018.1446641
11. García-Bello LB, Bedoya DM, Figueredo SJ, Centurión OA. Refractory heart failure despite optimized medical treatment which subsides only after surgical resection of a large left atrial myxoma. J Cardiol Curr Res. 2017. 9(6). DOI: 10.15406/jccr.2017.09.00344.
12. Kanda T, Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J. 2004;45:183–193. doi:10.1536/jhj.45.183
13. Umeyama S. Clinical and immunohistochemical findings in cardiac myxoma with special reference to the expression of interleukin-6. KITAKANTO Med J. 1994;44:475–484. doi:10.2974/kmj1951.44.475
14. Huang C-Y, Chang -Y-Y, Hsieh M-Y, Hsu C-P. Atrial myxoma presenting as total occlusion of the abdominal aorta and its major four branches. J Chin Med Assoc. 2012;75:349–352. doi:10.1016/j.jcma.2012.05.007
15. Shavit L, Appelbaum L, Grenader T. Atrial myxoma presenting with total occlusion of the abdominal aorta and multiple peripheral embolism. Eur J Intern Med. 2007;18:74–75. doi:10.1016/j.ejim.2006.12.009
16. Wang, J.-G., Li YJ, Liu H, et al. Clinicopathologic analysis of cardiac myxomas: seven years’ experience with 61 patients. J Thorac Dis. 2012;4:272–283.
17. van Cleemput J, Daenen W, de Geest H. Coronary angiography in cardiac myxomas: findings in 19 consecutive cases and review of the literature. Cathet Cardiovasc Diagn. 1993;29:217–220. doi:10.1002/(ISSN)1097-0304
18. Kim YK, Yong HS, Kang E-Y, Woo OH. Left atrial myxoma with neovascularization: detected on cardiac computed tomography angiography. Int J Cardiovasc Imaging. 2009;25(Suppl 1):95–98. doi:10.1007/s10554-008-9416-z
19. Lehrman KL, Prozan GB, Ullyot D. Atrial myxoma presenting as acute myocardial infarction. Am Heart J. 1985;110:1293–1295. doi:10.1016/0002-8703(85)90027-4
20. Panos A, Kalangos A, Sztajzel J. Left atrial myxoma presenting with myocardial infarction. Case report and review of the literature. Int J Cardiol. 1997;62:73–75. doi:10.1016/S0167-5273(97)00178-2
21. Al Zahrani IM, Alraqtan A, Rezk A, Almasswary A, Bella A. Atrial myxoma related myocardial infarction: case report and review of the literature. J Saudi Heart Assoc. 2014;26:166–169. doi:10.1016/j.jsha.2014.03.001
22. Patanè S, Marte F, Di Bella G. Revelation of left atrial myxoma during acute myocardial infarction. Int J Cardiol. 2008;128:134–136. doi:10.1016/j.ijcard.2007.06.033
23. Chaudhuri AA, Simmons CJ, Ellison D, Hemp J, Chung K. Atrial myxoma presenting as myocardial infarction diagnosed by echocardiography, managed endoscopically with robot-assisted surgery. Cureus. 2016. doi:10.7759/cureus.484
24. Ozaydin M, Dogan A, Altinbas A. Left atrial myxoma presenting with acute myocardial infarction. Angiology. 2005;56:767–769. doi:10.1177/000331970505600615
25. Ito S, Endo A, Okada T, et al. Acute myocardial infarction due to left atrial myxoma. Intern Med. 2016;55:49–54. doi:10.2169/internalmedicine.55.5179
26. Cannarile, P, Cresti A, Stefanelli S, et al. [Myocardial infarction as the first manifestation of an atrial myxoma: the bomb in the heart. Case report and literature review]. G Ital Cardiol. 2016;17:1012–1016.
27. Abascal VM, Kasznica J, Aldea G, Davidoff R. Left atrial myxoma and acute myocardial infarction. Chest. 1996;109:1106–1108. doi:10.1378/chest.109.4.1106
28. Reynen K, Daniel WG. Cardiac myxoma. Circulation. 1996;94:1137–1137. doi:10.1161/01.CIR.94.5.1137
29. Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610–1617. doi:10.1056/NEJM199512143332407
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.