Back to Journals » OncoTargets and Therapy » Volume 12
Atractylodes macrocephala polysaccharides regulate the innate immunity of colorectal cancer cells by modulating the TLR4 signaling pathway
Authors Feng Z, Yang R, Wu L, Tang S, Wei B, Guo L, He L, Feng Y
Received 15 June 2019
Accepted for publication 1 August 2019
Published 4 September 2019 Volume 2019:12 Pages 7111—7121
DOI https://doi.org/10.2147/OTT.S219623
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Zifang Feng*,1, Ruibin Yang*,1, Liusong Wu2, Shihua Tang1, Bin Wei1, Lijia Guo1, Ling He1, Yonghuai Feng3
1Department of Laboratory, Xingyi City People’s Hospital, Xingyi, People’s Republic of China; 2Department of Pediatrics, Affiliated Hospital of Zunyi Medical University, Zunyi, People’s Republic of China; 3Department of Haematology, Affiliated Hospital of Zunyi Medical University, Zunyi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghuai Feng
Department of Haematology, Affiliated Hospital of Zunyi Medical University, No. 149, Dalian Road, Huichuan District, Zunyi City, Guizhou Province 563003, People’s Republic of China
Tel +86 8 512 865 1472
Email [email protected]
Background: It has been well-recognized that the polysaccharides from Atractylodes macrocephala (PAM) are immune system enhancers, which can facilitate the proliferation of lymphocytes and stimulate immune cells. Nevertheless, the antitumor effects of PAM and their molecular mechanisms remain unclear.
Aim: Our research aimed to evaluate the anti-cancer effects of PAM on colorectal cancer (CRC).
Methods: We tested the effects of PAM on the growth and proliferation of CRC cells and macrophages by MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. The pro-inflammatory cytokines expression and secretion was analyzed by real-time RT-PCR and ELISA assay. We also used MC38 cells xenograft model to test the anti-cancer effects of PAM in vivo.
Results: We found that although PAM treatment did not significantly affect the growth of CRC cells or enhance the proliferation of bone marrow-derived macrophages (BMDMs), it could enhance the phagocytosis of BMDMs by CRC cells. Biochemical tests and immunoblotting assays revealed that exposing BMDMs to PAM promoted the production of interleukin-6 (IL-6), interferon λ (IFN λ), tumor necrosis factor α (TNF-α), and nitric oxide (NO) through the MyD88/TLR4-dependent signaling pathway. One noteworthy observation is that PAM treatment could significantly prevent tumorigenesis of MC38 cells in C57BL/6J mice and increase the survival duration of mice with tumors, without influence on the weight of those mice. However, the anti-cancer effects of PAM were compromised in TLR4 KO mice, further suggesting that TLR4 signaling plays a vital role in the anti-cancer effects of PAM.
Conclusion: Therefore, PAM may prove to be a potential candidate in cancer immunotherapy.
Keywords: polysaccharides from Atractylodes macrocephala, colorectal cancer, macrophages, TLR4, cancer immunotherapy
Introduction
Classical cancer therapies include radiation, surgery, and cytotoxic chemotherapy, and aim to eradicate cancer cells thoroughly. However, the growing pattern of malignant cells is aggregation but not isolation. Moreover, in the tumor microenvironment, stromal cells such as macrophages and T cells must also be considered to obtain effective treatment outcomes.1,2 One way of achieving such positive outcomes could be via cytotoxic CD8+ T cells (applied in checkpoint blocking treatment), which are the major effectors in the immune system. The outcomes can also be indirectly reached by specifically targeting other types of immune cells, for example, macrophages.2 Currently, immunotherapy is well-accepted as an approach in treating tumors, either alone or combined with more classical treatment choices such as chemotherapy.3 Reinforcement of the immune system plays an important part in cancer treatment reacting to drugs against tumors. Recently, proteoglycans and polysaccharides extracted from natural sources have been recognized for their prevention of tumor growth by activating effector cells in the immune system, which include macrophages. Such a mechanism is useful for developing anti-tumor drugs from proteoglycans and polysaccharides, which potentiate the immune system.
Polysaccharides from Atractylodes macrocephala (PAM) are major bioactive components which are considered to modulate immune system function through anti-stress and antioxidant mechanisms and to promote immune reactions.4,5 Furthermore, PAM can significantly enhance the proliferation of T-lymphocytes in peripheral blood (in mice, chickens, and humans) and T-lymphocytes in the spleen (in chickens and mice), inducing T-lymphocytes to transform to the G2/M and S phases, and efficiently elevating the ratio of CD8+ and CD4+ T cells.4,6 In addition, PAM can remarkably elevate the generation of IFN-γ, TNF-α, and IL-6 in the serum of mice, and the generation of IFN-γ, IL-2, IL-4, IL-6, and TNF-α in the serum of chickens.6,7 It has been demonstrated that the administration of PAM to mice immunized with hen egg-white lysozyme (HEL) can remarkably elevate the production of serum IgG and induce an antigen-specific humoral reaction.6 It has also been shown that PAM can regulate macrophage activities by initiating the degradation of the inhibitor kappa B (IκB) and activating NF-κB through the process of p65 nuclear translocation.8 Moreover, atractylenolide I (AO-I) is another principal bioactive constituent of Atractylodes macrocephala, which was shown to protect mice from acute pulmonary injury caused by lipopolysaccharide (LPS) though the inhibited expression of Toll-like receptor 4 (TLR4) and activation of NF-κB.9 However, there are no reports regarding the anti-cancer immune responses mediated by PAM.
In this research, we systematically characterized and identified the anti-tumor and immune-modulating effects of PAM in cells and animal models. Furthermore, this research also aimed to determine the mechanism and role of the TLR4 signaling pathway in tumor suppression and immune-regulation induced by PAM.
Materials and methods
Cell culture and reagents
We purchased MC38 cells, derived from C57BL/6 murine colon adenocarcinoma, and CT26 cells, derived from BALB/c murine colon carcinoma, from JENNIO Biological Technology (Guangzhou, China). The Raw264.7 cells, the macrophage from BALB/c mouse, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). As previously described, the primary bone marrow-derived macrophages (BMDMs) were extracted and induced.10 Mycoplasma testing was performed, and all cells were authenticated. DMEM with 10% fetal bovine serum (FBS) was used for cell cultures. Cells were maintained in a humidified atmosphere at 37 °C with 5% CO2.
PAM (purity 70%) was purchased from Tianyuan (Xi’an, China). Double distilled water (500 mL) was used to dissolve PAM. TAK-242, ST2825 and NBP1-77221, the MyD88 inhibitor and TRIF inhibitor, respectively, were purchased from Novus Biological (Centennial, CO, USA). MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) was purchased from Biodee Co., Ltd (Beijing, China). Antibodies against p65 (#8242), iNOS (#13120), TLR1 (#2029), TLR2 (#12276), TLR4 (#14358), TLR6 (#12717), and p-p65 (#3033) were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-TRIF (NB-120-13810) was purchased from Novus Biological. Anti-β-actin (sc-58673), MyD88 (sc-136970), and anti-rabbit (sc-2004) or mouse (sc-2031) secondary antibodies labeled with HRP were purchased from Santa Cruz Biotechnology (Dalla, TX, USA). The primary antibodies were diluted at 1:1000, and the secondary antibodies were diluted at 1:5000.
Assay of cell viability
The cells were seeded in 96-well plates at a density of 4×103–1×104 cells/well. After one day, PAM was added to the cells at the specified concentrations for multiple time points. After MTT was added and incubated for 4 h at 37 °C, DMSO (150 μl) was added to each well and a microplate reader was used to measure the optical density at 490 nm.
Phagocytosis assay
Flow cytometry-based phagocytosis tests were performed to measure the phagocytic capabilities of macrophages. After 6–8 days of differentiation, macrophages were obtained and were stained with CellTrace™ CFSE (Thermo Fisher, Shanghai, China). The MC38 cells were stained with CellTrace™ Far red (Thermo Fisher) and mixed with macrophages, incubated at 37 °C for 2 hrs, and finally analyzed using flow cytometry (BD Accuri C6).
Macrophage trans-well assay
We used Corning Costar Transwell plates (Corning, 3422) following the manufacturer’s protocol. After the process of trypsinization and cell counting, an aliquoted number of cells were suspended in the appropriate media in the upper chamber and seeded at 25,000 cells per chamber. After incubation in a 5% or 10% CO2 environment separately at 37 °C for 24 h, the upper chambers were removed and the non-migrating cells excised using cotton swabs. Subsequently, the cells that migrated to the bottom of the upper chamber were fixed and then stained with crystal violet. The results represent three independent tests with double technical replicates in every stimulation condition.
Enzyme-linked immunosorbent assay (ELISA)
BMDMs were cultured in 6-well plates. After one day, PAM was added to the cells at the specified concentrations for 24 hrs. The supernatants were collected from cell cultures. IL-6, TNF-α, and IFN-λ ELISA kits (Boster, Wuhan, China) were used to measure the levels of IL-6, TNF-α, and IFN-λ in the supernatants following the instructions from the manufacturer. Venous blood was obtained from the tumor-bearing mice for serum cytokine extraction. The same kits were applied to test concentrations of IL-6, TNF-α, and IFN-λ.
Nitric oxide measurements (NO)
BMDMs were cultured in 96-well plates with a density of 8×103 cells per well. After one day, PAM was added to the cells at the specified concentrations for 24 hrs. By measuring the concentration of nitrite from the cell culture supernatants, the generation of NO could be evaluated. The nitrite generation was assessed by the Griess reaction using a nitric oxide assay kit (Applygen, Beijing, China) following the protocol provided by the manufacturer.
Western blot analysis
Using cold PBS, the BMDMs were washed twice, and the proteins were obtained using a lysis buffer on ice (100 mM DTT, 2% SDS, 10% glycerol, and 10 mM Tris (pH 6.8)), and the proteins were boiled for ten minutes at 98 °C. The protein levels were measured using immunoblot analysis, as previously described (52). In short, the proteins were separated using SDS-PAGE and then transferred to PVDF membranes. Next, TBST with 3–5% nonfat milk powder was used to block the membranes for one hour at room temperature, and then the membranes were incubated with the primary antibodies in a 4 °C environment overnight. For the next step, secondary antibodies labeled with HRP were incubated for two hours at room temperature, followed by chemical luminescence determination.
Real-time quantitative PCR analysis
Following BMDM treatment with PAM at the specified concentrations for 24 hrs, TRIzol was used to extract the total RNA, and the RNA was transcribed into cDNA using the PrimeScript RT Reagent Kit following the instructions from the manufacturer. The primer sequences are listed below: IL-6: 5ʹ-AAATAGTCCTTCCTACCCCAA-3ʹ and 5ʹ-CCGAGTAGATCTCAAAGTGAC-3ʹ; iNOS: 5ʹ-CGGCAAACATGACTTCAGGC-3ʹ and 5ʹ-GCACATCAAAGCGGCCATAG-3ʹ; IFNλ: 5ʹ-CGGCACAGTCATTGAAAGCCTA-3ʹ, and 5ʹ-GTTGCTGATGGCCTGATTGTC-3ʹ; TNF-α: 5ʹ-CCCCTTTATTGTCTACTCCT-3ʹ and 5ʹ-AAAGCCCATTTGAGTCCTTG-3ʹ; GAPDH: 5ʹ-GCGACTTCAACAGCAACTCC-3ʹ and 5ʹ-CACCCTGTTGCTGTAGCCGT-3ʹ.
In vivo PAM experiments
TLR4 KO C57BL/6 or wildtype (6-week-old) mice (N=6 for each group) were housed in our laboratory. The C57BL/6 mice were inoculated using MC38 colorectal cancer cells (5 × 106 cells per mouse) in their flanks. Seven days after cell injection, the mice received intraperitoneal injection of PAM (500 mg/kg) or PBS as a negative control (Control). The drug injection was administered three times a week for a total of two weeks. The tumor volume (size) was calculated as 0.523× (length × width × height) and evaluated dynamically. This research was approved by the Institutional Animal Use and Care Committee in Affiliated Hospital of Zunyi Medical University and followed the Regulations for the Management of Animal Laboratories.
Statistical analysis
The statistical analysis was conducted by using graphpad prism 5 (San Diego, CA, USA). Paired Student’s tests were used to calculate p-values. Differences were statistically significant at a p<0.05 unless indicated otherwise.
Results
PAM enhanced the phagocytosis of tumor cells by macrophages
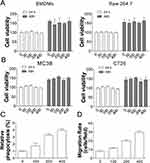
To study the anti-tumor immune effects of PAM, its effects on cell proliferation of BMDMs derived from MC38 tumor cells xenografted into C57BL/6J mice or mouse derived Raw264.7 cells were tested. As shown in Figure 1A, different concentrations of PAM produced no overt effects on BMDMs and Raw264.7 cell proliferation when compared with control cells. Similarly, PAM treatment also did not affect the proliferation of MC38 or CT26 tumor cells (Figure 1B). Next, we investigated whether PAM would induce phagocytosis of MC38 by BMDMs. The MC38 cells were co-incubated with BMDMs at a 1:1 ratio, and treated with different concentrations of PAM. As revealed by the flow cytometry results, PAM increased phagocytosis of MC38 cells by macrophages in a concentration-dependent manner (Figure 1C). To determine if these results are specific to contact with macrophages, tumor cells were co-cultured with BMDMs using trans-well plates. Our data suggested that PAM treatment induced migration of BMDMs when co-cultured with MC38 cancer cells (Figure 1D). These results collectively suggest that PAM treatment activates anti-cancer immunity of macrophages.
PAM activated the expression and secretion of immunomodulatory factors in BMDMs
We posited that the anti-cancer immune responses associated with PAM treatment were related to stimulation of factors secreted by BMDMs. For further investigation of the immunological responses of BMDMs, we measured the levels of TNF-α, IL-6, and interferon-λ in BMDM cells treated with PAM. PAM administration remarkably elevated the mRNA levels of IL-6, TNF-α, and IFN-λ in BMDMs (Figure 2A). NO (nitric oxide) is among the most multifunctional modulators of the immune system.11 We measured the effect of PAM on the production of NO by BMDM cells. The NO production by BMDMs was remarkably induced by PAM administration in a concentration-dependent manner (Figure 2B). Compared with the control group, the concentrations of NO in supernatants extracted from BMDM cells were significantly elevated to 23.93±1.18, 23.54±0.52, 23.09±0.49, and 22.63±0.45 μM by administering PAM at concentrations of 500, 200, 100, and 50 μg/mL, separately. Furthermore, our results suggested that the anti-cancer mechanism involves TPG-1-elevated protein and mRNA expression of inducible nitric oxide synthase (iNOS) from BMDMs (Figure 2C–E). Taken together, our results revealed that PAM treatment caused the activation of macrophages.
Induction of immunomodulatory factors by PAM is mediated by TLR4
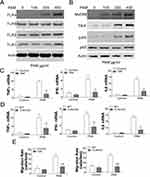
Toll-like receptors (TLRs) have an important role in the activation of macrophages and control of parasitic infections.12 Therefore, we investigated the regulation of multiple TLRs in BMDMs in response to PAM treatment, including TLR1, TLR2, TLR4, TLR6. Interestingly, only TLR4 expression was induced by PAM treatment in a concentration-dependent manner (Figure 3A), suggesting that the TLR4 pathway was activated by PAM. The TLR4 downstream effectors, such as MyD88, TRIF, and NF-κB were also activated by PAM (Figure 3B). After that, we investigated whether TLR4 pathway activation stimulated by PAM treatment of BMDMs involved increased IFN-λ, IL6, and TNF-α. The elevated expressions of IFN-λ, TNF-α, and IL6 stimulated by TPG-1 were remarkably suppressed by TAK-242 treatment of BMDMs (Figure 3C). To further investigate the role TLR4 plays in inflammatory cytokine expression, we obtained TLR4 KO BMDMs from MC38 xenografted TLR4 KO mice. By comparing the cytokine expression in WT and TLR4 KO BMDMs, we found that the induction of TNF-α, IFNλ, and IL6 induced by PAM in WT BMDMs was all abandoned in TLR4 KO BMDMs (Figure 3D). Moreover, inhibition or deletion TLR4 in BMDMs abolished its migration activity when co-cultured with MC38 cells (Figure 3E). Therefore, our data indicated that activation of the TLR4 pathway by PAM triggered macrophage maturation in response to PAM treatment.
PAM treatment led to tlr4-dependent activation of MyD88 signaling pathways in BMDM cells
Since both MyD88 and TRIF were upregulated in PAM-treated BMDMs, we further analyzed whether MyD88 and TRIF played a part in macrophage activation induced by PAM. BMDMs were previously treated with ST2825 and NBP1-77221, which are MyD88 and TRIF inhibitors, separately. Then, RT-PCR was performed to detect cytokine levels of IL-6, TNF-α, and IFN λ. As shown in Figure 4A, only the presence of a MyD88 inhibitor significantly suppressed cytokine production induced by PAM, when compared with cells without a MyD88 inhibitor or with a TRIF inhibitor (p < 0.05). To clarify the role of MyD88 in anti-tumor immunomodulatory effects of PAM, we obtained BMDMs from MyD88-KO (MyD88−/−) and WT C57BL/6J (MyD88+/+) tumor-bearing mice and administered PAM treatment. By comparing the cytokine expression in MyD88 KO and WT BMDMs, we found that the induction of TNF-α, IFNλ, and IL6 induced by PAM in WT BMDMs was also suppressed in MyD88 KO BMDMs (Figure 4B). Furthermore, depletion of MyD88 in BMDMs abolished NF-κB activation and the induction of iNOS (Figure 4C), suggesting that MyD88 acts as the downstream effector of TLR4 activation. Finally, we investigated the macrophage migration activity using the transwell assay, and found that BMDMs without MyD88 has lower migration activity when co-cultured with MC38 cells (Figure 4D). Collectively, our results indicated that PAM treatment activated the anti-cancer immunity of macrophages via the TLR4/MyD88 pathway.
In vivo anti-tumor activity of PAM
To determine whether the TLR4 signal pathway participates in the immunomodulatory and anti-tumor functions of APS in animal models, TLR4-deficient (TLR4−/−) and C57BL/J (TLR4+/+) tumor-bearing mice were used in this research. First, the anti-tumor effects of PAM in MC38 tumor-bearing C57BL/6J mice were investigated. We found that PAM administration remarkably suppressed the oncogenic effects of MC38 in vivo (Figure 5A). Consistently, PAM treatment significantly lengthened the survival time of mice with MC38 tumors (Figure 5B). The calculated body weight curve indicated that PAM treatment did not significantly affect body weights of mice (Figure 5C). In contrast, the effects of PAM in oncogenicity suppression and tumor-bearing mouse survival were absent in TLR4 KO mice (Figure 5A and B). The cytokines generated in serum were measured using ELISA. Consistent with the in vitro results, PAM induced a higher secretion of TNF-α, IFNλ, and IL6 in the serum of WT mice, but was unchanged in TLR4 KO mice (Figure 5D). Therefore, these data suggest that PAM induced anti-tumor immunity by triggering TLR4-dependent macrophage activation.
Discussion
Side-effects generate numerous challenges in radiotherapy and chemotherapy for CRC cancer treatment, which makes nontoxic herbal drugs as an alternative choice. Some herbs having anti-cancer effects have garnered increasing attention from the biomedical field owing to their multiple biological functions. In the case of Atractylodes macrocephala, its anti-tumor activity is recognized to be closely related to the presence of bioactive polysaccharides.13 This research aimed to explore if PAM, a polysaccharide derived from Atractylodes macrocephala, could suppress CRC cell growth and stimulate macrophages to kill cancer cells. These experiments suggested that although PAM did not remarkably suppress CRC cell growth, PAM-induced BMDM macrophages showed anti-tumor functions. Furthermore, PAM could increase the expression of iNOS, nitric oxide (NO), and some pro-inflammatory cytokines, including TNFα, IFNλ, and IL6, which suggested the anti-tumor immunity of PAM-treated macrophages. Further investigation demonstrated that PAM showed effective anti-cancer function because of the immune-potentiating effects through the TLR4/MyD88 signaling pathway. The in vivo anti-cancer effects of PAM were also elucidated, which will provide a therapeutic strategy for CRC cancer.
Polysaccharides in conventional Chinese medicine, eg, Schisandra chinensis (Turcz.) polysaccharides, curcuminoids, and Astragalus polysaccharides, have been shown to regulate the immune system via the TLR4 signal pathway, and finally to take part in modulating the activation of NF-κB (nuclear factor kappa B).9,14–16 PAM is one of the components in Atractylodes macrocephala. Research has shown that chronic use of Atractylodes macrocephala produces no adverse or toxic effects, and also PAM is an effective and safe bioactive component from Atractylodes macrocephala.17 Furthermore, PAM also protects the immune system and strengthens immune function.18 In this study, we first demonstrated that PAM also has anti-cancer effects in CRC via activation of macrophages to kill cancer cells. As key immune system cells, macrophages are significant for antitumor effects by generating some cytokines, for instance, NO, IL-6, and TNF-α.19 Much evidence supports that some proteoglycans and polysaccharides can strengthen immunity through the activation of macrophages. For example, SPS (Salicornia polysaccharides) can remarkably stimulate the production of NO and the transcription of iNOS within RAW264.7 cells.20 The proteoglycan extracted from Poria cocos activated RAW 264.7 cells by upregulating TNF-α.21 Moreover, the immunomodulatory function shares one of the potential mechanisms of the anti-cancer activity of polysaccharides from Atractylodes macrocephala.22 This study demonstrated that the proteoglycan PAM extracted from Atractylodes macrocephala largely increased the generation of IL-6, NO, and TNF-α in BMDM cells in the mouse. Furthermore, PAM treatment significantly increased the iNOS levels in BMDMs. Therefore, PAM is a potent immunostimulant for suppressing CRC tumor growth.
Subsequently, we explored the underlying mechanism of PAM and found that PAM activated the anti-cancer immunity of macrophages via the TLR4/MyD88 pathway. Toll-like receptors (TLRs) are one of the recognition molecules with evolutionarily conserved patterns.23 Previously, it was elucidated that TLR4 can recognize pathogen-associated molecular patterns (PAMPs), for example, endogenous damage-associated molecular patterns (DAMPs) such as hyaluronan and fibronectin, and gram-negative bacterial lipopolysaccharide (LPS), which are generated in inflammatory reactions including non-infectious and infectious conditions. There are some chronic inflammatory and infectious conditions considered to facilitate the formation of carcinoma. For example, viral hepatitis or Helicobacter pylori infections can lead to liver or gastric cancers, respectively.24,25 While antagonists for TLR4 could weaken the development of metastasis or carcinogenesis induced by inflammation, TLR4 agonists have been demonstrated to stimulate anti-tumor immune responses in models and patients with established tumors.26,27 A combination of LPS and E6020 with trastuzumab, paclitaxel, and a whole cell tumor cell vector improved the anti-cancer immune response in mice.28,29 Furthermore, it has been shown that TLR4 is of vital importance for the activation of macrophages following exposure to proteoglycans or polysaccharides extracted from Poria cocos and Platycodon grandiflorum.21,30 In this study, we found that TLR4 was necessary for anti-cancer macrophage activation induced by PAM. Moreover, our study demonstrated that PAM-induced elevation of IL-6, NO, and TNF-α are regulated by TLR4 via a pharmacological mechanism. Therefore, we conclude that PAM has promising immune-potentiating effects via the up-regulation of the TLR4 signal pathway.
There are two branches in TLR4 signal pathways: the TRIF-dependent signaling pathways and the MyD88-dependent pathway.31 In the cytoplasm, MyD88 contains a death domain, which is combined with the structural domain of TLR linked by MyD88 adaptor-like (MAL) protein. MAL protein is a fundamental adapter protein combined with MyD88 and can activate TRAF-6, which is its downstream molecule. TRAM shares the same role as MAL, which can bind with TLR and TRIF and specifically plays a part in the signaling pathway independent from MyD88.31 Considering these above, we measured the protein expression of TRIF, TLR4, and MyD88 in BMDMs. Compared with the control group in vitro, these results suggested that the levels of key proteins were dramatically upregulated by PAM. However, only inhibition of MyD88, rather than TRIF inhibition, suppressed inflammatory cytokine expression in BMDMs treated with PAM, suggesting that MyD88 mediates the anti-tumor immunity associated with PAM treatment.
In summary, our findings suggest that PAM can stimulate macrophages to produce an anti-cancer immune response by activating the TLR4-MyD88-dependent signaling pathway.
Conclusion
Thus, the TLR4-regulated MyD88-dependent signaling pathway may be one of the signal pathways induced by PAM underlying the anti-tumor and immunomodulatory functions of PAM both in cells and in animal models.
Ethics approval and informed consent
This research was approved by the Institutional Animal Use and Care Committee in Affiliated Hospital of Zunyi Medical University and followed the Regulations for the Management of Animal Laboratories.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest in this work.
References
1. Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(7596):651–655. doi:10.1038/nature17412
2. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi:10.1126/science.aaa8172
3. Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi:10.1146/annurev.immunol.021908.132544
4. Sun W, Meng K, Qi C, et al. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr Polym. 2015;126:91–96. doi:10.1016/j.carbpol.2015.03.034
5. Xu D, Li B, Cao N, Li W, Tian Y, Huang Y. The protective effects of polysaccharide of Atractylodes macrocephala Koidz (PAMK) on the chicken spleen under heat stress via antagonizing apoptosis and restoring the immune function. Oncotarget. 2017;8(41):70394–70405. doi:10.18632/oncotarget.19709
6. Son YO, Kook SH, Lee JC. Glycoproteins and polysaccharides are the main class of active constituents required for lymphocyte stimulation and antigen-specific immune response induction by traditional medicinal herbal plants. J Med Food. 2017;20(10):1011–1021. doi:10.1089/jmf.2017.3943
7. Liu J, Chen X, Yue C, et al. Effect of selenylation modification on immune-enhancing activity of Atractylodes macrocephala polysaccharide. Int J Biol Macromol. 2015;72:1435–1440. doi:10.1016/j.ijbiomac.2014.10.022
8. Ji GQ, Chen RQ, Zheng JX. Macrophage activation by polysaccharides from Atractylodes macrocephala Koidz through the nuclear factor-kappaB pathway. Pharm Biol. 2015;53(4):512–517. doi:10.3109/13880209.2014.929152
9. Zhang JL, Huang WM, Zeng QY. Atractylenolide I protects mice from lipopolysaccharide-induced acute lung injury. Eur J Pharmacol. 2015;765:94–99. doi:10.1016/j.ejphar.2015.08.022
10. Trouplin V, Boucherit N, Gorvel L, Conti F, Mottola G, Ghigo E. Bone marrow-derived macrophage production. J Vis Exp. 2013;81:e50966.
11. Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi:10.1038/ni1001-907
12. Gallego C, Golenbock D, Gomez MA, Saravia NG. Toll-like receptors participate in macrophage activation and intracellular control of Leishmania (Viannia) panamensis. Infect Immun. 2011;79(7):2871–2879. doi:10.1128/IAI.01388-10
13. Li W, Song K, Wang S, et al. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater Sci Eng C Mater Biol Appl. 2019;98:685–695. doi:10.1016/j.msec.2019.01.025
14. Zhou L, Liu Z, Wang Z, et al. Astragalus polysaccharides exerts immunomodulatory effects via TLR4-mediated MyD88-dependent signaling pathway in vitro and in vivo. Sci Rep. 2017;7:44822. doi:10.1038/srep44822
15. Lee SJ, Kim JJ, Kang KY, et al. Herbal preparation (HemoHIM) enhanced functional maturation of bone marrow-derived dendritic cells mediated toll-like receptor 4. BMC Complement Altern Med. 2016;16:67. doi:10.1186/s12906-016-1045-9
16. Zhao T, Feng Y, Li J, et al. Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int J Biol Macromol. 2014;65:33–40. doi:10.1016/j.ijbiomac.2014.01.018
17. Wang X, Li L, Ran X, et al. What caused the changes in the usage of Atractylodis Macrocephalae Rhizoma from ancient to current times? J Nat Med. 2016;70(1):36–44. doi:10.1007/s11418-015-0934-4
18. Li W, Guo S, Xu D, et al. Polysaccharide of Atractylodes macrocephala Koidz (PAMK) relieves immunosuppression in cyclophosphamide-treated geese by maintaining a humoral and cellular immune balance. Molecules. 2018;23(4).pii: E932.
19. Zong A, Cao H, Wang F. Anticancer polysaccharides from natural resources: a review of recent research. Carbohydr Polym. 2012;90(4):1395–1410. doi:10.1016/j.carbpol.2012.07.026
20. Lee KY, Lee MH, Chang IY, Yoon SP, Lim DY, Jeon YJ. Macrophage activation by polysaccharide fraction isolated from Salicornia herbacea. J Ethnopharmacol. 2006;103(3):372–378. doi:10.1016/j.jep.2005.08.037
21. Chang HH, Yeh CH, Sheu F. A novel immunomodulatory protein from Poria cocos induces toll-like receptor 4-dependent activation within mouse peritoneal macrophages. J Agric Food Chem. 2009;57(14):6129–6139. doi:10.1021/jf9011399
22. Chen M, May BH, Zhou IW, Xue CC, Zhang AL. FOLFOX 4 combined with herbal medicine for advanced colorectal cancer: a systematic review. Phytother Res. 2014;28(7):976–991. doi:10.1002/ptr.5092
23. Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi:10.1126/science.282.5396.2085
24. Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi:10.1016/j.canlet.2013.08.016
25. Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta-analysis of observational studies. World J Gastroenterol. 2013;19(26):4234–4241. doi:10.3748/wjg.v19.i26.4234
26. Onier N, Hilpert S, Arnould L, et al. Cure of colon cancer metastasis in rats with the new lipid A OM 174. Apoptosis of tumor cells and immunization of rats. Clin Exp Metastasis. 1999;17(4):299–306.
27. Gerard C, Baudson N, Ory T, Louahed J. Tumor mouse model confirms MAGE-A3 cancer immunotherapeutic as an efficient inducer of long-lasting anti-tumoral responses. PLoS One. 2014;9(5):e94883. doi:10.1371/journal.pone.0094883
28. Wang S, Astsaturov IA, Bingham CA, et al. Effective antibody therapy induces host-protective antitumor immunity that is augmented by TLR4 agonist treatment. Cancer Immunol Immunother. 2012;61(1):49–61. doi:10.1007/s00262-011-1090-7
29. Davis MB, Vasquez-Dunddel D, Fu J, Albesiano E, Pardoll D, Kim YJ. Intratumoral administration of TLR4 agonist absorbed into a cellular vector improves antitumor responses. Clin Cancer Res. 2011;17(12):3984–3992. doi:10.1158/1078-0432.CCR-10-3262
30. Yoon YD, Han SB, Kang JS, et al. Toll-like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum. Int Immunopharmacol. 2003;3(13–14):1873–1882. doi:10.1016/j.intimp.2003.09.005
31. Yamamoto M, Sato S, Hemmi H, et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4(11):1144–1150. doi:10.1038/ni986
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.