Back to Journals » Nature and Science of Sleep » Volume 12
Astroglial Mechanisms Underlying Chronic Insomnia Disorder: A Clinical Study
Authors Zhang P , Li YX, Zhang ZZ, Yang Y, Rao JX, Xia L, Li XY, Chen GH , Wang F
Received 28 May 2020
Accepted for publication 27 August 2020
Published 8 October 2020 Volume 2020:12 Pages 693—704
DOI https://doi.org/10.2147/NSS.S263528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sutapa Mukherjee
Ping Zhang,1,* Ying-Xue Li,1,* Zhe-Zhe Zhang,2 Ye Yang,1 Ji-Xian Rao,1 Lan Xia,3 Xue-Yan Li,1 Gui-Hai Chen,1 Fang Wang2
1Department of Sleep Disorders, The Affiliated Chaohu Hospital of Anhui Medical University, Hefei 238000, People’s Republic of China; 2Department of Neurology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, People’s Republic of China; 3Department of Neurology, The Second Affiliated Hospital of Anhui Medical University, Hefei 230601, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Wang
Department of Neurology, The First Affiliated Hospital of Anhui Medical University, Hefei 230022, People’s Republic of China
Tel/Fax +86-551-62922426
Email [email protected]
Gui-Hai Chen
Department of Sleep Disorders, The Affiliated Chaohu Hospital of Anhui Medical University, Hefei, (Chaohu) 238000, People’s Republic of China
Tel/Fax +86-551-82324252
Email [email protected]
Purpose: The objective of this study was to investigate whether the serum biomarkers S100 calcium binding protein B (S100B), glial fibrillary acidic protein (GFAP), brain-derived neurotrophic factor (BDNF), and glial cell line-derived neurotrophic factor (GDNF) change in patients with chronic insomnia disorder (CID), and if this is the case, whether the altered levels of these serum biomarkers are associated with poor sleep quality and cognitive decline in CID.
Patients and Methods: Fifty-seven CID outpatients constituted the CID group; thirty healthy controls (HC) were also enrolled. Questionnaires, polysomnography, Chinese-Beijing Version of Montreal Cognitive Assessment (MoCA-C) and Nine Box Maze Test (NBMT) were used to assess their sleep and neuropsychological function. Serum S100B, GFAP, BDNF, and GDNF were evaluated using enzyme-linked immunosorbent assay.
Results: The CID group had higher levels of S100B and GFAP and lower levels of BDNF and GDNF than the HC group. Spearman correlation analysis revealed that poor sleep quality, assessed by subjective and objective measures, was positively correlated with S100B level and negatively correlated with BDNF level. GFAP level correlated positively with poor subjective sleep quality. Moreover, S100B and GFAP levels correlated negatively with general cognitive function assessed using MoCA-C. GFAP level correlated positively with poor spatial working memory (SWM) in the NBMT; BDNF level was linked to poor SWM and object recognition memory (ORcM) in the NBMT. However, principal component analysis revealed that serum S100B level was positively linked to the errors in object working memories, BDNF and GDNF concentrations were negatively linked with errors in ORcM, and GFAP concentration was positively correlated with the errors in the SWM and spatial reference memories.
Conclusion: Serum S100B, GFAP, BDNF, and GDNF levels were altered in patients with CID, indicating astrocyte damage, and were associated with insomnia severity or/and cognitive dysfunction.
Keywords: cognition, S100 calcium binding protein B, glial fibrillary acidic protein, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor
Introduction
Insomnia is a common health problem characterized by subjective complaints about difficulties in initiating or maintaining sleep, early morning awakenings, and significant impairments in important areas of daytime functioning.1 Roughly 50% of insomnia patients with more severe symptoms have a chronic course.2 In most cases, chronic insomnia disorder (CID) is associated with medical and psychiatric disorders.3,4 Even though the burden of CID on both individuals and society is significant, the neural underpinnings of insomnia are poorly understood.5 Accordingly, there is an urgent need to determine the neural mechanisms of CID, which would inform future attempts to alleviate or treat insomnia symptoms.
In previous studies, CID was considered a functionally injurious disease causing different functional impairments, such as fatigue, irritability, and cognitive impairment.1,6 Patients with CID were more likely to report that cognitive impairments contributed to their reduced quality of life, highlighting the clinical significance of performance impairments.7,8 However, we could not reasonably explain why functional impairment, especially the obvious residual cognitive function impairment, became a prominent residual symptom although other symptoms of insomnia disappeared after effective treatment. In addition, it is still unknown whether these functional impairments in patients with CID are a direct consequence of sleep loss or that of brain injury indirectly related to insomnia.
A growing body of studies has investigated insomnia-related functional or structural alterations in the brain. In terms of changes in gray matter, neuroimaging studies have linked insomnia to a decrease in gray matter in the left orbitofrontal cortex9,10 and hippocampus.11 In addition, diffusion tensor imaging has been used to evaluate differences in white matter tracts between insomniacs and matched controls.12 Insomnia-related alterations in functional connectivity have also been investigated, but the results have been relatively inconsistent so far.13–15 In summary, most studies on brain structure have evaluated insomnia-related differences in specific regions, but neuroimaging studies have not been able to clearly conclude what kind of damage insomnia causes and in which areas this damage occurs.16 Furthermore, very few studies up till now have assessed the relevant microstructural changes in the brain, for example, at the cellular level.
Although CID can be assessed using objective means such as neuroimaging and polysomnography (PSG), which are regarded as standard methods for evaluation of objective sleep,17,18 these methods are often impractical, as clinical screening and research tools are expensive. Simpler methods, such as those based on serological indicators, may provide information that is just as useful to clinicians. Therefore, there is increasing interest in the expanding field of serum markers for CID.
Astrocytes are the most predominant glial cells in the brain, and play a crucial role in sleep regulation under physiological conditions. Astrocytes can release adenosine and pro-inflammatory cytokines, with direct influence on sleep pressure, intensity, and duration. Moreover, astrocytes determine the urge to sleep by clearing toxic substances through the glymphatic system.19,20 Serum S100 calcium-binding protein B (S100B) levels are neurobiochemical markers and can reflect the severity of astrocyte damage. Our preliminary study showed that patients with CID had significantly increased serum S100B levels, while their concentrations did not change significantly after effective therapy.21 Thus, we speculate that CID patients may have pathological changes in their astrocytes. Unfortunately, we did not control for potential non-cerebral sources of serum markers, and as various subtypes of leukocytes can also secrete S100B,22 this was a limitation of the experimental design.
There is a need to investigate other astrocyte-related markers, such as glial fibrillary acidic protein (GFAP),23 brain-derived neurotrophic factor (BDNF),24 and glial cell line-derived neurotrophic factor (GDNF),25 and study their role in CID. The aim of this study was to determine whether astrocyte-specific biomarker levels in patients with CID could serve as objective and accurate complementary diagnostic tools for the detection and assessment of insomnia severity.
Methods
Subjects
Fifty-seven outpatients with CID at the Clinics of Sleep Disorders of both the First Affiliated Hospital and the Affiliated Chaohu Hospital of Anhui Medical University were enrolled in this study. Patients were diagnosed with CID according to the International Classification of Sleep Disorders, third edition (ICSD-3).26 Patients were excluded if they had craniocerebral injury, spinal cord injury, neurodegenerative neurological diseases, intracranial vascular disease or any other psychiatric disorders. Thirty healthy subjects were selected as healthy controls (HC), and the inclusion criteria were as follows: (1) with similar demographics, (2) without subjective complaint of insomnia and mood disorder with a Pittsburgh sleep quality index (PSQI) score <7,27,28 a 17-item Hamilton depression rating scale (HAMD-17) score <7,29 and (3) a Chinese-Beijing Version of Montreal Cognitive Assessment (MoCA-C) score ≥26.30
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committees of the First Affiliated Hospital and the Affiliated Chaohu Hospital of Anhui Medical University (Number 201,805-kyxm-01). All subjects signed a written informed consent form.
Baseline Data Collection
A researcher-developed questionnaire was used to collect demographic characteristics (ie, age, gender and educational information), medical history, and family history.
Depression, Anxiety and Comprehensive Cognition Evaluations
The HAMD-17 was employed to assess the severity of depressive symptoms.29 The questionnaire contained 17 terms, and the total scores ranged from 0 to 52, where higher scores indicate greater depressive severity. In China, the corresponding grades to depressive states are as follows: no depression (0–7), mild depression (8–17), moderate depression (18–23), and severe depression (24–52).
The 14-item Hamilton anxiety rating scale (HAMA-14) was used to assess the severity of anxiety symptoms.31 The 14 items are rated from 0 to 4, and they incorporate groups of symptoms (ie, symptoms related to anxiety mood, fears, feelings of tension, sleep, cognitive function, depressed mood, and behavior in conversation and autonomic, respiratory, somatic, cardiovascular, sensory, gastrointestinal, and reproductive and urinary symptoms). In China, the total scores corresponding to different anxiety states are as follows: <7, no anxiety; ≥7, probable anxiety; ≥14 mild anxiety; ≥21 moderate anxiety; ≥29, severe anxiety.
The MoCA-C scale is a cognitive screening test that has high levels of reliability and validity and is widely used in China.30 It measures eight domains including visuospatial and executive functions, naming, attention, abstraction, short-term memory, delayed recall, language, and orientation. The maximum score is 30 points, and scores ≥26 are considered as normal cognitive function.
Subjective and Objective Insomnia Assessment
PSQI, a self-reported questionnaire, was employed to assess subjective quality of sleep.28 It contains 7 domains including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction. The total score of PSQI ranges from 0 to 21, with a higher score corresponding to poorer sleep quality.
PSG was used to evaluate object sleep on two consecutive nights. The first night was used to acclimatize to novel environment. The second night was to assess the aspects of sleep including sleep continuity and sleep structure. The former involved the parameters of total sleep time (TST), sleep efficiency (SE) and sleep onset latency (SOL); the latter included percentages of sleep stage 1 (N1%), stage 2 (N2%), and slow wave sleep (SWS, N3%), time awake after sleep onset, and time (REMT) and percentage (REM%) of rapid eye movement sleep.32
Multi-Dimensional Memory Evaluation
The Nine Box Maze Test (NBMT) was modified to evaluate multi-dimensional memory, including spatial/object working memories (SWM/OWM), spatial/object reference memories (SRM/ORM), and object recognition memory (ORcM).33,34 The experiment was conducted according to our previous studies.21,35 The test included object-familiarization, training and testing periods. The numbers of errors were recorded as the performance of SWM, OWM, SRM, ORM, and ORcM, whereby a higher number of errors indicates poor memory.
Blood Sample Collection
Blood samples were collected in the morning (between 07:30 h and 08:00 h) following the second PSG. Samples were separated immediately by centrifugation, and the serum was frozen at –80°C until assay. According to the manufacturer’s instructions (Millipore Corporation; USA), a quantitative sandwich enzyme-linked immunosorbent assay was used to quantify serum levels of GFAP, BDNF, GDNF, and S100B.
Statistical Analysis
SPSS® 22.0 for Windows was used for statistical analyses. The Student’s t-test was selected to analyze normally distributed data expressed as mean ± standard deviation (SD); the Mann−Whitney U-test was selected to analyze nonparametric data expressed as the 25th, 50th, and 75th percentiles (P25, P50, and P75, respectively). Spearman correlation analysis was used to analyze the correlations between serum S100B, GFAP, GDNF, and BDNF levels and sleep quality, general cognition, and NBMT performances. A multivariate data reduction technique of principal component analysis was performed to analyze the relationships among parameters from patients with CID. In addition, receiver operating characteristics (ROC) analysis was used to compare the diagnostic information provided by different biomarkers according to the calculated areas under the curves (AUCs), optimal cut-off points, sensitivity, and specificity. Two-tailed P-values of ≤0.05 were considered statistically significant.
Results
Background Data
There were no significant differences in sex, age and educated levels between the two groups (p > 0.05, Table 1). Compared to the HC, patients with CID had significantly higher HAMD-17 and HAMA-14 (p < 0.001).
 |
Table 1 Background Data, Subjective Sleep Quality |
Subjective and Objective Insomnia
Individuals in CID group had significantly higher PSQI scores than the HC (p < 0.001, Table 1). Although limited PSG samples were available, the PSG results showed that the patients with CID had significantly longer SOL and significantly lower SE and TST than the HCs (p < 0.01, Table 2).
 |
Table 2 The Objective Sleep Quality |
Comprehensive Cognition and Nine Box Maze Performance
Compared to the HC, patients with CID had significantly lower MoCA-C scores (p < 0.01) and more errors in tests of SWM, OWM, and ORcM (p < 0.01, Table 3).
 |
Table 3 The Cognitive Performance |
Changes of Serum S100B, GFAP, GDNF and BDNF
Compared to the HC, patients with CID had higher S100B (p < 0.001) and GFAP (p < 0.05) levels and lower GDNF and BDNF (p < 0.001) levels (Figure 1AD).
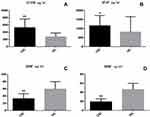 |
Figure 1 (A–D). Comparison of serum levels between chronic insomnia disorder patients (CID) and healthy controls (HC). |
Correlations Among Serum Biomarkers, Sleep Parameters and Cognitive Performances
The correlations between serum biomarkers of astrocyte damage and subjective sleep parameters are shown in Table 4. In patients with CID, S100B (r = 0.352, p < 0.01) and GFAP (r = 0.287, p < 0.05) levels correlated positively with PSQI score. Serum BDNF level (r = 0.369, p < 0.01) was negatively correlated with PSQI score.
 |
Table 4 The Correlations Between S100B/GFAP/GDNF/BDNF and Subjective Sleep Quality, Cognitive Performance |
The correlations between serum biomarkers and objective sleep parameters are displayed in Figure 2AE. The levels of S100B negatively correlated with the TST, SE, N3 and N3% in the CID (r = −0.485, −0.454, r = −0.433 and −0.423; p < 0.05). The BDNF positively correlated with the TST (r = 0.386, p < 0.001).
 |
Figure 2 (A–E). Scatter plots: Correlation between BDNF and TST in the CID; Correlation between S100B and TST, SE, N3, N3% in the CID. |
The correlations between serum biomarkers and cognitive parameters are shown in Table 4. S100B and GFAP concentrations (r = −0.301, p < 0.05 and r = −0.307, p < 0.05, respectively) were negatively correlated with MoCA-C scores in the CID group. Regarding the performance in the NBMT, the CID group had significant positive correlations between serum GFAP level and SWM errors (r = 0.317, p < 0.05) and negative correlations between serum BDNF level and the errors of SWM and ORcM (r = −0.456, p < 0.01 and r = −0.410, p < 0.01, respectively).
Principal Component Analysis for CID
The Kaiser–Meyer–Olkin value, as a measure of sampling adequacy, was 0.501, and p was lower than 0.001 in Bartlett’s test of sphericity, suggesting that these measures selected in the CID group (the number of patients who completed the PSG evaluation is 27) are appropriate for principle components analysis. Thus, five significant factors were obtained, and the component loadings of variables on the rotated factors are shown in Table 5. The PSG parameters of sleep (TST, SE, and REMT), PSQI score, OWM, and S100B level loaded highly on factor 1. REM%, ORcM, and GDNF and BDNF level loaded on factor 4; GFAP level, MoCA-C score, SRM, and SWM loaded on factor 5.
 |
Table 5 Principal Component Analysis for CID |
Appropriateness of Serum Indexes as Diagnostic Biomarkers for CID
The results of the ROC analysis are listed in Table 6. The AUCs of S100B, GFAP, GDNF, and BDNF levels were all larger than 0.70, which were greater for predicting CID compared to HC. The optimal cut-off values for S100B and GFAP were 323.9 pg/mL and 888.5 pg/mL, respectively, indicating that the subject should be considered as having CID other than health sleeper when their serum biomarker levels exceed the corresponding cut-off values. The optimal cut-off values for GDNF and BDNF were 24.5 pg/mL and 26.7 ng/mL, indicating that the subject should be considered as having CID other than health sleeper when their serum biomarker levels are lower than the corresponding cut-off values.
 |
Table 6 Characteristics of Potential Blood Biomarkers for CID in ROC Analysis* |
Discussion
CID Seems to Be an Organic Condition Characterized by Prominent Dysfunction of Astrocytes
S100B, GFAP, BDNF, and GDNF are deemed to be astrocyte-related biomarkers, as shown in Table 7. In the present study, we found that serum levels of S100B and GFAP in CID patients were significantly higher than those in healthy individuals, while levels of GDNF and BDNF in CID were significantly lower when compared to healthy individuals. Therefore, it is reasonable to assume that patients with CID have some degree of astrocyte damage.
 |
Table 7 List of the Astrocyte Associated Proteins, Normal Function, and Association with Disease |
S100B is crucial for the maintenance of proper neuronal function, particularly under pathological conditions. Our preliminary study21 showed that patients with CID had significantly increased S100B serum levels when compared to healthy individuals; these were found to reflect the poor subjective and objective sleep quality (TST, SE, N3 and N3%) in patients with CID. In addition, higher serum S100B concentrations were associated with poor general cognition, as assessed using MoCA-C, and poor SRM, as indicated by the number of errors. In the present study, our findings also indicate that elevated S100B levels were negatively correlated with MoCA-C score in patients with CID. However, higher serum S100B concentrations were linked to poor OWM, rather than poor SRM. These conflicting findings may be due to several factors such as sample size, as a bigger sample was used in the current study; severity of impaired cognitive function; duration of illness; education level; depressive symptoms; among others. Since S100B serum levels cannot be used as a specific marker of astrocytes damage,22 we selected other astrocyte-related serum biomarkers to test this conclusion.
GFAP is only released into the blood stream after cell death, which suggests that GFAP is specific to the central nervous system (CNS) and indicative of definite astroglial injury.44 To the best of our knowledge, the current study is the first to explore the correlation between serum GFAP levels and sleep quality and cognitive impairment in patients with CID. Our results show that serum GFAP levels positively correlate with subjective sleep quality, while they had no correlations with objective sleep parameters. GFAP serum levels were also associated with both general cognition dysfunction and spatial memory impairment, including SWM and SRM. The reason may be that astrocytes are actively involved in normal memory functions and in the abnormal processes, including astroglial injury, leading to cognitive impairment in pathological conditions.53 Even a few hours of sleep deprivation led to astrocytic processes extending closer to the synaptic cleft. These structural changes potentially represent the importance of positioning astrocytes adjacent to synapses for the clearance of neurotransmitters via astrocytic transporters or for the transmission of glial-derived signals to support synaptic plasticity.54
BDNF is a critical growth factor involved in the maturation of the CNS.45 Giese’s pilot study suggested that peripheral BDNF could be regarded as a biomarker for subjective insomnia, and that the more severe the insomnia, the lower the serum BDNF level.55 In our study, decreased BDNF levels directly correlated with poor subjective and objective sleep quality in patients with CID. However, a recent study suggests that BDNF is related to subjective sleep perception but not to objective sleep continuity.56 This discrepancy may be attributed to some factors such as genetic background, the duration of illness, and comorbidities. BDNF mediates long-term potentiation, a form of synaptic plasticity that is associated with enhanced learning and memory.57–59 A recent study in China found that decreased peripheral BDNF might mediate impaired neuropsychological performances (including brief visuospatial memory, spatial span, managing emotions, fluency, and continuous performance) in insomniacs with short sleep duration.60 Our results indicate that decreased serum BDNF concentration was linked to poor SWM and ORcM. Interestingly, both SWM and ORcM are categories of visual memory, and SWM has similar components to the brief visuospatial memory detected in the aforementioned study.60
GDNF has a potential neuroprotective effect in the adult brain. In the present study, patients with CID had a significant reduction of serum GDNF when compared to healthy controls, and it had no correlation with sleep quality. Moreover, decreased serum concentrations of GDNF were linked to poor ORcM in patients with CID. GDNF may play an important role in cognitive abilities. For instance, GDNF heterozygous mutant mice show impaired water-maze learning performance.61–63 Furthermore, GDNF exposure in hippocampal astrocytes led to improved spatial cognitive abilities in aged, cognitively impaired rats.64 These studies are consistent with our findings.
According to our results, we consider that patients with CID have some degree of astrocytes dysfunction. The nature of the relationship remains unclear, that is, whether astrocyte dysfunction causes insomnia or insomnia causes astrocyte dysfunction. The important role of astrocytes in sleep is to regulate sleep homeostasis and clean the brain parenchyma. They release ATP and increase levels of adenosine to stimulate sleep in the brain.65–69 In addition, astrocytes, through regulation of extracellular glutamate, are involved in triggering a slow neuronal rhythm in the brain.70 Moreover, experimental activation of astrocytes, and thus induction of calcium transients, results in slow oscillations which have been shown to be important in sleep and memory formation.71–73 Therefore, it is plausible that astrocyte dysfunction observed in CID impairs astrocytes sleep-regulating capabilities. On the other hand, sleep has been proposed to be “the brain’s housekeeper”, serving to restore and repair the brain. The restorative function of sleep may be a consequence of the enhanced removal of potentially neurotoxic waste products that accumulate in the awake CNS.74 There is little evidence that CID presents organic brain damage.20,21 Therefore, insomnia may have a direct impact upon dysfunction of astrocytes, which may begin at the early course or even the prodromal stage of the illness.
In addition, stress can induce structural and functional modifications of astrocytes.75,76 At the same time, current consensus is that exposure to stressful events impairs normal sleep function.77,78 Therefore, it is plausible that, at least in some individuals, the mechanism leading to insomnia may be accumulating stress events across the lifetime that could result in the dysfunction of astrocytes, in turn causing the onset and development of insomnia.
In summary, we conclude that patients with CID have pathological astrocyte-related changes, which may be a trait of CID patients. Astrocyte dysfunction correlates with poor sleep quality, on parameters such as decrease of total sleep time, sleep efficiency, and N3 sleep. Furthermore, astrocyte dysfunction may be involved in the underlying mechanism of cognitive impairment in CID.
The Possible Diagnostic and Prognostic Value of These Serum Indexes
ROC analysis was used to calculate the cut-off values of different serum biomarkers to discriminate between true and false CID diagnosis as established by the ICSD-3 criteria. The results showed that the AUCs of all four proteins were over 0.7 between patients with CID and HC. The optimal cut-off values for S100B and GFAP indicated that subjects should be considered to have CID if their serum biomarkers levels were over the corresponding cut-off values. The optimal cut-off values for GDNF and BDNF indicated that subjects should be considered to have CID if their serum biomarkers levels were lower than the corresponding cut-off values. These results might provide strong cues for considering CID relative to healthy sleep, implying a possibility that these serum indexes act as objective diagnostic markers of CID. Combining these four indicators may provide a more specific and sensitive method to better manage and offer more accurate prognosis of patients with CID.
Conclusion
Patients with CID have altered serum levels of astrocyte-related biomarkers, with increased S100B and GFAP and decreased BDNF and GDNF serum levels, indicating pathological changes involving astrocytes. In addition, these serum markers of astrocyte injury were associated with insomnia severity and/or cognitive dysfunction.
Acknowledgment
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was financially supported by the National Natural Science Foundation of China (81671316), Natural Science Foundation for the Youth of China (81301094), the Annual Research Plan of Anhui Province (1301043041), Natural Science Foundation of Anhui Medical University (2018xkj029), and Natural Science Foundation of Anhui Medical University (2018xkj066).
Disclosure
The authors have indicated no conflicts of interest for this work.
References
1. Wardle-Pinkston S, Slavish DC, Taylor DJ. Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med Rev. 2019;48:101205. doi:10.1016/j.smrv.2019.07.008.
2. Winkelman JW. Insomnia Disorder. N Engl J Med. 2015;373(15):1437–1444. doi:10.1056/NEJMcp1412740.
3. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379:1129–1141. doi:10.1016/S0140-6736(11)60750-2.
4. Blanken TF, Benjamins JS, Borsboom D, et al. Insomnia disorder subtypes derived from life history and traits of affect and personality. Lancet Psychiatry. 2019;6(2):151–163. doi:10.1016/S2215-0366(18)30464-4.
5. Sivertsen B, Overland S, Neckelmann D, et al. The long-term effect of insomnia on work disability: the HUNT-2 historical cohort study. Am J Epidemiol. 2006;163:1018–1024. doi:10.1093/aje/kwj145
6. Sutton EL. Insomnia. Med Clin North Am. 2014;98:565–581. doi:10.1016/j.mcna.2014.01.008.
7. Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med Rev. 2012;16(1):83–94. doi:10.1016/j.smrv.2011.03.008.
8. Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol a Biol Sci Med Sci. 2006;61(4):405–410. doi:10.1093/gerona/61.4.405
9. Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. doi:10.1016/j.biopsych.2009.08.003.
10. Stoffers D, Moens S, Benjamins J, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. doi:10.3389/fneur.2012.00105.
11. Riemann D, Voderholzer U, Spiegelhalder K, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30(8):955–958. doi:10.1093/sleep/30.8.955
12. Jespersen KV, Stevner A, Fernandes H, et al. Reduced structural connectivity in Insomnia Disorder. J Sleep Res. 2019;12:e12901. doi:10.1111/jsr.12901.
13. Zhao L, Wang E, Zhang X, et al. Cortical structural connectivity alterations in primary insomnia: insights from MRI-based morphometric correlation analysis. Biomed Res Int. 2015;2015:817595. doi:10.1155/2015/817595.
14. Spiegelhalder K, Riemann D. Losing sleep. Lancet Neurol. 2015;14(6):571. doi:10.1016/S1474-4422(15)00065-4.
15. Tagliazucchi E, van Someren EJW. The large-scale functional connectivity correlates of consciousness and arousal during the healthy and pathological human sleep cycle. Neuroimage. 2017;160:55–72. doi:10.1016/j.neuroimage.2017.06.026.
16. Kay DB, Buysse DJ. Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies. Brain Sci. 2017;7(3):
17. Kay DB, Karim HT, Soehner AM, et al. Subjective-objective sleep discrepancy is associated with alterations in regional glucose metabolism in patients with insomnia and good sleeper controls. Sleep. 2017;40:11. doi:10.1093/sleep/zsx155.
18. Hermans LWA, Leufkens TR, van Gilst MM, et al. Sleep EEG characteristics associated with sleep onset misperception. Sleep Med. 2019;57:70–79. doi:10.1016/j.sleep.2019.01.031
19. Haydon PG. Astrocytes and the modulation of sleep. Curr Opin Neurobiol. 2017;44:28–33. doi:10.1016/j.conb.2017.02.008.
20. Garofalo S, Picard K, Limatola C, et al. Role of glia in the regulation of sleep in health and disease. Compr Physiol. 2020;10:2. doi:10.1002/cphy.c190022
21. Zhang P, Tan CW, Chen GH, et al. Patients with chronic insomnia disorder have increased serum levels of neurofilaments, neuron-specific enolase and S100B: does organic brain damage exist? Sleep Med. 2018;48:163–171. doi:10.1016/j.sleep.2017.12.012
22. Di C, Zeng Y, Mao J, Gu W. Dynamic changes and clinical significance of serum S100B protein and glial fibrillary acidic protein in patients with delayed encephalopathy after acute carbon monoxide poisoning. Pak J Med Sci. 2018;34(4):945–949. doi:10.12669/pjms.344.15363
23. Breitling B, Brunkhorst R, Verhoff M, Foerch C. Post-mortem serum concentrations of GFAP correlate with agony time but do not indicate a primary cerebral cause of death. PLoS One. 2018;13(10):e0205323. doi:10.1371/journal.pone.0205323
24. Deuschle M, Schredl M, Wisch C, et al. Serum brain-derived neurotrophic factor (BDNF) in sleep disordered patients: relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J Sleep Res. 2018;27(1):73–77. doi:10.1111/jsr.12577
25. Tang X, Zhou C, Gao J, et al. Serum BDNF and GDNF in Chinese male patients with deficit schizophrenia and their relationships with neurocognitive dysfunction. BMC Psychiatry. 2019;19(1):254. doi:10.1186/s12888-019-2231-3
26. Morin CM, Drake CL, Harvey AG, et al. Insomnia disorder. Nat Rev Dis Primers. 2015;1:15026. doi:10.1038/nrdp.2015.26
27. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi:10.1016/j.smrv.2015.01.009
28. Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1):5–13. doi:10.1016/s0022-3999(97)00298-5
29. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi:10.1136/jnnp.23.1.56
30. Chen X, Zhang R, Xiao Y, Dong J, Niu X, Kong W. Reliability and validity of the Beijing version of the Montreal Cognitive Assessment in the evaluation of cognitive function of adult patients with OSAHS. PLoS One. 2015;10:e0132361. doi:10.1371/journal.pone.0132361
31. Zimmerman M, Martin J, Clark HJ, McGonigal P, Harris L, Holst CG. Measuring anxiety in depressed patients: A comparison of the Hamilton anxiety rating scale and the DSM-5 anxious distress specifier interview. Psychiatr Res. 2017;93:59–63. doi:10.1016/j.jpsychires.2017.05.014
32. Hori T, Sugita Y, Koga E, et al. Proposed supplements and amendments to ‘A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects’, the Rechtschaffen & Kales (1968) standard. Psychiatry Clin Neurosci. 2001;55:305–310. doi:10.1046/j.1440-1819.2001.00810.x
33. Abrahams S, Pickering A, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;35:11–24. doi:10.1016/s0028-3932(96)00051-6
34. Porter VR, Buxton WG, Avidan AY. Sleep, cognition and dementia. Curr Psychiatry Rep. 2015;17:97. doi:10.1007/s11920-015-0631-8
35. Chen GH, Xia L, Wang F, et al. Patients with chronic insomnia have selective impairments in memory that are modulated by cortisol. Psychophysiology. 2016;53:1567–1576. doi:10.1111/psyp.12700
36. Tsoporis JN, Marks A, Haddad A, Dawood F, Liu PP, Parker TG. S100B expression modulates left ventricular remodeling after myocardial infarction in mice. Circulation. 2005;111(5):598–606. doi:10.1161/01.CIR.0000154554.65287.F5
37. Carvalho DZ, Schönwald SV, Schumacher-Schuh AF, et al. Overnight S100B in Parkinson’s disease: a glimpse into sleep-related neuroinflammation. Neurosci Lett. 2015;608:57–63. doi:10.1016/j.neulet.2015.10.010
38. Bouvier D, Giguère Y, Pereira B, et al. Cord blood S100B: reference ranges and interest for early identification of newborns with brain injury. Clin Chem Lab Med. 2019. doi:10.1515/cclm-2019-0737
39. Faridaalee G, Keyghobadi Khajeh F. Serum and cerebrospinal fluid levels of S-100β is a biomarker for spinal cord injury; a systematic review and meta-analysis. Arch Acad Emerg Med. 2019;7(1):e19.
40. Morera-Fumero AL, Díaz-Mesa E, Abreu-Gonzalez P, Fernandez-Lopez L, Cejas-Mendez MDR. Day/night changes in serum S100B protein concentrations in acute paranoid schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;75:207–212. doi:10.1016/j.pnpbp.2017.02.007
41. Potokar M, Morita M, Wiche G, Jorgačevski J. The diversity of intermediate filaments in astrocytes. Cells. 2020;9(7):E1604. doi:10.3390/cells9071604
42. McMahon PJ, Panczykowski DM, Yue JK, et al. Measurement of the glial fibrillary acidic protein and its breakdown products GFAP-BDP biomarker for the detection of traumatic brain injury compared to computed tomography and magnetic resonance imaging. J Neurotrauma. 2015;32(8):527–533. doi:10.1089/neu.2014.3635
43. Gan ZS, Stein SC, Swanson R, et al. Blood biomarkers for traumatic brain injury: a quantitative assessment of diagnostic and prognostic accuracy. Front Neurol. 2019;10:446. doi:10.3389/fneur.2019.00446
44. Papa L, Lewis LM, Falk JL, et al. Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann Emerg Med. 2012;59(6):471–483. doi:10.1016/j.annemergmed.2011.08.021
45. Zagrebelsky M, Tacke C, Korte M. BDNF signaling during the lifetime of dendritic spines [published online ahead of print, 2020 Jun 14]. Cell Tissue Res. 2020;10. doi:10.1007/s00441-020-03226-5.
46. Wei C, Sun Y, Chen N, Chen S, Xiu M, Zhang X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology. 2019;111:104473. doi:10.1016/j.psyneuen.2019.104473
47. Costa CM, Oliveira GL, Fonseca ACS, Lana RC, Polese JC, Pernambuco AP. Levels of cortisol and neurotrophic factor brain-derived in Parkinson’s disease. Neurosci Lett. 2019;708:134359. doi:10.1016/j.neulet.2019.134359
48. Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28(15):4088–4095. doi:10.1523/JNEUROSCI.5510-07.2008
49. Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–258. doi:10.1124/pr.111.005108
50. Walker M, Xu XM. History of Glial Cell Line-Derived Neurotrophic Factor (GDNF) and its use for spinal cord injury repair. Brain Sci. 2018;8(6):
51. Ibáñez CF, Andressoo JO. Biology of GDNF and its receptors - Relevance for disorders of the central nervous system. Neurobiol Dis. 2017;97(Pt B):80–89. doi:10.1016/j.nbd.2016.01.021
52. Duarte Azevedo M, Sander S, Tenenbaum L. GDNF, A neuron-derived factor upregulated in glial cells during disease. J Clin Med. 2020;9(2):456. doi:10.3390/jcm9020456
53. Ayanlaja AA, Zhang B, Ji G, et al. The reversible effects of glial cell line-derived neurotrophic factor (GDNF) in the human brain. Semin Cancer Biol. 2018;53:212–222. doi:10.1016/j.semcancer.2018.07.005
54. Frank MG. Astroglial regulation of sleep homeostasis. Curr Opin Neurobiol. 2013;23(5):812–818. doi:10.1016/j.conb.2013.02.009
55. Giese M, Unternährer E, Hüttig H, et al. BDNF: an indicator of insomnia? Mol Psychiatry. 2014;19(2):151–152. doi:10.1038/mp.2013.10
56. Mikoteit T, Brand S, Eckert A, Holsboer-Trachsler E, Beck J. Brain-derived neurotrophic factor is a biomarker for subjective insomnia but not objectively assessable poor sleep continuity. J Psychiatr Res. 2019;110:103–109. doi:10.1016/j.jpsychires.2018.12.020
57. He YY, Zhang XY, Yung WH, Zhu JN, Wang JJ. Role of BDNF in central motor structures and motor diseases. Mol Neurobiol. 2013;48(3):783–793. doi:10.1007/s12035-013-8466-y
58. Meis S, Endres T, Lessmann V. Postsynaptic BDNF signalling regulates long-term potentiation at thalamo-amygdala afferents. J Physiol. 2012;590(1):193–208. doi:10.1113/jphysiol.2011.220434
59. Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20(18):7116–7121. doi:10.1523/JNEUROSCI.20-18-07116.2000
60. Fan TT, Chen WH, Shi L, et al. Objective sleep duration is associated with cognitive deficits in primary insomnia: BDNF may play a role. Sleep. 2019;42:1. doi:10.1093/sleep/zsy192
61. Gerlai R, McNamara A, Choi-Lundberg DL, et al. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14(7):1153–1163. doi:10.1046/j.0953-816x.2001.01724.x
62. Naumenko VS, Kondaurova EM, Bazovkina DV, et al. Effect of GDNF on depressive-like behavior, spatial learning and key genes of the brain dopamine system in genetically predisposed to behavioral disorders mouse strains. Behav Brain Res. 2014;274:1–9. doi:10.1016/j.bbr.2014.07.045
63. Nanobashvili A, Airaksinen MS, Kokaia M, et al. Development and persistence of kindling epilepsy are impaired in mice lacking glial cell line-derived neurotrophic factor family receptor alpha 2. Proc Natl Acad Sci U S A. 2000;97:12312–12317. doi:10.1073/pnas.97.22.12312
64. Pertusa M, García-Matas S, Mammeri H, et al. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29(9):1366–1379. doi:10.1016/j.neurobiolaging.2007.02.026
65. Deng Q, Terunuma M, Fellin T, Moss SJ, Haydon PG. Astrocytic activation of A1 receptors regulates the surface expression of NMDA receptors through a Src kinase dependent pathway. Glia. 2011;59(7):1084–1093. doi:10.1002/glia.21181
66. Zhang JM, Wang HK, Ye CQ, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40(5):971–982. doi:10.1016/S0896-6273(03)00717-7
67. Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26(20):5370–5382. doi:10.1523/JNEUROSCI.5255-05.2006
68. Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi:10.1126/science.276.5316.1265
69. Halassa MM, Florian C, Fellin T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61(2):213–219. doi:10.1016/j.neuron.2008.11.024
70. Pál B. Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell Mol Life Sci. 2018;75(16):2917–2949. doi:10.1007/s00018-018-2837-5
71. Poskanzer KE, Yuste R. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci USA. 2016;113(19):E2675E2684. doi:10.1073/pnas.1520759113
72. Pelluru D, Konadhode RR, Bhat NR, Shiromani PJ. Optogenetic stimulation of astrocytes in the posterior hypothalamus increases sleep at night in C57BL/6J mice. Eur J Neurosci. 2016;43(10):1298–1306. doi:10.1111/ejn.13074
73. Zhou X, Oishi Y, Cherasse Y, et al. Extracellular adenosine and slow-wave sleep are increased after ablation of nucleus accumbens core astrocytes and neurons in mice. Neurochem Int. 2019;124:256–263. doi:10.1016/j.neuint.2019.01.020
74. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–377. doi:10.1126/science.1241224
75. Murphy-Royal C, Gordon GR, Bains JS. Stress-induced structural and functional modifications of astrocytes-Further implicating glia in the central response to stress. Glia. 2019;67(10):1806–1820. doi:10.1002/glia.23610
76. Duenas Z, Caicedo-Mera JC, Torner L. Global effects of early life stress on neurons and glial cells. Curr Pharm Des. 2018;23(39):6042–6049. doi:10.2174/1381612823666170224111641
77. Pillai V, Roth T, Mullins HM, Drake CL. Moderators and mediators of the relationship between stress and insomnia: stressor chronicity, cognitive intrusion, and coping. Sleep. 2014;37(7):1199–1208. doi:10.5665/sleep.3838
78. Dolsen MR, Crosswell AD, Prather AA. Links between stress, sleep, and inflammation: are there sex differences? Curr Psychiatry Rep. 2019;21(2):8. doi:10.1007/s11920-019-0993-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
