Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Astragalus–Saffron–Rhubarb Mixture Delays the Progress of Diabetic Nephropathy in db/db Mice
Authors Zhou XC, Liang YJ, Qin L, Wei GH, Wang JQ
Received 25 August 2021
Accepted for publication 2 November 2021
Published 1 December 2021 Volume 2021:14 Pages 4679—4690
DOI https://doi.org/10.2147/DMSO.S334662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Xiao-Chun Zhou, Yao-Jun Liang, Long Qin, Guo-Hua Wei, Jian-Qin Wang
Department of Nephrology, Lanzhou University Second Hospital, Lanzhou, 730030, People’s Republic of China
Correspondence: Jian-Qin Wang
Department of Nephrology, Lanzhou University Second Hospital, No. 82 of Cuiying Men, Chengguan District, Lanzhou, 730030, People’s Republic of China
Tel +8613919038189
Email [email protected]
Objective: This study aimed to investigate the protective effect of astragalus–saffron–rhubarb mixture (Bao’shen recipe, BSR) on diabetic nephropathy (DN) in db/db mice and preliminarily explore the possible underlying mechanism.
Methods: A total of 125 8-week-old male db/db mice with DN were randomly divided into five groups: model group, irbesartan group and high-, medium- and low doses of BSR group, while 25 male db/m mice were used as a blank control. At 8, 12, 16, 20, and 24 weeks of feeding, the animals were sacrificed and blood as well as urine samples were collected for blood glucose, urea nitrogen, creatinine and urinary albumin excretion rate (UAER) measurement via blood glucose meter or corresponding detection kits, respectively. The renal tissues of each mouse underwent hematoxylin and eosin (H&E), Masson, periodic acid Schiff (PAS) staining. Renal homogenate was used to detect IL-6, TNF-α, TNF-1R and TNF-2R by enzyme-linked immunosorbent assay. Additionally, the data obtained was statistically analyzed via one-way analysis of variance.
Results: BSR could effectively reduce the body weight, blood glucose, UAER, blood urea nitrogen and creatinine levels, relieve the proliferation of mesangial tissue, and lower the levels of IL-6, TNF-α, TNF-1R, and TNF-2R in renal tissue of db/db mice with DN. Of note, the high-dose BSR treatment group has advantages over irbesartan treatment group in improving above-mentioned aspects.
Conclusion: BSR could effectively delay the progress of DN, partly related to its anti-inflammation effect.
Keywords: diabetic nephropathy, treatment, inflammatory factors, traditional Chinese medicine
Introduction
Diabetic nephropathy (DN) is the most common and serious chronic complication of diabetes. In developed countries, DN has become the main cause of renal replacement therapy in patients with end-stage renal failure (ESRF).1 It is estimated that diabetes will become a major disease in developing countries in the next 20 years. About 25–40% of patients with type 2 diabetes mellitus (T2DM) have renal damage and chronic kidney disease.2,3 DN has multiple pathophysiologic mechanisms involving microvascular, macrovascular changes, the disorder of glucose and lipid metabolism, oxidative stress, inflammation, fibrosis, coagulation, and other factors that play important roles in the progress of DN.4–8 Even so, the precise mechanisms in DN are not fully understood. When targeting the pathogenesis of DN, comprehensive management causes the blood glucose, blood lipid, blood pressure, and other indicators to reach the standards, those means of treatment can only delay the pathological progression of diabetes mellitus, but it fails to reduce the incidence of ESRD in patients with DN.4 Therefore, finding new treatment measures is the key to the prevention and treatment of DN.
Traditional Chinese Medicine (TCM) plays an important role in the prevention and treatment of chronic kidney disease and T2DM in China, which have been demonstrated by multiple pharmacological and clinical studies.9–12 Previous reports showed that Astragalus, saffron crocus and Rhubarb were drugs commonly used for diabetes mellitus and chronic kidney disease,13–15 suggesting the possibly protective effects of the above three drugs on DN by mixing them in appropriate proportions. However, the three drugs mentioned above were currently administered mainly as a form of single drug, and little information about mixing them into the Chinese herbal compound for the treatment of DN. Against this backdrop, we previously prepared Astragalus–Saffron–Rhubarb Mixture, named Bao’shen recipe (BSR), by combining the afore-mentioned drugs in appropriate proportion and used for patients with chronic renal failure. Additionally, we found that BSR could prevent progress of DN by decreasing the expressions of CTGF and increasing the expressions of MMP-9 in rats with DN.16
Based on this, the aim of this study was further to investigate the protective effect of BSR on the kidneys of db/db DN mice and its effects on kidney tissue’s inflammatory cytokines associated with the progression of DN, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), tumor necrosis factor α receptor-1 (TNF-1R), and tumor necrosis factor-α receptor 2 (TNF-2R).
Materials and Methods
Drug Preparation
Astragalus, Rhubarb and Saffron were purchased from Lanzhou Huanghe Pharmaceutical Co., Ltd., and the composition of BSR were as follows: Astragalus 60g, Rhubarb 12g, and Saffron 6g. The above-mentioned three drugs were soaked in pure water for 30min and boiled over high heat until boiling, followed by simmering for 30 minutes. After filtering through four layers of gauze, the BSR was concentrated at a dose of 2.235 g/mL and placed in a refrigerator at −20°C for further use.
Animal Feeding, Grouping, and Medication
Animal maintenance and experimental procedures were carried out in accordance with the US National Institutes of Health guidelines for use of experimental animals and approved by the Animal Care Committee of the Second Hospital of Lanzhou University (Lanzhou, China). One hundred and twenty-five male db/db diabetic mice in C57BL/6 background and their nondiabetic littermate control db/m mice (n = 25) were obtained from Nanjing Model Animal Research Institute. Mice were housed in a room at a constant temperature of 22 ± 2°C with 12-h light/dark cycles.
These 6-week-old male db/db mice and db/m mice were firstly reared adaptively for two weeks, following by randomly divided into five groups of 25 mice per group: control group (distilled water, db/m), model group (distilled water, db/db-m), positive group (irbesartan, 0.05 mg/kg, db/db-me), high (3.3525g/kg of crude drug, db/db-hd), medium (2.235g/kg of crude drug, db/db-md) and low (1.1175g/kg of crude drug, db/db-ld) doses of BSR groups.
Measurement of Body Weight and Fasting Blood Glucose (FBG) Levels
Body weight and FBG levels obtained by blood glucose meters (Sinocare Inc., China) were measured at 0, 4, 8, 12, and 24 weeks.
Measurement of Biochemical Parameters
Five mice from each group were killed at the same point by exsanguination under anesthesia before blood samples were collected, followed by centrifuging blood at 3000 rpm for 10 min at 4°C to obtain serum. Urea nitrogen and creatinine were detected using urea nitrogen test kits and creatinine test kits (Nanjing Jiancheng Bioengineering Research Institute, China), respectively. Microalbuminuria was detected using mouse urine microalbuminuria detection kits (Shanghai Enzyme-linked Biotechnology Co., Ltd., China).
Measurement of Inflammatory Cytokines in Renal Tissue Homogenate by Enzyme-Linked Immunosorbent Assay (ELISA)
Renal tissue was weighed, added with 10 mL of tissue lysate (the ratio of the radio-immunoprecipitation assay [RIPA]: phenylmethylsulfonyl fluoride [PMSF] was 100:1), and ground at 4°C for 30 minutes by electronic tissue homogenizer (Shanghai Jingxin Industrial Development Co., Ltd., China). The tissue homogenate was obtained by centrifuging the suspension at 12,000 rpm for ten minutes (Beckman Coulter, USA, model: Allegra X-30R), followed by detecting the levels of IL-6, TNF-α, TNF-1R, and TNF-2R by corresponding ELISA Kit [Mouse IL-6 (ab100713), mouse TNF-α ELISA Kit (ab208348), mouse sTNF-1R ELISA kit (TNFRSF1A, ab202408), mouse sTNF-2R ELISA kit (TNFRSF1b, ab202412), Abcam, UK], respectively. All procedures were performed strictly according to the instructions.
Histopathological Evaluation
Renal tissue was harvested and fixed in 4% phosphate-buffered paraformaldehyde. After being embedded in paraffin, 3 μm thick sections of renal tissue were prepared and deparaffinized in xylene as well as rehydrated in graded ethanol. Then, haematoxylin and eosin (H&E), PAS and Masson’s trichrome stained samples were separately used for histopathological examination.
Statistical Methods
Data were statistically analyzed using statistical software SPSS25.0. Data with a normal distribution and variance homogeneity were expressed as mean ± standard deviation (x ± SD), compared within one group using one-way analysis of variance, and compared between two groups using t-tests. P < 0.05 was considered statistically significant.
Results
Tolerance of db/db Mice to BSR
The db/db mice were treated with irbesartan or BSR starting at the 8th week of modeling. No mice developed infections or bites resulting in death during the treatment period, indicating that the mice were well tolerated to BSR.
Effects of BSR on Body Weight, Blood Glucose, and UAER in db/db Mice
Change of Body Weight
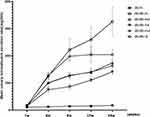
The body weight of the mice in the blank control (db/m) group did not change significantly (P > 0.05) in the feeding process. In sharp contrast, the body weight of the mice in the model group was much higher than that of the control group at 8th, 12th, 16th, 20th, and 32nd weeks of feeding (P < 0.01 for all). No significant difference in body weight could be found between the db/db-me group and the db/db-m group (P > 0.05). Of note, mouse treated with high- and medium doses of BSR performed a lower body weight as compared with mouse in db/db-m group (P < 0.05 for both) at the same time point (P < 0.01 for all). In the db/db-ld group, there was no significant change in body mass before and after medication, and the body mass was the same as that in the db/db-me group at the same time point (P > 0.05 for all, Figure 1). The results revealed that medium and high doses of BSR could reduce the body weight of mice, and the body weight did not significantly change in the positive control group.
Change of Blood Glucose in db/db Mice
The blood glucose level of mice in the db/db-m group was significantly increased as compared with the almost stable blood glucose (P > 0.05) level of the mice in the db/m group during the experiment, which showed statistical difference at the 8th, 12th, 16th, 20th, and 32nd week of feeding (P < 0.01 for all). Intervention with high- and medium doses of BSR could improve the elevated blood glucose levels of mice in db/db-m group (P < 0.05 for both), and the blood glucose levels were lower in these three groups than that of in db/db-me group at the same time point (P < 0.01 for all). In the db/db-ld group, no significant change could be found in blood glucose levels before and after medication, and the blood glucose levels were not statistically significant as compared with the db/db-me group at the same time point (P > 0.05 for all, Figure 2). Additionally, there was no significant difference in blood glucose levels between the db/db-me group and the db/db-m group (P > 0.05). The results revealed that medium and high doses of BSR have the ability to reduce the blood glucose level of mice.
Change of UAER in db/db Mice
Similar to the tendency towards blood glucose of mice, the UAER of the mice in the db/m group also did not change significantly (P > 0.05) in the feeding process. In sharp contrast, the UAER of the mice in the db/db-m group showed significant elevation (P < 0.01) at 12 weeks of feeding as compared with the db/m group. What is of interest was that UAER of the mouse in db/db-me group and three doses of BSR group were remarkably decreased at 4, 8, 12, and 24 weeks of medication (P < 0.01). The UAER in the db-hd group was significantly lower than that of in the db/db-me group (P < 0.01). There was no significant difference in UAER between the db/db-md group and the db/db-me group (P > 0.05, Figure 3).
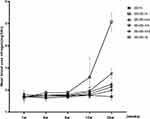
Effects of BSR on the Level of Blood Urea Nitrogen and Serum Creatinine in db/db Mice
The blood urea nitrogen (Figure 4) and serum creatinine (Figure 5) level of the db/m group did not change significantly (P > 0.05) in the feeding process, while a significant elevation could be observed in the db/db-m group at 12 weeks of feeding (P < 0.01). Treating with positive drug and three doses of BSR could remarkably improve (P < 0.01 for all) the elevated levels of both blood urea nitrogen and serum creatinine in db/db mouse. Of note, animals in the db/db-hd group and the db/db-md group showed lower levels of both indicators as compared with that of in db/db-me group (P < 0.01 for all), suggesting the pronounced improving renal function effects of both high- and medium doses of BSR.
Effects of BSR on Histopathological Damage of DN in db/db Mice
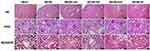
H&E staining was used to analyze the renal histopathological changes of db/db mice before and after intervention with BSR. In the normal control group, the morphology and structure of the renal tissue were normal. Renal histopathological damages including proliferation of glomerular mesangial cells, dilation of the mesangial matrix, thickening of the capillary basement membrane, stenosis of partial capillaries, vacuolar degeneration of the epithelial cells of renal tubules, focal tubular atrophy, and interstitial fibrosis in db/db mouse with DN could be improved at different degree by intervention with positive drug and three doses of BSR. Especially, in animals in db/db-hd group, a series of improvements such as basically normal glomerular volume, unobstructed lumen of most of the capillaries, intact renal tubular epithelium and mild degree of interstitial fibrosis could be observed (Figure 6).
Additionally, PAS staining was performed by blind method to quantify mesangial dilatation, and twenty glomeruli were observed. The degree of mesangial dilatation was scored from 0 to 3, where 0 represented no mesangial dilatation, and 3 represented severe mesangial dilatation. The results revealed that the renal tissues of db/db-md group and db/db-hd group were significantly better than that of db/db-me group in improving renal histopathological damage (Table 1).
 |
Table 1 Effect of BSR on Mesangial Matrix Dilation Score of db/db DN Mice (After 24 Weeks of Treatment) |
Effects of BSR on TNF-α, IL-6, TNF-1R, and TNF-2R in Renal Tissue Homogenate of db/db Mice
In this study, IL-6, TNF-α, TNF-1R, and TNF-2R in renal homogenate were detected by ELISA to further demonstrate the protective effect of BSR on DN. The results revealed that in the db/m group, the levels of IL-6, TNF-α, TNF-1R, and TNF-2R in renal homogenate did not significantly change with the extension of the experiment time (P < 0.05). However, the levels of above-mentioned indicators of animals in db/db-m group exhibited significant elevation as compared with those in the control group at 8th, 12th, 16th, 20th, and 32nd weeks of feeding (corresponding to those at 0, 4, 8, 12, and 24 weeks of medication in the treatment group) (P < 0.01 for all). It is noteworthy that intervention with high- (P < 0.01), medium- (P < 0.05) and low (P < 0.05) doses of BSR could significantly reduce the levels of IL-6, TNF-α, TNF-1R, and TNF-2R, with high dose BSR having the best effect. Additionally, the levels of IL-6, TNF-α, TNF-1R, and TNF-2R in renal homogenate in the db/db-md group were similar to db/db-me group (P > 0.05). These results revealed that medium and high doses of BSR could significantly down-regulate the levels of IL-6, TNF-1R, and TNF-2R in renal tissue (Figure 7, Tables 2–5).
 |
Table 2 Effects of BSR on the Levels of IL-6 in Renal Tissue Homogenate of db/db Mice (pg/mL) |
 |
Table 3 Effects of BSR on the Levels of TNF-α in Renal Tissue Homogenate of db/db Mice (pg/mL) |
 |
Table 4 Effects of BSR on the Levels of TNF-1R in Renal Tissue Homogenate of db/db Mice (pg/mL) |
 |
Table 5 Effects of BSR on the Levels of TNF-2R in Renal Tissue Homogenate of db/db Mice (pg/mL) |
Discussion
With the clinical application of angiotensin-converting enzyme inhibitor (ACEI) and/or an angiotensin II receptor blocker (ARB), great changes have taken place in the treatment of diabetic nephropathy. However, the clinical application of ACEI and/or ARB has failed to reduce the incidence of ESRD in patients with diabetic nephropathy.
What is of great interest is that traditional Chinese herbal medicines with a long history of applications are widely used treatment for chronic kidney disease (CKD) in China and other Asian regions. They show good potential in improving clinical symptoms, controlling CKD proteinuria, and improving renal function. Additionally, the definite efficacies of Chinese herbal medicines on various renal diseases have been further confirmed via randomized case-control trials14–16 in recent years, which provided a beneficial strategy for treatment of various renal diseases such as DN.
In this study, BSR was obtained by compatibility of Astragalus, saffron, and rhubarb in a certain ratio. According to the theories of traditional Chinese medicine (TCM), the deficiency of spleen and kidney is the key to the development of DN, which determines the development and prognosis of this disease. Blood stasis, present throughout the course of diabetes, is not only the pathological product of diabetes, but also further lead to the occurrence of diabetes, which is also the main cause of various complications such as DN of diabetes. The compatibility of the three Chinese herbal medicines perform the effects of invigorating qi and strengthening spleen, activating blood circulation, and removing blood stasis, dampness, and turbidity, which is consistent with the pathogenesis of DN. Additionally, applying BSR on DN is also in accordance with the basic principles of TCM on the syndrome differentiation and treatment of DN.
ACEIs and receptor antagonists are commonly used in the treatment of proteinuria. A study reported that in adults, ACEI or ARB could reduce proteinuria by 30–50%. Current guidelines also recommend these for the diagnosis and treatment of DB.17–19 Therefore, in this study, irbesartan was used as a positive control to evaluate the clinical effect of BSR. This study revealed that the blood glucose level of db/db mice began to increase at the 8th week, but the UAER did not increase significantly. Renal histology revealed that there were no significant changes in mesangial cells and matrix. In the 12th week, the UAER was increased. In addition, renal pathological results also reveal an increase of mesangial cells and dilation of the matrix. These indicated the formation of DN. This is consistent with the early changes of DN in db/db mice reported by Simonson20 at the age of 8–16 weeks. In this study, db/db mice models of DN were treated with low, medium, and high doses of BSR, and the ARB drug irbesartan was used as a positive control. The mice were observed for 24 weeks. The results revealed that the body weight, blood glucose, UAER, blood urea nitrogen, and creatinine levels of db/db mice were significantly decreased in the db/db-ld group, the db/db-md group, and the db/db-hd group. Compared with the db/db-me group, the db/db-hd group and the db/db-md group had obvious advantages in controlling body weight, blood glucose, UAER, blood urea nitrogen, and creatinine levels. Renal pathological results at the same time points also revealed that the db/db-md group and db/db-hd group had significant advantages in the reduction of mesangial matrix proliferation and the degree of interstitial fibrosis. These results indicate that BSR can improve DN, control proteinuria, and improve renal function, and is better than ARB drugs in controlling blood glucose and reducing body weight.
Modern TCM studies revealed that astragalus in the prescription could improve cisplatin-induced acute kidney injury (AKI)21 and enhance LPS-induced NO production in macrophages of patients with renal failure.22 Astragalus polysaccharide, an active component of astragalus, can significantly improve the renal pathology and renal fibrosis index of streptozotocin-induced DN.23 Rhubarb in the prescription has a variety of active components.24 One of these components, emodin, can reduce 24-hour proteinuria and improve renal pathology in diabetic rats, inhibit the p38 mitogen-activated protein kinase (MAPK) pathway, decrease the expression of fibronectin in renal tissue of DN rats, and improve the proliferation of glomerular basement membrane. It can prevent the synthesis of extracellular matrix and down-regulated expressions of TNF-α, IL-6, and Toll-like receptor 4 in renal tissue.25–27 Another component, rhein, can down-regulate the expression of oxidative stress products, relieve inflammation in the kidney, reduce the synthesis of TXB2, and improve renal hemodynamics through anticoagulation, playing a role in renal protection.24 Rhein can reduce the expression of TNF-α and IL-1 β in renal tissue of sepsis rats, inhibit NF-kB pathway activity,28 prevent and treat AKI, and reduce the levels of TGF-β and α-smooth muscle actin in renal tissue of rats with obstructive nephropathy and DN.29,30 Chrysophanol can improve renal interstitial fibrosis by inhibiting the TGF-β/Smad signaling pathway, reducing the formation of reactive oxygen-free radicals.31 Saffron in the prescription has anti-inflammatory, antioxidant, and insulin-sensitizing effects.32 Milajerdi et al33 conducted a randomized, three-blinded, controlled study on 54 patients with T2DM. The result revealed that saffron could down-regulate blood glucose and HbA1c. It has been found that saffron extract contains a variety of active components. Among these, β-carotene, with its many biological properties, can reduce the apoptosis of islet β cells by eliminating free radicals, inhibiting oxidative stress, and inhibiting the expression of p53, protecting islet beta cells and improving islet function. Saffron extracts can also inhibit the plasma creatinine concentration, malondialdehyde level, TNF-α, ICAM-1 expression, and leukocyte infiltration of ischemia-reperfusion AKI models.34,35 Based on the above studies, BSR, the prescription in this study, may play a renal protective role in DN by improving islet function, lowering blood glucose levels, inhibiting oxidative stress, improving blood coagulation, and inhibiting inflammation and fibrosis.
Activation of the innate immune system and chronic low-grade inflammation are important components of the pathogenesis of T2DM. Improving oxidative stress is one of the main therapeutic targets of DN. The serum level of IL-6 in patients with DN is significantly increased and significantly correlated with albuminuria excretion and glomerular basement membrane thickening.36 TNF-α is a pleiotropic cytokine that can activate a variety of downstream molecules related to vascular endothelial dysfunction, such as ICAM-1, VCAM-1, PAI-1, and systemic inflammatory markers such as IL-6 and C-reactive protein. The level of circulating markers of the TNF pathway is closely related to the risk of UAER, impaired renal function, and cardiovascular death.37,38 A cohort study conducted observations for 8–12 years of 410 patients with T2DM. The result revealed that 59 patients developed ESRF and the baseline TNF-1R and TNF-2R levels were closely related to the risk of ESRF.39 In this study, IL-6, TNF-α, TNF1R, and TNF2R in renal tissue homogenate of db/db DN model mice were detected by ELISA to further clarify the anti-inflammatory effect in the treatment of DN. The levels of these factors in renal tissue homogenate were monitored in this study. The results revealed that at 12 weeks of age, TNF1R, TNF2R, and IL-6 in renal tissue homogenate of db/db mice in the db/db-m group were increased significantly. After the mice were treated with BSR, the levels of IL-6, TNF1R, and TNF2R in renal tissue were significantly decreased, and the corresponding pathological findings of renal tissue revealed that the proliferation of mesangial cells and matrix decreased. These results indicate that BSR may delay the progression of DN by inhibiting inflammation.
Although our data showed that BSR could delay the pathology and reduce the expression of inflammatory in mice with DN, in-depth mechanisms such as the potential signal pathway, target and molecular mechanism of BSR on protection the renal damage induced by DN still need to be further explored.
Conclusion
In summary, BSR, a traditional Chinese medicine prescription, could effectively delay the pathological damage induced by DN, mainly manifested as reducing the levels of a series of indicators including blood glucose, body weight, urinary albumin, blood urea nitrogen, creatinine and improving renal pathology. Additionally, the down-regulation effects of BSR on renal levels of IL-6, TNF-α, TNF-1R, and TNF-2R indicated that BSR may perform nephroprotective effects partly through anti-inflammation mechanism. This study not only demonstrates the protective effect of BSR on DN from the perspective of anti-inflammation, but also provides a theoretical basis for the development of traditional Chinese medicine herb drug therapy for DN.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study was conducted with approval from the Ethics Committee of Lanzhou University Second Hospital.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
Lanzhou Science and Technology Bureau talent innovation project (2014-RC-64).
Disclosure
The authors declare that they have no competing interests.
References
1. Ansell D, Feehally J, Feest TG, et al. UK Renal Registry Report. Bristol, UK: UK Renal Registry; 2008:49–74.
2. Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346(15):1145–1151. doi:10.1056/NEJMcp011773
3. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi:10.1016/j.diabres.2009.10.007
4. Altemtam N, Russe J, El Nahas M. A study of the natural history of diabetic kidney disease (DKD). Nephrol Dial Transplant. 2012;27(5):1847–1854. doi:10.1093/ndt/gfr561
5. Domingueti CP, Dusse LMS, Carvalho MG, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–7454. doi:10.1016/j.jdiacomp.2015.12.018
6. Yaribeygi H, Atkin SL, Sahebkar A. A review of the molecular mechanisms of hyperglycemia‐induced free radical generation leading to oxidative stress. J Cell Physiol. 2018;234(2):
7. Song P, Sun C, Jinbo L, et al. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J Sci Food Agric. 2021;101(4):1598–1608. doi:10.1002/jsfa.10779
8. Ziyuan N, Guo L, Liu F, et al. Allium tuberosum alleviates diabetic nephropathy by suppressing hyperglycemia-induced oxidative stress and inflammation in high fat diet/streptozotocin treated rats. Biomed Pharmacother. 2019;112:108678. doi:10.1016/j.biopha.2019.108678
9. Olatunji OJ, Chen H, Zhou Y. Lycium chinense leaves extract ameliorates diabetic nephropathy by suppressing hyperglycemia mediated renal oxidative stress and inflammation. Biomed Pharmacother. 2018;102:1145–1151. doi:10.1016/j.biopha.2018.03.037
10. Chen Y, Deng Y, Ni Z, et al. Efficacy and safety of traditional Chinese medicine (Shenqi particle) for patients with idiopathic membranous nephropathy: a multicenter randomized controlled clinical trial. Am J Kidney Dis. 2013;62(6):1068–1076. doi:10.1053/j.ajkd.2013.05.005
11. Zou C, Lu ZY, Wu YC, et al. Colon may provide new therapeutic targets for treatment of chronic kidney disease with Chinese medicine. Chin J Integr Med. 2013;19(2):86–91. doi:10.1007/s11655-013-1351-8
12. Huang KC, Su YC, Sun MF, et al. Chinese herbal medicine improves the long-term survival rate of patients with chronic kidney disease in Taiwan: a nationwide retrospective population-based cohort study. Front Pharmacol. 2018;9:1117. doi:10.3389/fphar.2018.01117
13. Liu W, Wang J, Wang X, et al. Effects of shenbao recipe on expressions of CTGF and MMP-9 in diabetic nephropathy rats. Zhongguo Zhong Yao Za Zhi. 2010;35(14):1874–1877.
14. Zhang Z, Zhang L, Hansong X. Effect of Astragalus polysaccharide in treatment of diabetes mellitus: a narrative review. J Tradit Chin Med. 2019;39(1):133–138.
15. Azimi P, Ghiasvand R, Feizi A, et al. Effects of cinnamon, cardamom, saffron, and ginger consumption on markers of glycemic control, lipid profile, oxidative stress, and inflammation in Type 2 diabetes patients. Rev Diabet Stud. 2014;11(3–4):258–266. doi:10.1900/RDS.2014.11.258
16. Régnier M, Rastelli M, Morissette A, et al. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansia muciniphila in mice. Nutrients. 2020;12(10):2932. doi:10.3390/nu12102932
17. Lewis EJ, Hunsicker LG, Bain RP, et al.; The Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–1462. doi:10.1056/NEJM199311113292004.
18. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi:10.1056/NEJMoa011161
19. Doshi SM, Friedman AN. Diagnosis and management of Type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12(8):1366–1373. doi:10.2215/CJN.11111016
20. Simonson MS, Tiktin M, Debanne SM, et al. The renal transcriptome of db/db mice identifies putative urinary biomarker proteins in patients with type 2 diabetes: a pilot study. Am J Physiol Renal Physiol. 2012;302(7):F820–9. doi:10.1152/ajprenal.00424.2011
21. Hui D, Rui-Zhi T, Jian-Chun L, et al. Astragalus propinquus Schischkin and Panax notoginseng (A&P) compound relieved cisplatin-induced acute kidney injury through inhibiting the mincle maintained macrophage inflammation. J Ethnopharmacol. 2020;252:112637. doi:10.1016/j.jep.2020.112637
22. Liao H, Hu L, Cheng XN, et al. Are the therapeutic effects of Huangqi (Astragalus membranaceus) on diabetic nephropathy correlated with its regulation of macrophage iNOS activity? J Immunol Res. 2017;2017:3780572.
23. wang ZS, Xiong F, Xie XH, et al. Astragaloside IV attenuates proteinuria in streptozotocin-induced diabetic nephropathy via the inhibition of endoplasmic reticulum stress. BMC Nephrol. 2015;16(1):44. doi:10.1186/s12882-015-0031-7
24. Cao YJ, Pu ZJ, Tang YP, et al. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin Med. 2017;12(1):36. doi:10.1186/s13020-017-0158-5
25. Dou F, Liu YT, Liu LM, et al. Aloe-emodin ameliorates renal fibrosis via inhibiting PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Rejuvenation Res. 2019;22(3):218–229. doi:10.1089/rej.2018.2104
26. Wang JP, Huang HQ, Liu PQ, et al. Inhibition of phosphorylation of p38 MAPK involved in the protection of nephropathy by emodin in diabetic rats. Eur J Pharmacol. 2006;553(1–3):297–303. doi:10.1016/j.ejphar.2006.08.087
27. Zhu XL, Wang YJ, Yang YZ, et al. Suppression of lipopolysaccharide-induced upregulation of toll-like receptor 4 by emodin in mouse proximal tubular epithelial cells. Mol Med Rep. 2012;6(3):493–500. doi:10.3892/mmr.2012.960
28. Yu C, Qi D, Sun JF, et al. Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci Rep. 2015;5(1):11822. doi:10.1038/srep11822
29. He D, Lee L, Yang J, et al. Preventive effects and mechanisms of rhein on renal interstitial fibrosis in obstructive nephropathy. Biol Pharm Bull. 2011;34(8):1219–1226. doi:10.1248/bpb.34.1219
30. Gao Q, Qin WS, Jia ZH, et al. Rhein improves renal lesion and ameliorates dyslipidemia in db/db mice with diabetic nephropathy. Planta Med. 2010;76(1):27–33. doi:10.1055/s-0029-1185948
31. Dou F, Ding Y, Wang C, et al. Chrysophanol ameliorates renal interstitial fibrosis by inhibiting the TGF-β/Smad signaling pathway. Biochem Pharmacol. 2020;180:114079. doi:10.1016/j.bcp.2020.114079
32. Yaribeygi H, Zare V, Butler AE, et al. Antidiabetic potential of saffron and its active constituents. J Cell Physiol. 2019;4(6):8610–8617. doi:10.1002/jcp.27843
33. Milajerdi A, Jazayeri S, Hashemzadeh N, et al. The effect of saffron (Crocus sativus L.) hydroalcoholic extract on metabolic control in type 2 diabetes mellitus: a triple‐blinded randomized clinical trial. J Res Med Sci. 2018;23(1):16. doi:10.4103/jrms.JRMS_286_17
34. Mahmoudzadeh L, Najafi H, Ashtiyani SC, et al. Anti-inflammatory and protective effects of saffron extract in ischaemia-reperfusion induced acute kidney injury. Nephrology. 2017;22(10):748–754. doi:10.1111/nep.12849
35. Rameshrad M, Razavi BM, Hosseinzadeh H. Saffron and its derivatives, crocin, crocetin and safranal: a patent review. Expert Opin Ther Pat. 2018;28(2):147–165. doi:10.1080/13543776.2017.1355909
36. Satirapoj B. Tubulointerstitial biomarkers for diabetic nephropathy. J Diabetes Res. 2018;2018:2852398. doi:10.1155/2018/2852398
37. Ernandez T, Mayadas TN. Immunoregulatory role of TNF-alpha in inflammatory kidney diseases. Kidney Int. 2009;76(3):262–276. doi:10.1038/ki.2009.142
38. Navarro JF, Mora-Fernández C. The role of TNF-alpha in diabetic nephropathy: pathogenic and therapeutic implications. Cytokine Growth Factor Rev. 2006;17(6):441–450. doi:10.1016/j.cytogfr.2006.09.011
39. Niewczas MA, Gohda T, Skupien J, et al. Circulating TNF receptors 1 and 2 predict ESRD in Type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507–515. doi:10.1681/ASN.2011060627
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.