Back to Journals » Cancer Management and Research » Volume 12
Astragalus Polysaccharide Promotes Adriamycin-Induced Apoptosis in Gastric Cancer Cells
Authors Song J , Chen Y, He D, Tan W, Lv F, Liang B, Xia T, Li J
Received 3 November 2019
Accepted for publication 10 March 2020
Published 1 April 2020 Volume 2020:12 Pages 2405—2414
DOI https://doi.org/10.2147/CMAR.S237146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Jie Song,1,* Youming Chen,2,* Donghong He,1,* Wenhui Tan,1 Fang Lv,1 Biao Liang,1 Tingting Xia,3 Jing Li1,4
1Center of Digestive Endoscopy, Guangdong Second Provincial General Hospital, Guangzhou, People’s Republic of China; 2Department of Infectious Diseases, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China; 3Center for Reproductive Medicine, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China; 4Guangdong-Hongkong-Macau Institute of CNS Regeneration, Ministry of Education CNS Regeneration Collaborative Joint Laboratory, Jinan University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Li; Tingting Xia
Email [email protected]; [email protected]
Purpose: Astragalus polysaccharide (APS), a common Chinese herbal compound extracted from Astragalus membranaceus, has been proposed to increase the tumour response of and stabilize chemotherapy drugs while reducing their toxicity. Here, we examined the effects of APS on apoptosis in gastric cancer (GC) cells in the presence or absence of adriamycin (0.1 μg/mL).
Methods: GC cells cultured in the presence or absence of adriamycin (0.1 μg/mL) were administered APS (50– 200 μg/mL) for 24– 72 h and subjected to an MTT assay to examine cell viability. Active caspase-3 expression and DNA fragmentation were assessed to evaluate apoptosis, and real-time PCR was used to analyse the expression levels of multidrug resistance (MDR1) genes and tumour suppressor genes. Western blot analysis was applied to detect cleaved caspase-3 and phosphorylated AMPK (p-AMPK).
Results: Cellular viability was profoundly reduced by APS, and GC cell apoptosis was strongly increased by APS in a time- and dose-dependent manner; these changes may be linked to an increase in p-AMPK levels because the AMPK inhibitor compound C blocked the effects of APS. Similarly, adriamycin-induced decreases in cellular viability and apoptosis of GC cells were enhanced by APS administration. The expression of tumour suppressor genes (SEMA3F, P21WAF1/CIP1, FBXW7), but not of MDR1, was increased by APS compared to the control, and p-AMPK levels were lower in adriamycin-resistant GC cells than in either adriamycin-sensitive GC cells or an immortalized human gastric epithelial cell line.
Conclusion: APS induces apoptosis independently and strengthens the proapoptotic effect of adriamycin on GC cells, suggesting that APS may act as a chemotherapeutic sensitizer.
Keywords: Astragalus polysaccharide, gastric cancer, apoptosis, chemotherapy
Introduction
As one of the most common cancers worldwide, gastric cancer (GC) is the second leading cause of cancer-related death.1 Although the overall incidence of GC has been decreasing in North America and Europe, the prevalence of proximal GC has increased remarkably,2 and GC is the cancer with the highest incidence in East Asia.3 Despite the rapid progress in the diagnosis and treatment of this disease, the 5-year survival rate of GC patients is still low.4 Because few symptoms of early GC can be readily observed, many clinical patients present with advanced stage GC at initial diagnosis, which disqualifies them for surgical resection of early stage GC—this procedure is crucial for effectively treating GC.5 Cancer chemoresistance has become a key obstacle in tumour treatment; thus, conquering chemoresistance and enhancing the sensitivity of tumours to chemotherapeutic drugs via chemosensitizers are urgently needed.
Astragalus membranaceus, a popular herbal compound, has long been used in traditional Chinese and Western medicine.6 In China, medicinal herbs have been commonly combined with chemotherapy to treat non-small-cell lung cancer, enhance the cancer response and/or reduce adverse reactions to cytotoxic antineoplastic drugs.7 Currently, A. membranaceus is frequently administered as an immunomodulating agent in mixed herbal decoctions to address influenza, fatigue, anorexia, and diarrhoea and has also been used to treat patients with cardiac diseases.8,9 Researchers have added A. membranaceus to platinum-based chemotherapies to reduce chemotherapeutic toxicity, improve the stabilization and performance status of the drugs, and increase the tumour response.10,11
Astragalus polysaccharide (APS), a compound extracted from the radix of A. membranaceus, has been widely studied due to its immunopotentiation activities, such as enhancing cytokine production and B cell proliferation.12 APS may enhance host immune function by potentiating natural killer cell and macrophage activity. Moreover, clinical trials indicated that APS could improve the immune recognition ability of lung cancer cells by reducing the level of T-helper cell type 2 cytokines.13 An increasing number of studies have shown that APS may exhibit antitumorigenic potential. For example, a previous report indicated that the aqueous fraction of Astragalus could prevent rat hepatocarcinogenesis, and the key component was APS.14 Other studies have demonstrated that APS may restore T cell function, which is suppressed in carcinomatosis patients.15 A series of case studies indicated that Astragalus could be safely combined with chemotherapy,16,17 and some clinical studies have reported that APS could enhance the curative effect of chemotherapy in non-small-cell lung cancer patients15,16 while reducing associated side effects.18
As a highly conserved serine/threonine protein kinase, AMP-activated protein kinase (AMPK) is a central key regulator of energy and cellular metabolism involving glucose, lipids, and proteins.19 AMPK is also a heterotrimeric enzyme comprising two noncatalytic b and c subunits and a catalytic subunit. AMPK activation requires the phosphorylation of threonine 172 in the catalytic subunit.20 AMPK is activated in energy metabolism by regulating gene transcription and changing cellular metabolism and ultimately promotes ATP conservation. As a pharmacological target, owing to its remarkable effect in treating metabolic disorders, including insulin resistance, type II diabetes, and obesity, the AMPK pathway has attracted widespread attention.21 Several other reports have indicated that AMPKα expression decreases during the early stage of GC, and GC patients with positive AMPK expression tended to have a favourable prognosis.4,22 In addition, etoposide-induced apoptosis was accelerated by AMPK activation in both lung and prostate cancer.23
Previous studies have reported that APS activates AMPK;6,24 however, it remains unknown whether APS can increase the sensitivity of cells to chemotherapeutic drugs and cause apoptosis in GC cells. To answer this question, we cultured the human gastric carcinoma cell line SGC-7901 with multiple concentrations of APS for various durations in the presence or absence of adriamycin, the most commonly used chemotherapeutic drug for treating gastric carcinoma.
Materials and Methods
Cell Lines and Cell Culture
APS was purchased from Tianjin Cinorch Pharmaceutical Co., Ltd. (C832678; China). SGC-7901 cells (ATCC®CRL-1739™; USA), human gastric epithelial cells (GES-1, ATCC®CRL-7407; USA) and the adriamycin-resistant GC cell line SGC-7901/ADR (ATCC®TCP-1008™; USA) were purchased from the American Type Culture Collection. SGC-7901 and SGC-7901/ADR cells were used in cell viability tests, quantitative real-time PCR, caspase-3 activity assays, qualitative DNA fragmentation and apoptotic tests, respectively, whereas all three cell lines were used for Western blot analysis. Adriamycin was purchased from Sigma-Aldrich (D1515; USA). Compound C was purchased from Tocris Bioscience (3093/10, UK). All cells were cultured in complete DMEM supplemented with streptomycin (100 U/mL), 10% foetal bovine serum, and penicillin (100 U/mL) and incubated at 37°C in a humidified atmosphere with 5% CO2. For all experiments, the cells were seeded in 6-well plates at a density of 1×106 cells/well in 2 mL of media and then treated with APS (100–400 μg/mL) in the presence or absence of adriamycin (0.1 µg/mL)25 for 24–72 h.
Cell Viability Assay
Cells were seeded into 96-well plates at a density of 4 × 104 cells per well and incubated overnight. Cellular viability was determined by MTT assays (Cell Signaling Technologies, USA). Cells were treated with APS (100–400 μg/mL) in the presence or absence of adriamycin (0.1 µg/mL)25 for 24, 48 or 72 h. Then, 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) from a 5 mg/mL solution was added to each well and cultured for 4 h. Next, the purple formazan crystals were dissolved in DMSO for 10 min, and the absorbance at 490 nm in each well was measured with a spectrophotometer.
Apoptosis Assay
A Cellular DNA Fragmentation ELISA Kit (11585045001; Roche Applied Science, CH) was used to measure cytoplasmic histone-associated DNA fragments, which reflect the percentage of apoptotic cells.26 This assay is based on the quantitative detection of BrdU-labelled DNA fragments. Using camptothecin (50 μM, 24 h) as a positive control, the optimal apoptotic response was detected after administration. These methods were then applied to evaluate the proportion of apoptotic cells after treatment with APS in the presence or absence of adriamycin (0.1 µg/mL) for 24 and 48 h. DNA fragmentation was determined according to the manufacturer’s instructions. Absorbance values at 450 nm were measured with an ELx808 ELISA reader (BioTek).
Caspase-3 Activity Assay
A caspase-3 assay kit purchased from Sigma-Aldrich (235419; USA) was used to detect caspase-3 activity in cells. Cells cultured in the presence or absence of APS were collected via extraction buffer and incubated on ice for 25 min, and the resulting lysates were centrifuged at 12,000 rpm for 10 min. DEVD-AFC and sample buffer were added to the reaction mixture following the instructions. Then, the samples were incubated at 37°C for 2 h, and the caspase-3 activity was examined with a fluorometric plate reader at a wavelength of 494 nm.
Western Blot Analysis
PIPA lysis buffer (Biouniquer Technology, China) was used to extract proteins from cells according to the manufacturer’s instructions. A NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) was used to quantify the protein content. PIPA lysis buffer alone was used as a control, and absorbance at 280 nm was recorded. Proteins were separated via sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis through 8% gels and then transferred to polyvinylidene difluoride membranes. Next, the membranes were blocked in 5% skim milk for 2 h and incubated with anti-p-AMPK (SAB4503754; Sigma-Aldrich, USA; 1:500), anti-AMPK (SAB4502329; Sigma-Aldrich, USA; 1:500), anti-β-actin (A1978; Sigma-Aldrich, USA; 1:1000) and anti-cleaved caspase-3 (MFCD01321906; Sigma-Aldrich, USA; 1:500) antibodies at 4°C overnight, followed by incubation with peroxidase-conjugated anti-mouse IgG (whole molecule) secondary antibody (A9044; Sigma-Aldrich, USA; 1:20,000) for 2 h at room temperature. An enhanced chemiluminescence (ECL) plus kit (Millipore, America) was applied to visualize the protein bands, which were quantitatively analysed with ImageJ (version 1.8.0).
Quantitative Real-Time PCR
To extract total RNA, we used TRIzol in line with the manufacturer’s instructions. A transcription first-strand cDNA synthesis kit (Shiga, Japan) was used to generate cDNA. Primers specific for the WD repeat domain containing 7 (FBXW7), P21WAF1/CIP1, semaphorin III/F (SEMA3F) and multidrug resistance 1 (MDR1) were applied for PCR amplification.27–29 The reverse transcription reaction protocol was performed using three sequential incubations of 6 min at 23°C, 25 min at 41°C and 5 min at 85°C. The thermocycling conditions were as follows: denaturation at 60°C for 30 s and 95°C for 5 s, followed by 40 cycles. Primers were designed in Primer Bank, and their sequences were as follows: FBXW7, Forward Primer 5’-GGCCAAAATGATTCCCAGCAA-3ʹ and Reverse Primer 5′-ACTGGAGTTCGTGACACTGTTA-3′; p21Cip1, Forward Primer 5′-TGTCCGTCAGAACCCATGC-3′ and Reverse Primer 5′-AAAGTCGAAGTTCCATCGCTC-3′; SEMA3F, Forward Primer 5′-AACACAACCGACTACCGAATC-3′ and Reverse Primer: GGCTGCCCAGTGTATAATGAG-3′; and Mdr1, Forward Primer 5′- TTGCTGCTTACATTCAGGTTTCA-3′ and Reverse Primer 5′- AGCCTATCTCCTGTCGCATTA-3ʹ. β-Actin mRNA expression served as a control. To determine the relative levels of specific mRNAs, we used a Thermo PIKOREAL 96 real-time PCR detection system with Qiagen SYBR Green Supermix (Valencia, USA) according to the manufacturer’s instructions. The results were normalized to the corresponding amounts of β-actin. The relative mRNA expression levels were calculated using the comparative quantification cycle 2−ΔΔcq method.27
Qualitative DNA Fragmentation
Cells were suspended in 100 μL of lysis buffer containing 10 mM NaCl, 10 mM EDTA, 10 mM Tris-HCl, and 2% SDS; incubated with 50 μg/mL proteinase K at 4°C for 30 min; and then centrifuged at 12,000 rpm for 30 min. The soluble DNA in the resulting supernatant was precipitated with ethanol at −20°C; after centrifugation, the DNA pellet was rinsed with 70% ethanol and dissolved in sterile ddH2O. Next, 5 µg of genomic DNA was run on a 1.5% ethidium bromide-treated agarose gel at 80 volts (Power Supply Biorad, Model 200/2.0) and visualized under a UV transilluminator (Stratagene, USA) to observe nuclear DNA fragmentation (ladder), which is characteristic of apoptosis.
Statistical Analysis
GraphPad Prism 7 software was used for statistical analyses. All the data represent the means ± standard deviation (SD), and the results were analysed using one-way analysis of variance (ANOVA) with Tukey’s multiple comparison tests. p<0.05 was regarded as statistically significant.
Results
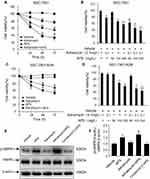
Effect of APS on the Viability of SGC-7901 and SGC-7901/ADR Cells
We investigated the effect of APS and/or adriamycin on the viability of SGC-7901 or SGC-7901/ADR cells by MTT assays. We incubated the cells with adriamycin, APS, or both for 24 h, 48 h, and 72 h. Cellular viability was reduced by either adriamycin (0.1 µg/mL) or APS (100 µg/mL) in a time-dependent manner (Figure 1A and C). Similarly, cellular viability was significantly reduced by APS (50–200 µg/mL) in a concentration-dependent manner (Figure 1B and D). Moreover, the ability of adriamycin to reduce cellular viability was significantly improved by APS (Figure 1A–D). These effects of APS may be due to increased p-AMPK levels because the observed effects were abrogated by treatment with the AMPK inhibitor compound C (Figure 1E and F). Taken together, our data showed that APS decreases cellular viability, and, more importantly, APS strengthens the sensitivity of cells to adriamycin to induce cell death via the AMPK pathway.
Effect of APS in the Presence or Absence of Adriamycin on Apoptosis in SGC-7901 Cells and SGC-7901/ADR Cells
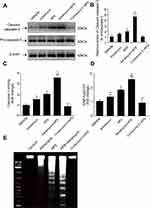
We employed several methods to detect APS-induced apoptosis in SGC-7901 cells and SGC-7901/ADR cells. First, the apoptosis rate of cells treated with APS was examined by a photometric ELISA and compared to that of cells treated with camptothecin (50 μM, assuming 100% apoptotic cells). The results indicated that APS alone led to an increased rate of apoptotic GC cells in a time- and concentration-dependent manner, APS administration enhanced adriamycin-mediated apoptosis (Figure 2). Then, GC cells treated with APS (200 µg/mL) for 24 h in the presence or absence of adriamycin were analysed for cleaved caspase-3 levels by Western blotting, with each lane containing the same quantity of purified protein. Western blotting showed that either adriamycin or APS increased the levels of cleaved caspase-3 in GC cells. Furthermore, the effect was enhanced when APS was combined with adriamycin, but the effect of APS was inhibited by the AMPK inhibitor compound C (Figure 3A and B). To further confirm these results, we performed caspase-3 expression and DNA fragmentation analyses (Figure 3C–E), both of which confirmed the proapoptotic effect of APS. In conclusion, our findings demonstrated that APS not only induces apoptosis but also improves the sensitivity of GC cells to adriamycin.
Effect of APS on the Expression of MDR1 and Tumour Suppressor Genes in Gastric Cancer Cells
The ability of APS to both induce apoptosis and increase the sensitivity of GC cells to adriamycin prompted us to investigate the possible underlying mechanism. After cells were cultured in the presence or absence of APS (100 µg/mL) for 24 h, the expression of tumour suppressor genes, such as SEMA3F, P21WAF1/CIP1, and FBXW7, was determined by real-time PCR. The results indicated that the genes above exhibited significantly increased expression compared to that in the vehicle group. Additionally, we examined the expression of the MDR1 gene, which was reduced upon APS treatment (Figure 4A). Altogether, these results suggest that regulating tumour suppressor genes plays a pivotal role in the proapoptotic effect of APS.
p-AMPK Levels Were Reduced in Adriamycin-Resistant SGC-7901/ADR Cells
The expression of MDR1 was reduced by APS treatment (Figure 4A), which might contribute to APS-induced apoptosis. However, it is unclear whether AMPK activation is altered in adriamycin-resistant and/or adriamycin-sensitive GC cells. We investigated p-AMPK levels in adriamycin-sensitive GC SGC-7901 cells, adriamycin-resistant GC SGC-7901/ADR cells, and normal gastric epithelial cells (GES-1). The Western blot results revealed that the p-AMPK levels were significantly decreased in SGC-7901/ADR cells compared to GES-1 and SGC-7901 cells (Figure 4B and C). This decrease suggests that chemoresistance to adriamycin is related to reductions in AMPK activity.
Discussion
In the present study, we investigated whether APS affects cellular viability and apoptosis in human GC cells. The results indicated that APS indeed decreases cellular viability and induces apoptosis in a time- and dose-dependent manner. Consistent with our findings, a previous study reported the proapoptotic effects of AMPK activation in many cancer cells, including GC cells.19 In particular, we present the first evidence that APS administration can augment the ability of adriamycin to reduce the cellular viability of and induce apoptosis in GC cells. Data from the current study suggested that APS is a potentially useful chemosensitizer in adriamycin-based chemotherapy for GC. Apoptosis, also known as programmed cell death, is a major physiological mechanism for self-induced cell destruction. This process plays an important role in renewing normal cells, eliminating abnormal cells and maintaining homeostasis in multicellular organisms. Caspase-3, a member of the caspase family of proteases, is an important contributing factor for apoptosis due to its capacity to cleave most of the caspase substrates in the apoptotic pathway. As caspase-3 is activated in response to proapoptotic stimuli, its activity is crucial for inducing apoptosis. Once DNA damage is beyond the capacity of DNA repair, caspase-3 plays an important role in the induction of apoptosis. In this work, caspase-3 and DNA fragmentation assays were used to detect apoptosis, and the results indicated that APS treatment induced apoptosis in human GC cells. This change probably contributes to the decrease in cell viability observed upon APS administration. The underlying mechanism of the proapoptotic function of APS is related to the increased expression of several genes, including SEMA3F, the tumour repressor P21WAF1/CIP1 and FBXW7. AMPK phosphorylates nuclear receptor RAR-related orphan receptor a (RORa), a potential tumour suppressor that is mainly studied in breast cancer.23 This work showed that RORa phosphorylation may be an important mechanism involved in AMPK-mediated regulation of the SEMA3F, MDR1, FBXW7, and P21WAF1/CIP1 genes. However, few studies to date have elucidated the pathway (intrinsic or exogenous) by which APS induces apoptosis in GC cells. In addition, whether APS exerts proapoptotic effects in GC in vivo has not been described previously.
Notably, some proteins, such as Topo II, p-glycoprotein (encoded by the MDR1 gene) and GST-pi protein, are involved in the chemosensitivity of GC.26 Likewise, these findings are consistent with previous studies showing that chemotherapeutic sensitivity is enhanced by both rubimaillin and puerarin, which inhibit the expression of the mdr1 gene in breast cancer via the AMPK pathway.30,31 Analogously, APS promotes glucose uptake in L6 myotubes through AMPK activation and, consequently, AS160/TBC1D4 phosphorylation.24 However, it remains unclear whether APS regulates chemotherapeutic sensitivity of GC to adriamycin via the AMPK pathway. As a result, the purpose of this study was to examine the effects of APS on chemotherapeutic sensitivity of GC to adriamycin and reveal the underlying mechanism. Our results suggested that APS treatment enhanced adriamycin-induced apoptosis and decreased the viability of GC cells. Moreover, the levels of p-AMPK were decreased in adriamycin-resistant GC SGC-7901/ADR cells compared to normal human gastric epithelial cells and adriamycin-sensitive SGC-7901 cells. The findings demonstrated that AMPK activation reduces chemoresistance, while APS enhances chemosensitivity to adriamycin by reducing MDR1 gene expression. Further studies are needed to determine whether APS can enhance the chemosensitivity of GC cells to other chemotherapeutic agents and to clarify how APS affects SGC-7901 cells. HPLC analysis is also needed to determine the major effective components.
Previous research has shown that these bioactivities are related to the chemical structures of APS, which have been identified as acidic heteropolysaccharides mainly composed of glucose, galactose, arabinose, rhamnose, mannose, xylose, fucose, fructose, ribose, glucuronic acid, and galacturonic acid, with molecular weights in the range of 8.7×103–4.8×107 g mol−1. 32–34 This wide variety of compounds can enter the cell or act on receptors on the cell surface in different ways. However, few studies on the molecular-cellular relationship of APS have been undertaken. At present, we are further studying the mechanism by which these molecules play a role in cells. We hope to overcome this problem in the future.
Collectively, our results demonstrate that APS promotes apoptosis and decreases the viability of GC cells via the AMPK pathway, either independently or with adriamycin. This study provides a basis of targeting AMPK in combination with APS to improve patient outcome in GC.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 81370535, 81672701), the Science and Technology Planning Project of Guangdong Province, China (No. 20160909, 411308023039 and 2016A020215221), the Research and Development Project of Applied Science and Technology of Guangdong Province, China (No. 2016B020237004), the Medical Scientific Research Foundation of Guangdong Province (No. A2016435), the Guangzhou Science and Technology Project (1561000155), and the Doctoral workstation foundation of Guangdong Second Provincial General hospital (2019BSGZ011). The abstract for this paper was published online as part of a conference presentation at the following link: https://gut.bmj.com/content/68/Suppl_1/A25.3.
Author Contributions
All authors contributed to data analysis, drafted and revised the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209.
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi:10.1002/ijc.29210
3. Sankaranarayanan R, Ramadas K, Qiao YL. Managing the changing burden of cancer in Asia. BMC Med. 2014;12. doi:10.1186/1741-7015-12-3
4. Kim YH, Liang H, Liu X, et al. AMPKalpha modulation in cancer progression: multilayer integrative analysis of the whole transcriptome in Asian gastric cancer. Cancer Res. 2012;72:2512–2521. doi:10.1158/0008-5472.CAN-11-3870
5. Yamashita K, Sakuramoto S, Nemoto M, et al. Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol. 2011;17:3390–3397. doi:10.3748/wjg.v17.i29.3390
6. Wang N, Zhang DL, Mao XQ, Zou F, Jin H, Ouyang JP. Astragalus polysaccharides decreased the expression of PTP1B through relieving ER stress induced activation of ATF6 in a rat model of type 2 diabetes. Mol Cell Endocrinol. 2009;307:89–98. doi:10.1016/j.mce.2009.03.001
7. Wu P, Dugoua JJ, Eyawo O, Mills EJ. Traditional Chinese Medicines in the treatment of hepatocellular cancers: a systematic review and meta-analysis. J Exp Clin Cancer Res. 2009;28:112. doi:10.1186/1756-9966-28-112
8. Liu QY, Yao YM, Yu Y, Dong N, Sheng ZY. Astragalus polysaccharides attenuate postburn sepsis via inhibiting negative immunoregulation of CD4+ CD25(high) T cells. PLoS One. 2011;6:e19811. doi:10.1371/journal.pone.0019811
9. Chen W, Li YM, Yu MH. Astragalus polysaccharides inhibited diabetic cardiomyopathy in hamsters depending on suppression of heart chymase activation. J Diabetes Complications. 2010;24:199–208. doi:10.1016/j.jdiacomp.2008.12.003
10. Ben-Arye E, Attias S, Tadmor T, Schiff E. Herbs in hemato-oncological care: an evidence-based review of data on efficacy, safety, and drug interactions. Leuk Lymphoma. 2010;51(8):1414–1423. doi:10.3109/10428194.2010.487622
11. Tin MM, Cho C-H, Chan K, James AE, Ko JK. Astragalus saponins induce growth inhibition and apoptosis in human colon cancer cells and tumor xenograft. Carcinogenesis. 2007;28(6):1347–1355. doi:10.1093/carcin/bgl238
12. Liu QY, Yao YM, Zhang SW, Sheng ZY. Astragalus polysaccharides regulate T cell-mediated immunity via CD11c(high)CD45RB (low) DCs in vitro. J Ethnopharmacol. 2011;136:457–464. doi:10.1016/j.jep.2010.06.041
13. Clement-Kruzel S, Hwang SA, Kruzel MC, Dasgupta A, Actor JK. Immune modulation of macrophage pro-inflammatory response by goldenseal and Astragalus extracts. J Med Food. 2008;11:493–498. doi:10.1089/jmf.2008.0044
14. Cui R, He J, Wang B, et al. Suppressive effect of Astragalus membranaceus Bunge on chemical hepatocarcinogenesis in rats. Cancer Chemother Pharmacol. 2003;51:75–80. doi:10.1007/s00280-002-0532-5
15. McCulloch M, See C, Shu XJ, et al. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: meta-analysis of randomized trials. J Clin Oncol. 2006;24:419–430. doi:10.1200/JCO.2005.03.6392
16. Cassileth BR, Rizvi N, Deng G, et al. Safety and pharmacokinetic trial of docetaxel plus an Astragalus-based herbal formula for non-small cell lung cancer patients. Cancer Chemother Pharmacol. 2009;65:67–71. doi:10.1007/s00280-009-1003-z
17. Yang AK, He SM, Liu L, Liu JP, Wei MQ, Zhou SF. Herbal interactions with anticancer drugs: mechanistic and clinical considerations. Curr Med Chem. 2010;17:1635–1678. doi:10.2174/092986710791111279
18. Duan P, Wang ZM. [Clinical study on effect of Astragalus in efficacy enhancing and toxicity reducing of chemotherapy in patients of malignant tumor]. Chin J Integr Traditional West Med. 2002;22:515–517. Chinese.
19. Jorgensen SB, Viollet B, Andreelli F, et al. Knockout of the alpha (2) but not alpha(1) 5 ‘-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside- but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi:10.1074/jbc.M306205200
20. Viollet B, Andreelli F, Jorgensen SB, et al. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–98. doi:10.1172/JCI16567
21. Friedrichsen M, Mortensen B, Pehmoller C, Birk JB, Wojtaszewski JFP. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013;366:204–214. doi:10.1016/j.mce.2012.06.013
22. Kim JG, Lee SJ, Chae YS, et al. Association between phosphorylated AMP-activated protein kinase and MAPK3/1 expression and prognosis for patients with gastric cancer. Oncology-Basel. 2013;85:78–85. doi:10.1159/000351234
23. Luo L, Huang W, Tao R, Hu N, Xiao ZX, Luo Z. ATM and LKB1 dependent activation of AMPK sensitizes cancer cells to etoposide-induced apoptosis. Cancer Lett. 2013;328:114–119. doi:10.1016/j.canlet.2012.08.034
24. Liu J, Zhang JF, Lu JZ, et al. Astragalus polysaccharide stimulates glucose uptake in L6 myotubes through AMPK activation and AS160/TBC1D4 phosphorylation. Acta Pharmacol Sin. 2013;34:137–145. doi:10.1038/aps.2012.133
25. Yin F, Du YL, Hu WH, et al. Mad2 beta, an alternative variant of Mad2 reducing mitotic arrest and apoptosis induced by adriamycin in gastric cancer cells. Life Sci. 2006;78:1277–1286. doi:10.1016/j.lfs.2005.06.034
26. Geng M, Wang L, Chen X, Cao RX, Li PF. The association between chemosensitivity and Pgp, GST-pi and Topo II expression in gastric cancer. Diagn Pathol. 2013;8:198. doi:10.1186/1746-1596-8-198
27. Calcagno DQ, Freitas VM, Leal MF, et al. MYC, FBXW7 and TP53 copy number variation and expression in gastric cancer. BMC Gastroenterol. 2013;13:141. doi:10.1186/1471-230X-13-141
28. Onoyama I, Tsunematsu R, Matsumoto A, et al. Conditional inactivation of Fbxw7 impairs cell-cycle exit during T cell differentiation and results in lymphomatogenesis. J Exp Med. 2007;204:2875–2888. doi:10.1084/jem.20062299
29. Mendes-da-Cruz DA, Brignier AC, Asnafi V, et al. Semaphorin 3F and Neuropilin-2 Control the Migration of Human T-Cell Precursors. PLoS One. 2014;9:e103405. doi:10.1371/journal.pone.0103405
30. Zhu H, Zheng Z, Zhang J, et al. Anticancer effect of 2,7-dihydroxy-3-methylanthraquinone on human gastric cancer SGC-7901 cells in vitro and in vivo. Pharm Biol. 2016;54:285–292. doi:10.3109/13880209.2015.1033563
31. Yue L, Haroun S, Parent JL, de Brum-fernandes AJ. Prostaglandin D-2 induces apoptosis of human osteoclasts through ERK1/2 and Akt signaling pathways. Bone. 2014;60:112–121. doi:10.1016/j.bone.2013.12.011
32. Li SG, Zhang YQ. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr Polym. 2009;78:343–348. doi:10.1016/j.carbpol.2009.04.013
33. Zhu ZY, Liu RQ, Si CL, et al. Structural analysis and anti-tumor activity comparison of polysaccharides from Astragalus. Carbohydr Polym. 2011;85:895–902. doi:10.1016/j.carbpol.2011.04.020
34. Jin ML, Zhao K, Huang QS, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi:10.1016/j.ijbiomac.2013.12.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.