Back to Journals » Drug Design, Development and Therapy » Volume 14
Astragaloside IV: An Effective Drug for the Treatment of Cardiovascular Diseases
Received 15 July 2020
Accepted for publication 1 September 2020
Published 15 September 2020 Volume 2020:14 Pages 3731—3746
DOI https://doi.org/10.2147/DDDT.S272355
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Yu-Qing Tan,1,2 Heng-Wen Chen,1,* Jun Li1,*
1Department of Cardiology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing 100053, People’s Republic of China; 2Graduate School of Beijing University of Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Heng-Wen Chen; Jun Li Email [email protected]; [email protected]
Abstract: Cardiovascular disease (CVD), the number one cause of death worldwide, has always been the focus of clinical and scientific research. Due to the high number of deaths each year, it is essential to find alternative therapies that are safe and effective with minimal side effects. Traditional Chinese medicine (TCM) has a long history of significant impact on the treatment of CVDs. The mode of action of natural active ingredients of drugs and the development of new drugs are currently hot topics in research on TCM. Astragalus membranaceus is a commonly used Chinese medicinal herb. Previous studies have shown that Astragalus membranaceus has anti-tumor properties and can regulate metabolism, enhance immunity, and strengthen the heart. Astragaloside IV (AS-IV) is the active ingredient of Astragalus membranaceus, which has a prominent role in cardiovascular diseases. AS-IV can protect against ischemic and hypoxic myocardial cell injury, inhibit myocardial hypertrophy and myocardial fibrosis, enhance myocardial contractility, improve diastolic dysfunction, alleviate vascular endothelial dysfunction, and promote angiogenesis. It can also regulate blood glucose and blood lipid levels and reduce the risk of cardiovascular diseases. In this paper, the mechanism of AS-IV intervention in cardiovascular diseases in recent years is reviewed in order to provide a reference for future research and new drug development.
Keywords: Astragalus membranaceus, astragaloside IV, traditional Chinese medicine, cardiovascular diseases, cardiomyocytes
Introduction
Cardiovascular diseases (CVDs) are disorders of the heart and blood vessels.1 As the number one cause of death, CVDs pose multiple threats to health, overburdening the global economic system. An estimated 17.9 million people died from CVDs in 2016, accounting for 31% of all deaths worldwide. More than three quarters of CVD deaths occur in low- and middle-income countries.1 Given present trends, the annual number of deaths from CVD will increase to 22.2 million by 2030. One-third of deaths occur in people under the age of 70.2 However, a large number of people fail to recognize the risks of CVD. Individuals at risk for CVD may present weight issues, high blood pressure, and altered glucose or lipid levels. Studies on the pathogenesis and pharmacological mechanisms of CVD have made great progress, but the morbidity and mortality associated with CVD remains high. Drugs with better therapeutic efficacy and minimal side effects are urgently needed to prevent and treat these diseases.
Therefore, therapeutic strategies for intervening in chronic diseases such as CVDs are important for prolonging healthy aging, and various approaches are being used to develop effective drugs for the treatment or prevention of CVDs. Natural products may have advantages over traditional compound-based drugs, such as fewer side effects, decreased long-term toxicity, and variable bioavailability.3 Bioactive natural products come from a wide variety of sources. The history of traditional Chinese medicine (TCM) can be traced back thousands of years in Eastern countries. Modern research has confirmed that TCM contains a variety of active constituents with strong pharmacological effects that play a significant role in the prevention and treatment of cardiac metabolic diseases.4
Astragalus membranaceus, the dried roots of Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao or Astragalus membranaceus (Fisch.) Bge.,5 is one of the most frequently used TCMs, with a history of use of more than 2000 years. Astragalus membranaceus was first recorded in Shen Nong Ben Cao Jing. Modern pharmacological research suggests the Astragalus membranaceus has positive effects such as improving cardiac function,6 promoting angiogenesis,7 regulating blood glucose,8 being an anti-tumor agent,9 and increasing immunomodulatory activity.10 The principle active constituents of Astragalus membranaceus are saponins, flavonoids, and polysaccharides,11 but it also includes components such as anthraquinones, alkaloids, amino acids, β-sitosterol, and metallic elements.12 Astragaloside IV (AS-IV) is a lanolin alcohol-derived tetracyclic triterpene saponin extracted from Astragalus membranaceus. It is a white powder with the molecular formula C41H68O14. The relative molecular weight is 784.97, and the CAS number is 84,687–43-4. The melting point is 299–300°C (in MeOH). AS-IV has a structure similar to steroidal drugs with very low solubility. It is easily soluble in methanol, ethanol, acetone, and sparingly soluble in weakly polar organic solvents such as chloroform or ethyl acetate. [α] +22.0° (c 0.92, MeOH); FAB-MS m/z: 807.0 [M+Na]+, HR-FAB-MS m/z: C41H68O14Na (required 807.4507, [M+Na]+ at m/z 807.4529). IR (film) νmax cm−1: 3395 (OH), 1930, 1038. 1H NMR (400 MHz, C5D5N) data and 13C NMR (100 MHz, C5D5N) data, as shown in Table 1. AS-IV (8 mg·kg−1) was injected into the tail vein of rats, the content was determined by HPLC-ELSD, and the pharmacokinetic parameters were analyzed by DAS2.0 software. The results are shown in Table 2. The results showed that the two-compartment model was conformed in vivo after the tail vein injection of AS-IV in rats.13–15 The absolute bioavailability of AS-IV is 2.2%. Glycosyl hydrolysis can occur through the transformation of intestinal flora, and it is hardly metabolized in the liver. There is no first-pass effect after oral administration.16–19 In the Chinese Pharmacopoeia, the content of AS-IV is the standard for quality testing of Astragalus membranaceus.20 Figure 1 shows the structural formula of AS-IV. In this review, we discuss the therapeutic implications of the use of AS-IV in CVDs in greater detail. This study analyzes the effects and possible mechanisms of AS-IV on the heart and blood vessels. At the same time, the effects of AS-IV on blood glucose, blood lipids and its antiviral effects are reviewed.
 |
Table 1 1H and 13C NMR Spectroscopic Data of AS-IV |
 |
Table 2 Pharmacokinetic Parameters of AS-IV (8 mg·kg−1) Injected into the Tail Vein of Rats |
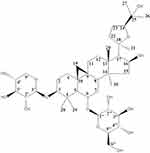 |
Figure 1 The structural formula of AS-IV. |
Protect Heart Structure and Function
According to existing literature, Astragalus membranaceus preparations (traditional decoctions, granule preparations, and injections) are widely used to treat diseases of the cardiac system, such as heart failure, odium and water retention, and arrhythmias.21–23 As the main active ingredient of the Chinese medicine Astragalus membranaceus, AS-IV has a good regulatory effect on the heart. It can protect against ischemic and hypoxic cardiomyocytes, protect the heart structure, and enhance heart function.
Protect Ischemic and Hypoxic Cardiomyocytes
Prevention and treatment of myocardial injury caused by ischemia and hypoxia have always been the focus of clinical and scientific research. A large number of research reports have shown that Astragalus membranaceus has a significant protective effect on myocardial cell injury caused by ischemia and hypoxia, and its mechanism may be related to antioxidant injury,24,25 energy metabolism,26,27 and ion balance regulation.28,29 Hypoxic-ischemic injury leads to the accumulation of oxygen-free radicals and the reduction of superoxide dismutase (SOD) content, leading to structural damage to the heart.30 The oxidative stress response can cause damage to mitochondria and plays an important role in cardiac pathological processes.31 The heart is a high oxygen-consuming, high energy-consuming organ. During the course of life, the heart’s orderly diastolic contraction activity relies on cardiomyocytes to increase energy support. There are a large number of mitochondria in cardiomyocytes, which increases available energy for the ordered activity of the heart. Mitochondria produce more than 95% of the adenosine triphosphate (ATP) used by the heart, and the production of ATP is essential for normal cardiac function.32 The regulation of ion-specific channels and electrophysiological balance are very important for the generation and conduction of electrical pulses, but their regulation is also related to energy regulation.33
AS-IV can improve cell viability, decrease malondialdehyde (MDA) content, reduce the activity of creatine phosphokinase (CPK) and lactic dehydrogenase (LDH), increase the activity of Glutathione peroxidase (GSH-Px) and SOD, and reduce the production of reactive oxygen species (ROS) and the loss of mitochondria.34 The extensive clearance of ROS may be related to the regulation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling pathway.35 AS-IV can increase the activity and expression of SOD-1 under normoxic and hypoxic conditions.24 Quercetin is a natural antioxidant.36 However, in vitro culture of rat cardiomyocytes has shown that AS-IV has a better protective effect on the myocardium than quercetin, and both of them are better than vitamin E.37
The ratio of ATP/adenosine diphosphate (ADP) and ATP/adenosine monophosphate (AMP) is decreased by ischemia and hypoxia.38 It is well known that during ischemia or hypoxia, the increase in ATP levels in the interstitial spaces within the heart is due to its release from many cell types including cardiomyocytes. Results show that ATP is released through the maxi-anion channel.39 AS-IV upregulates the expression of B-cell lymphoma 2 (Bcl-2), promotes the translocation of Bcl-2 to mitochondria, maintains the membrane potential of mitochondria, and inhibits mitochondrial permeability transition pore (mPTP) opening. This compound also inhibits the activation of caspase-3, decreases the expression of Bcl-2 associated X protein (Bax), reduces the release of cytochrome C (Cyt-C), increases ATP production, and ultimately reduces cell apoptosis and attenuates ischemia-hypoxia injury.26,27,34,39 When observing cell viability and morphology, it was found that AS-IV could upregulate the gene expression of GATA-4 and the survival factors Bcl-2 and P62 while also inhibiting apoptosis and autophagy genes, such as poly ADP-ribose polymerase (PARP), caspase-3, and Beclin-1. AS-IV can stimulate the expression of the transcription factor GATA-4, thereby enhancing the protective effect on the myocardium.40
The imbalance in coronary blood flow mechanisms, including misregulation of ion channels, leads to the disruption of cardiac structure and loss of myocardial function. Coronary ion channels may represent arterial microvascular dysfunction.41 Ca2+ is released from the sarcoplasmic reticulum (SR) via the ryanodine receptors (RyRs), and other ions (especially K+, Mg2+ and Cl−) provide counter-ion flux during systole.42 Hypoxic-ischemic injury inhibits SR Ca2+-ATPase activity in cardiomyocytes and decreases the mRNA and protein expression of SR Ca2+-ATPase.43 After treatment with AS-IV, the capacity for the decreased kinase activity of protein kinase A (PKA) can be restored. The expression of PKA specific phosphorylated Ser (16) phosphorylated phospholamban (Ser (16)-PLN) is upregulated in cardiomyocytes, restoring the function of SR Ca2+-ATPase (SERCA2a),29 regulating Ca2+ homeostasis, decreasing the expression of [Ca2+] (i) and CaSR, increasing the phosphorylation level of extracellular regulated protein kinases (ERK) to inhibit cell apoptosis.28,44
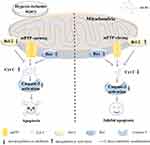
There have been numerous experiments that have been used to study the possible pathways involved in the inhibition of myocardial ischemia-hypoxia injury by AS-IV. Studies have shown that in cardiomyocytes injured by hypoxia/reoxygenation, AS-IV can promote cell proliferation, upregulate the expression of miR-101a, inhibit the expression of transforming growth factor-beta receptor 1 (TGFBR1) and toll-like receptor 2 (TLR2), and inhibit the downstream genes of the mitogen-activated protein kinase (MAPK) signaling pathway. These results indicate that AS-IV may play a role through the miR-101a/TGFBR1/TLR2/MAPK signaling pathway.45 Some studies have examined the expression of heme oxygenase (HO-1) by Western blot as well as the expression of Nrf2 and BTB and CNC homology 1 (Bach1) proteins in the nucleus. AS-IV can regulate the expression of Nrf2 and Bach1 proteins and significantly increase the expression of HO-1 protein (P < 0.01). Moreover, Phosphatidylinositol-3-kinase (PI3K) inhibitors have been found to have a certain reversal effect on the therapeutic effect of AS-IV. It was concluded that the PI3K/protein kinase B (AKT)/HO-1 signaling pathway may participate in the protective mechanism of ischemia and hypoxia.46,47 AS-IV can upregulate the p-AKT/AKT ratio and the phosphorylated glycogen synthase kinase/glycogen synthase kinase 3β (GSK-3β) ratio, and the mechanism of alleviating I/R damage may be related to the PI3K/AKT/GSK-3β signaling pathway.48 Similarly, studies have found that the inhibition of PI3K/mammalian target of rapamycin (mTOR) or mTOR can reverse AS-IV-induced downregulation of long non-coding RNA (lncRNA) growth arrest-specific transcript 5 (GAS5) in cardiomyocytes. AS-IV may play a protective role by activating the PI3K/mTOR pathway.49 Bcl-2 and Bcl-2-like protein 2 (BCL2L2) correspond to the target genes of miR-23a and miR-92a. Knockdown of miR-23a and miR-92a is associated with the PI3K/AKT and MAPK/ERK signaling pathways. AS-IV may play a protective role in cardiomyocytes by downregulating the target genes of Bcl-2 and BCL2L2 (miR-23a and miR-92a) and activating the PI3K/AKT and MAPK/ERK signaling pathways.50 In addition, after AS-IV treatment, the mRNA and protein expression levels of hypoxia-inducible factor-1α (HIF1α), the Notch signal receptor 1 (Notch1) and the Notch ligand Jag 1 (Jagged1) are significantly increased in rats (P < 0.01 or P < 0.05).51 Upregulation of Hes1 protein expression suggests that the Notch1/Hes1 signaling pathway may be involved in the prevention of myocardial ischemia and hypoxia injury.34 AS-IV downregulates the expression of toll-like receptor 4 (TLR4) and nuclear factor kappa-B (NF-κB), suggesting that it may play a role in inhibiting apoptosis and reducing ischemia and hypoxia injury in rats through the TLR4/NF-κB signaling pathway.52 In summary, the protective effect of AS-IV against ischemia and hypoxia injury may involve multiple pathways, such as the MAPK, PI3K/AKT, Notch1, and NF-κB pathways, and its mechanism is relatively complex. Figure 2 summarizes the effect of hypoxic-ischemic injury on myocardial mitochondria and the protective mechanism of AS-IV.
Regulation of the Heart Structure and Inhibition of Cardiac Hypertrophy and Myocardial Fibrosis
Hypertrophy includes physiological and pathological effects, and early compensatory myocardial hypertrophy is an adaptive response. Over time, pathological cardiac hypertrophy alters cardiac structure, leading to heart failure.53,54 Similarly, excessive myocardial fibrosis can also lead to ventricular remodeling and heart failure.55 AS-IV can inhibit ventricular remodeling and improve cardiac function.56
There are many experimental studies that have successfully created models of cardiac hypertrophy by taking multiple approaches and have demonstrated the protective effect of AS-IV on cardiac hypertrophy in vivo or in vitro. Recent research has used isoproterenol (ISO) or a calcium-sensing receptor agonist (GdCl3) to induce hypertrophy in rat and heart H9C2 cells and found that AS-IV can attenuate the cardiac function and mitochondrial structural damage caused by the inducer. Moreover, AS-IV can affect the expression of [Ca2+] (i) and calcium-sensing receptor, thereby inhibiting myocardial hypertrophy and apoptosis.57 AS-IV may exert an inhibitory effect on ISO-induced cardiac hypertrophy by reducing oxidative stress and calpain-1 activation. These researchers also used the same research subjects induced with ISO. Their results show that AS-IV increased Bcl-2 expression, decreased Bax and calpain-1 protein expression and calpain activity, and increased the activities of mitochondrial superoxide dismutase (mito-SOD) and mitochondrial catalase (mito-CAT).58 AS-IV also regulates the Ca2+-mediated calcineurin (CaN) signaling pathway. Lipopolysaccharide (LPS) induces cardiac hypertrophy, increases the level of resting Ca2+, and promotes CaN activation. The addition of AS-IV can reduce cardiac hypertrophy and inhibit CaN activation before lipopolysaccharide induction. It inhibits the level of resting Ca2+ similarly to verapamil.59 AS-IV may also prevent ISO-induced cardiac hypertrophy by mediating the NF-κB/peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) signaling pathway. AS-IV was used in combination with ISO to treat Sprague-Dawley (SD) rats or neonatal rat ventricular myocytes (NRVM). The results show that pathological changes were alleviated and the subunit transfer of transcription factor p65 (p65) and NF-κB were inhibited.60 AS-IV can upregulate Nrf2, stimulate the Nrf2/HO-1 signaling pathway, and improve ventricular function and structure.61 SD rats and neonatal rat cardiomyocytes were next treated with ISO in this report. In vivo, AS-IV could regulate the changes caused by ISO treatment, increase TLR4 and p65 protein expression, increase NF-κB inhibitor α (IκBα), and significantly reduce the expression of atrial natriuretic peptide (ANP), TLR4 mRNA, tumor necrosis factor-α (TNF-α), and interleukin 6 (IL-6) in serum. In vitro, AS-IV could regulate cell size and had a similar effect to propranolol. The mechanism of action may be related to the inhibition of TLR4/NF-κB signaling pathway and the attenuation of inflammation effects.62 Other studies have used an aortic band (AB) surgery-induced mouse myocardial hypertrophy model. IKKepsilon inhibitory factor (SIKE) is enhanced and TANK1-binding kinase 1 (TBK1)/PI3K/AKT activity is inhibited after AS-IV treatment so as to prevent heart hypertrophy.63 AS-IV could inhibit endothelin-1 (ET-1) induced cardiomyocyte hypertrophy and affect the expression of signaling molecules in the vitamin D axis.64 Table 3 summarizes the research subjects of all the experiments mentioned above, the induction methods of the respective cardiac hypertrophy models and the possible mechanisms of AS-IV.
 |
Table 3 Mechanisms of AS-IV in the Treatment of Cardiac Hypertrophy |
Myocardial fibrosis is caused by an excessive accumulation of collagen in the myocardial interstitium and exists in different stages of a variety of cardiomyopathies. In order to determine the therapeutic effects of AS-IV on viral myocarditis, a mouse model of viral myocarditis was established with coxsackievirus B3 (CVB3). Studies have shown that AS-IV can attenuate the expression of FAS, FASL, caspase-8 and caspase-3, and inhibit CVB3-induced myocardial apoptosis, which may be related to the inhibition of fatty acid synthetase/Fas ligand (FAS/FASL) signaling pathway activation.65 For CVB3-induced myocardial fibrosis in dilated cardiomyopathy, AS-IV treatment significantly improved survival. AS-IV can downregulate the expression of transforming growth factor-β1 (TGF-β1) and its downstream phosphorylated mothers against decapentaplegic protein 2/3 (pSmad2/3) and Smad4 in the myocardium, as well as reducing the level of type I collagen. The preventive fibrosis effect of AS-IV may be related to the downregulation of the TGF-β1-Smad signaling pathway.66 AS-IV can reduce mortality and improve myocardial fibrosis in mice with CVB3-induced chronic myocarditis. AS-IV can also reduce the collagen volume fraction. Its mechanism of action may be downregulation of the expression of TGF-β1 and matrix metalloproteinase-1 (MMP-1) and upregulation of MMP-13 and MMP-14 expression.67 It was observed that AS-IV significantly downregulated the mRNA expression of NLR family pyrin domain containing 3 (NLRP3), caspase-1, IL-18 and IL-6 in mouse heart tissue. AS-IV may exhibit ISO-induced inhibition of myocardial fibrosis in mice by inhibiting the NLRP3 pathway.68 Hypoxia can lead to the upregulation of transient receptor potential melastatin 7 (TRPM7) protein, which is one of the targets of miR-135a. In vivo and in vitro studies have shown that AS-IV can reduce the expression of TRPM7 and its mRNA, inhibit the activation of the TGF-β/Smads pathway, and inhibit myocardial fibrosis.69,70 The mechanism by which AS-IV inhibits ISO-induced myocardial fibrosis may be related to ROS-mediated responses. It has been found that both increased ROS content and upregulated cardiotrophin 1 (CT1) expression, both of which were inhibited after the addition of astragaloside, which inhibited cardiac fibroblast proliferation and collagen production.71 Inhibition of ROS-mediated MAPK activation can also inhibit myocardial fibrosis. AS-IV can significantly inhibit ISO-induced cardiac fibroblast proliferation and collagen I synthesis, reduce ROS levels, and inhibit the phosphorylation of extracellular signal-regulated kinase, p38MAPK, and cJun N-terminal kinase. Acetylcysteine, an ROS scavenger, has a similar effect to AS-IV in regulating ROS.72 AS-IV can significantly reduce the cardiac mass index and left ventricular mass index, metabolites of type I collagen, as well as TGF-β1, Smad 2/3, and Smad 4 protein expression levels (P < 0.05), but it can upregulate Smad 7 protein levels (P < 0.05). AS-IV combined with electroacupuncture had better effects on regulation than propranolol in some aspects. The attenuation of myocardial hypertrophy and myocardial fibrosis by AS-IV in rats may be related to the TGF-β1/Smad signaling pathway.73 Table 4 summarizes the research subjects of all the experiments mentioned above, the induction methods of myocardial fibrosis model, and the possible mechanisms of AS-IV.
 |
Table 4 Mechanisms of AS-IV Action in the Treatment of Myocardial Fibrosis |
Enhancement of Heart Function
Previous studies have found that AS-IV has a positive inotropic effect similar to cardiac glycosides.74 Gene chip control assays were used to investigate the regulatory effects of AS-IV on myocardial gene expression profiles in rats. It was found that AS-IV could upregulate and enhance myocardial function.75 AS-IV could also restore diastolic dysfunction in rats with chronic heart failure, and its mechanism of action was to downregulate calcium-sensitive receptors and protein kinase C-α.76 AS-IV inhibited L-type calcium channels, which could reduce extracellular calcium influx, and at the same time promote the release of internal calcium, bidirectionally regulating the level of Ca2+ in cardiomyocytes and maintaining a steady state.74,77 AS-IV could also inhibit Na+/K+-ATPase activity, which could produce immediate myocardial contractile effects.26,78
AS-IV alleviates oxidative stress, increases the production of nitric oxide (NO) and cyclic guanosine phosphate (cGMP) in the myocardium, and improves diastolic dysfunction.79 AS-IV increases left ventricular systolic pressure, maximum rates of increase and decrease of left ventricular pressure, and significantly improves the survival rate of rats with heart failure.80,81 Cardiac output, heart rate, stroke volume, mean aortic pressure, and systolic aortic pressure gradually return to normal levels after treatment.82 AS-IV can restore normal cardiac parameters, enhance myocardial contractility, and improve cardiac diastolic dysfunction.
Effects on Blood Vessels
Gene chip control assays revealed that AS-IV had the greatest effect on vascular developmental function.75 The effect of astragaloside on vascular regulation was mainly to improve vascular endothelial dysfunction and promote angiogenesis. Vascular endothelial cells are a barrier between circulating blood and the inner wall of blood vessels. A study of SD rats induced by ISO found that AS-IV increased the endothelial nitric oxide synthase (eNOS) dimer/monomer ratio and NO in serum and decreased the nuclear-cytoplasmic protein expression ratio of NF-κB p65. AS-IV downregulated the expression of IL-1β, IL-6, and TNFα mRNA. The mechanism may have been through the attenuation of the oxidative stress response and inhibit the ROS-NF-κB pathway.83 Dysglycemia and hyperhomocysteinemia are risk factors for cardiovascular disease, and AS-IV can also improve the endothelial dysfunction caused by them. For diabetic rats induced by streptozotocin (STZ), AS-IV can reduce the level of ROS, increase the production of NO and the expression of eNOS, and improve the activity of SOD and GSH-px. AS-IV ameliorated endothelial dysfunction in diabetic rats by decreasing oxidative stress and calpain-1.84 The effects of AS-IV on human umbilical vein endothelial cells (HUVECs) were similar to those of TLR4 and NF-κB p65 inhibitors. AS-IV could significantly reduce the content of IL-6 and TNF-α and decrease the expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), TLR4, and nuclear NF-κB p65. AS-IV may play a protective role through the TLR4/NF-κB signaling pathway.85 Rats fed with fructose develop metabolic syndrome. High doses of AS-IV could improve glucose tolerance and endothelium-dependent vasodilation as well as increase the levels of NOx and cGMP; this mechanism may be associated with the NO/cGMP-related pathways.86 AS-IV significantly ameliorated homocysteine-induced inactivation of the nitric oxide synthase (NOS) signaling pathway by increasing SOD activity, which was similar to SOD pretreatment. AS-IV may be beneficial for the treatment of the endothelial dysfunction caused by the NO/NOS pathway disorder in hyperhomocysteinemia.87 Table 5 summarizes the research subjects of all the experiments mentioned above, the induction methods of endothelial dysfunction, and possible mechanisms of AS-IV.
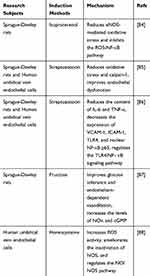 |
Table 5 Mechanisms of AS-IV in the Treatment of Endothelial Dysfunction |
AS-IV promotes angiogenesis, increases vascular density, and promotes the mRNA expression of CD31 and vascular endothelial growth factor (VEGF). AS-IV induces Janus kinase (JAK) and signal transducer, and is an activator of transcription 3 (STAT3) phosphorylation, which may promote angiogenesis possibly related to the JAK-STAT3 pathway.88 AS-IV increases the mRNA and protein expression levels of VEGF and basic fibroblast growth factor (bFGF) (P < 0.01 or P < 0.05). AS-IV can also significantly increase microvessel density (P < 0.05).89 AS-IV can alleviate ultrastructural damage and promote angiogenesis, promote cell proliferation and tube formation, and induce the activation of the phosphatase and tensin homologue deleted on chromosome (PTEN)/PI3K/Akt signaling pathway.90 After AS-IV administration, myocardial morphology is significantly improved and the number of new blood vessels is increased. The mRNA and protein expressions of Protein kinase D1 (PKD1), Histone Deacetylase 5 (HDAC5), and VEGF in myocardial tissue are significantly increased. AS-IV may promote angiogenesis through the PKD1-HDAC5-VEGF pathway.91 TCM has a coordinated and synergistic effect, and multiple medicines are used together for better efficacy. AS-IV combined with tanshinone IIA (an active ingredient of Salvia miltiorrhiza Bge) can significantly increase the proliferation and tube formation ability of EC-like cells compared with either drug alone. Tanshinone IIA and AS-IV can promote the angiogenesis of EC-like cells by upregulating the expression of connexin 37 (Cx37), Cx40, and Cx43.92 The search for vascular substitutes has always been an area of intense research. TCM in the fiber scaffold can provide abundant biological factors. AS-IV and ferulic acid (the main component of Angelica sinensis) in a ratio of 7:3 could activate the cell viability of endothelial and smooth muscle cells, promote extracellular matrix secretion, increase vascular density, and reduce inflammatory responses.93 Table 6 summarizes the research subjects of all the above experiments, the methods of promoting angiogenesis, and the possible mechanisms of AS-IV.
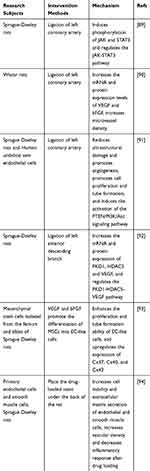 |
Table 6 Mechanisms of AS-IV Promotion of Angiogenesis |
Other Effects of AS-IV
Antiviral Effects
Through the high-throughput screening of classic prescriptions from The Treatise on Cold-Induced and Miscellaneous Diseases, effective antiviral compounds were recently identified. It was found that AS-IV could activate p65 phosphorylation and nuclear translocation and had antiviral effects.94 AS-IV can effectively alleviate myocardial fibrosis and inhibit myocardial cell apoptosis in the treatment of viral myocarditis. AS-IV upregulates the expression of Interferon γ (IFN-γ) mRNA, reduces the viral clearance of CVB3, and inhibits the proliferation of CVB3, thus exerting antiviral effects.95 The effectiveness of AS-IV in the intervention of viral myocarditis was also systematically reviewed, and the results indicated that it might play a role through anti-myocardial remodeling, anti-myocardial fibrosis, anti-viral, anti-inflammatory, or anti-oxidation mechanisms96 AS-IV decreases the protein expressions of Bax and caspase-3 and increases Bcl-2 protein expression. The mechanism may involve the inhibition of human adenovirus type 3 (HAdV-3) replication, and the virus inhibition rate was positively correlated with the concentration of AS-IV.97 In addition, AS-IV could also inhibit the secretion of hepatitis B virus surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) and reduce serum duck hepatitis B virus (DHBV) DNA level. Astragalus membranaceus, a TCM, helps to increase the negative conversion rate of HBeAg and Hepatitis B virus (HBV) DNA.98
Regulation of Blood Glucose and Lipid Levels
Dysglycemia and dyslipidemia are risk factors for cardiovascular disease, and regulating blood glucose and lipid stabilization helps to maintain cardiovascular health. AS-IV significantly increases the content of hepatic glycogen and insulin, decreases the content of blood glucose glycated serum protein, and significantly improves pancreatic pathological changes in streptozotocin-induced diabetic mice.99 By downregulating the expression of IL-6 and TNF-α and inhibiting the NLRP3 inflammasome in the pancreas of gestational diabetic mice, AS-IV can reduce glucose and insulin levels in gestational diabetic mice.100 Moreover, it can inhibit the expression of integrin-linked kinases in diabetic rats and restore the expression of integrin α3β1 to reduce diabetic nephropathy.101
The protective mechanism of AS-IV on myocardial injury in type 2 diabetes may be achieved by regulating abnormal energy and lipid metabolism, regulating the release of PGC-1α and nuclear respiratory factor 1 (NRF1), reversing hyperglycemia-induced oxidative stress and autophagy, and improving the accumulation of cardiac lipids.102–104 AS-IV intervenes in apoE (-/-) mice raised on a high-fat diet, and studies found that the levels of triacylglycerol (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) were downregulated and the levels of HDL-C were increased in the blood of mice. Thus, AS-IV can regulate blood lipids and protect against vascular injury.105
Discussion
In recent years, clinical and pathological research on CVDs has developed rapidly, and great achievements have been made in the research and development of therapeutic methods and drug research. However, the incidence and mortality of CVDs are still high and seeking treatments with high efficiency, low toxicity, and few side effects is still the focus of research. As a characteristic therapy, TCM plays an important role in the treatment of CVDs. Astragalus membranaceus, as a commonly used TCM, has obvious protective effects on the heart, brain, liver, kidney, and lung. In addition, Astragalus membranaceus has anti-inflammatory, antioxidant, and immune-enhancing effects, which are good therapeutic effects against cancer and immune diseases.106–108
Single drugs or compound preparations containing Astragalus membranaceus are widely used in the treatment of CVDs. Astragalus injection, the main component of which is Astragalus membranaceus, where the content of AS-IV is as high as 11.30 mg·mL−1, is often used in the treatment of CVDs. It is used to treat coronary heart disease, angina pectoris, and viral myocarditis, and can improve myocardial injury indicators [Cardiac troponin I (cTnI), high-sensitivity C-reactive protein (hs-CRP), creatine kinase-MB (CK-MB)], reduce TNF-α, IL-6, hs-CRP levels, improve immune function, improve blood lipid metabolism (reduce TC, LDL-C, TG; increase high-density lipoprotein cholesterol (HDL-C)), reduce blood agglutination, and decrease blood cell aggregation index, fibrinogen levels, and blood cell viscosity.109–111 There are many compound Chinese patent medicine preparations that use Astragalus membranaceus as the monarch herb, such as Qili Qiangxin Capsules, Qishen Yiqi Dripping Pills, Naoxintong Capsules, Yangxinshi Tablets, Buxinqi Oral Liquid, and Xintong Oral Liquid, which all can be used to treat coronary heart disease, angina pectoris, heart failure, and adverse cardiovascular events after intervention. Different Chinese patent medicines can be used according to TCM syndrome differentiation.112–117
The main active ingredient of Astragalus membranaceus is AS-IV, which is sparingly soluble in water and has an oral bioavailability of less than 3%. Structural modification and new formulations to improve the bioavailability of oral drugs are commonly used methods at present that can increase stability. Astragalosidic acid, a novel water-soluble derivative of AS-IV, also has obvious cardioprotective effects, with twice the relative bioavailability of AS-IV. It has better permeability and is easier to absorb in the intestine. Moreover, high-dose administration has no obvious acute toxicity. Improving the oral availability of AS-IV is a key direction of new drug research and development.16,19 There are few reports on the toxicology of AS-IV, and it is impossible to determine the possible chronic accumulation caused by its long-term use. Although of great significance, the limited number of studies have been mostly based on animal or cell experiments, and there is insufficient evidence for clinical application of the effective ingredients of Astragalus membranaceus. The human body is a living body, and the target pathways are more complicated than model organisms. At present, the targets and signaling pathways for CVDs are complex, and the research is relatively scant in humans. The research on the pharmacological effects and mechanism of AS-IV on a certain sites is not very deep, and there is a lack of in-depth study of network regulation relationships. The dosage of AS-IV used in each study varies greatly, and the safe range and most effective dose of AS-IV still needs to be further determined.
In conclusion, this article briefly analyzed the structure and pharmacokinetics of AS-IV and expounded on the cardiovascular protective mechanisms from its regulatory effects on the heart, blood vessels, blood sugar, and blood lipid levels. AS-IV can eliminate ROS, increase SOD activity, and help cells resist oxidative damage, regulate mitochondrial energy metabolism, and ion homeostasis. AS-IV also plays a protective role against ischemia-hypoxic damage by regulating multiple pathways such as the MAPK, PI3K/AKT, Notch1, and NF-κB pathways. AS-IV can act on ion channels or reduce the inflammatory response to inhibit myocardial hypertrophy, but also inhibits FAS/FASL and TGF-β/Smads pathway activation and reduces the level of collagen and collagen volume fraction to anti-myocardial fibrosis. Its positive inotropic effect can significantly improve myocardial diastolic dysfunction and restore normal cardiac parameters.
The effect on vascular development is more obvious. AS-IV can prevent vascular endothelial dysfunction by weakening oxidative stress response and reducing inflammation, increase the ability of cell proliferation and tube formation, increase blood vessel density, and promote angiogenesis. It has a wide range of inhibitory effects on a variety of viruses, inhibits virus proliferation, and improves the body’s immunity. The risk factors of CVDs also see significant improvement after AS-IV treatment, which can regulate metabolic abnormalities, improve lipid accumulation, and reduce vascular injury. From multiple levels, different dimensions of disease development play a therapeutic role, which is worthy of further promotion and application.
Conclusion
The study of the therapeutic effects and mechanisms of Astragalus membranaceus and its active ingredients has attracted much attention in the pharmacokinetic field. AS-IV displays good therapeutic effects, especially in the cardiovascular field. It can significantly reduce myocardial injury caused by ischemia and hypoxia, improve energy metabolism, improve endothelial dysfunction, promote angiogenesis, improve immune function, and regulate blood glucose and blood lipid levels. The mechanism by which AS-IV exerts its therapeutic effect on CVDs is complicated, and some therapeutic effects that may involve a combination of multiple pathways are still unclear. AS-IV has a wide range of therapeutic effects and has good development prospects. Based on current research, it can be applied to hypoxic and ischemic injury, physical damage caused by viral diseases, or novel proangiogenic agents. The research on new drugs and mechanisms of AS-IV continues to progress, and more new alternative drugs derived from AS-IV are expected in the future.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data. All authors took part in drafting the article and revising it critically for important intellectual content. All authors agreed on the journal to which the article will be submitted. All authors gave final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Disclosure
All authors declare that there are no conflicts of interest in publishing this work in whole or in part and report no conflicts of interest for this work.
References
1. Cardiovascular diseases (CVDs): health Topics; Updated May 2017. Available from: https://www.who.int/cardiovascular_diseases/en/.
2. WHO library cataloguing-in-publication data: hearts: technical package for cardiovascular disease management in primary health care. Available from: https://www.who.int/cardiovascular_diseases/hearts/Hearts_package.pdf.
3. Kim BM. The role of Saikosaponins in therapeutic strategies for age-related diseases. Oxid Med Cell Longev. 2018;2018:8275256. doi:10.1155/2018/8275256
4. Lyu M, Wang YF, Fan GW, Wang XY, Xu SY, Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front Microbiol. 2017;8(1):2146. doi:10.3389/fmicb.2017.02146
5. Liu P, Shan G, Zhang F, Chen JN, Jia TZ. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed Astragali Radix by UPLC-QTOF-MS combined with novel informatics UNIFI platform. Chin J Nat Med. 2018;16(9):714–720. doi:10.1016/S1875-5364(18)30111-0
6. Ma X, Zhang K, Li H, Han S, Ma Z, Tu P. Extracts from Astragalus membranaceus limit myocardial cell death and improve cardiac function in a rat model of myocardial ischemia. J Ethnopharmacol. 2013;149(3):720–728. doi:10.1016/j.jep.2013.07.036
7. Zhang L, Ling Y, Wang Y, Gao X. Astragalus membranaceus extract promotes neovascularisation by VEGF pathway in rat model of ischemic injury. Pharmazie. 2011;66(2):144–150. doi:10.1691/ph.2011.0738
8. Zhang K, Pugliese M, Pugliese A, Passantino A. Biological active ingredients of traditional Chinese herb Astragalus membranaceus on treatment of diabetes: a systematic review. Mini Rev Med Chem. 2015;15(4):315–329. doi:10.2174/1389557515666150227113431
9. Zhou R, Chen H, Chen J, Chen X, Wen Y, Xu L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement Altern Med. 2018;18(1):83. doi:10.1186/s12906-018-2148-2
10. Yang B, Xiao B, Sun T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int J Biol Macromol. 2013;62:287–290. doi:10.1016/j.ijbiomac.2013.09.016
11. Ibrahim LF, Marzouk MM, Hussein SR, Kawashty SA, Mahmoud K, Saleh NAM. Flavonoid constituents and biological screening of Astragalus bombycinus Boiss. Nat Prod Res. 2013;27(45):386–393. doi:10.1080/14786419.2012.701213
12. Li X, Qu L, Dong Y, et al. A Review of recent research progress on the Astragalus genus. Molecules. 2014;19(11):18850–18880. doi:10.3390/molecules191118850
13. Li Y, Li Z, Yan S, Su Y. Chemical constituents in roots of Astragalus membranaceus. Chin Tradit Herb Drugs. 2017;48(13):2601–2607. doi:0.7501/j.issn.0253-2670.2017.13.003
14. Hirotani M, Zhou Y, Lui H, Furuya T. Astragalosides from hairy root cultures of Astragalus membranaceus. Phytochemistry. 1994;36(3):665–670. doi:10.1016/S0031-9422(00)89793-9
15. Tan C, Liu X, Fu Z. Study on the pharmacokinetics of Astragaloside IV in rats. Strait Pharm J. 2013;25(08):40–41.
16. Yu J, Zhang Y, Zhang C, Han J, Sun S, Wang R. Pharmacokinetics and absolute bioavailability of Astragaloside IV inclusion compound. Chin Pharm J. 2011;46(08):615–618.
17. Sun G, Zhao Y, Miao P, et al. Stability study in biological samples and metabolites analysis of Astragaloside IV in rat intestinal bacteria in vitro. Chin J Chin Mater Med. 2014;39(21):4258–4264. doi:10.4268/cjcmm20142133
18. Gu Y, Wang G, Pan G, Fawcett JP, A J SJ. Transport and bioavailability studies of Astragaloside IV, an active ingredient in Radix Astragali. Basic Clin Pharmacol Toxicol. 2004;95(6):295–298. doi:10.1111/j.1742-7843.2004.t01-1-pto950508.x
19. Qing L, Chen T, Sun W, Luo P, Zhang Z, Ding L. Pharmacokinetics comparison, intestinal absorption and acute toxicity assessment of a novel water-soluble Astragaloside IV derivative (Astragalosidic Acid, LS-102). Eur J Drug Metab Pharmacokinet. 2019;44(2):251–259. doi:10.1007/s13318-018-0515-5
20. Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China. Beijing: China medical science and technology press; 2015:302.
21. Li S, Nong Y, Gao Q, et al. Astragalus Granule prevents Ca2+ current remodeling in heart failure by the downregulation of CaMKII. Evid Based Complement Alternat Med. 2017;2017:7517358. doi:10.1155/2017/7517358
22. Zhang JG, Yang N, He H, et al. Effect of Astragalus injection on plasma levels of apoptosis-related factors in aged patients with chronic heart failure. Chin J Integr Med. 2005;11(3):187–190. doi:10.1007/BF02836502
23. Ma J, Peng A, Lin S. Mechanisms of the therapeutic effect of Astragalus membranaceus on sodium and water retention in experimental heart failure. Chin Med J. 1998;111(1):17–23.
24. Hu JY, Han J, Chu ZG, et al. Astragaloside IV attenuates hypoxia-induced cardiomyocyte damage in rats by upregulating superoxide dismutase-1 levels. Clin Exp Pharmacol Physiol. 2009;36(4):351–357. doi:10.1111/j.1440-1681.2008.05059.x
25. Zhou JY, Fan Y, Kong JL, Wu DZ, Hu ZB. Effects of components isolated from Astragalus membranaceus Bunge on cardiac function injured by myocardial ischemia reperfusion in rats. Chin J Chin Mater Med. 2000;25(5):300–302.
26. Luo Y, Wan Q, Xu M, et al. Nutritional preconditioning induced by Astragaloside IV on isolated hearts and cardiomyocytes against myocardial ischemia injury via improving Bcl-2-mediated mitochondrial function. Chem Biol Interact. 2019;309:108723. doi:10.1016/j.cbi.2019.06.036
27. Si J, Wang N, Wang H, et al. HIF-1alpha signaling activation by post-ischemia treatment with Astragaloside IV attenuates myocardial ischemia-reperfusion injury. PLoS One. 2014;9(9):e107832. doi:10.1371/journal.pone.0107832
28. Jiang S, Jiao G, Chen Y, Han M, Wang X, Liu W. Astragaloside IV attenuates chronic intermittent hypoxia-induced myocardial injury by modulating Ca 2+++ homeostasis. Cell Biochem Funct. 2020;38:710–720. doi:10.1002/cbf.3538
29. Zhang DW, Bian ZP, Xu JD, et al. Astragaloside IV alleviates hypoxia/reoxygenation-induced neonatal rat cardiomyocyte injury via the protein kinase a pathway. Pharmacology. 2012;90(12):95–101. doi:10.1159/000339476
30. Kasparova D, Neckar J, Dabrowska L, et al. Cardioprotective and nonprotective regimens of chronic hypoxia diversely affect the myocardial antioxidant systems. Physiol Genomics. 2015;47(12):612–620. doi:10.1152/physiolgenomics.00058.2015
31. Sharmila Queenthy S, Stanely Mainzen Prince P, John B. Diosmin prevents isoproterenol-induced heart mitochondrial oxidative stress in rats. Cardiovasc Toxicol. 2018;18(2):120–130. doi:10.1007/s12012-017-9422-2
32. Kolwicz SC
33. Abriel H, Rougier JS, Jalife J. Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death. Circ Res. 2015;116(12):1971–1988. doi:10.1161/CIRCRESAHA.116.305017
34. Huang H, Lai S, Wan Q, Qi W, Liu J. Astragaloside IV protects cardiomyocytes from anoxia/reoxygenation injury by upregulating the expression of Hes1 protein. Can J Physiol Pharmacol. 2016;94(5):542–553. doi:10.1139/cjpp-2015-0457
35. Jiang M, Ni J, Cao Y, Xing X, Wu Q, Fan G. Astragaloside IV Attenuates myocardial ischemia-reperfusion injury from oxidative stress by regulating succinate, lysophospholipid metabolism, and ROS scavenging system. Oxid Med Cell Longev. 2019;2019:9137654. doi:10.1155/2019/9137654
36. Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of Quercetin and its complexes for medicinal application. Molecules. 2019;24(6):1123. doi:10.3390/molecules24061123
37. Hu JY, Huang YS, Song HP, et al. Protective effects of Astragaloside and Quercetin on rat myocardial cells after hypoxia. Chin J Burns. 2007;23(3):175–178.
38. Tu L, Pan CS, Wei XH, et al. Astragaloside IV protects heart from ischemia and reperfusion injury via energy regulation mechanisms. Microcirculation. 2013;20(8):736–747. doi:10.1111/micc.12074
39. Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559(Pt 3):799–812. doi:10.1113/jphysiol.2004.069245
40. Yang J, Zhang X, Ma X, et al. Astragaloside IV enhances GATA-4 mediated myocardial protection effect in hypoxia/reoxygenation injured H9c2 cells. Nutr Metab Cardiovasc Dis. 2020;30(5):829–842. doi:10.1016/j.numecd.2020.01.009
41. Severino P, D’Amato A, Pucci M, et al. Ischemic heart disease and heart failure: role of coronary ion channels. Int J Mol Sci. 2020;21(9):3167. doi:10.3390/ijms21093167
42. Berti C, Zsolnay V, Shannon TR, Fill M, Gillespie D. Sarcoplasmic reticulum Ca2+, Mg2+, K+, and Cl− concentrations adjust quickly as heart rate changes. J Mol Cell Cardiol. 2017;103:31–39. doi:10.1016/j.yjmcc.2016.10.018
43. Xu XL, Chen XJ, Ji H, et al. Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca-ATPase. Pharmacology. 2008;81(4):325–332. doi:10.1159/000121335
44. Yin B, Hou XW, Lu ML. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. 2019;40(5):599–607. doi:10.1038/s41401-018-0082-y
45. Wu Y, Fan Z, Chen Z, et al. Astragaloside IV protects human cardiomyocytes from hypoxia/reoxygenation injury by regulating miR-101a. Mol Cell Biochem. 2020;470(12):41–51. doi:10.1007/s11010-020-03743-5
46. Yang P, Zhou YP, Chang XC, Wang F, Li GW. Astragaloside IV regulates Nrf2/Bach1/HO-1 signaling pathway and inhibits H9c2 cardiomyocyte injury induced by hypoxia-reoxygenation. Chin J Chin Mater Med. 2019;44(11):2331–2337. doi:10.19540/j.cnki.cjcmm.20190312.001
47. Yang P, Zhou Y, Xia Q, Yao L, Chang X. Astragaloside IV regulates the PI3K/Akt/HO-1 signaling pathway and inhibits H9c2 cardiomyocyte injury induced by hypoxia-reoxygenation. Biol Pharm Bull. 2019;42(5):721–727. doi:10.1248/bpb.b18-00854
48. Wei D, Xu H, Gai X, Jiang Y. Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3β signaling pathways. Acta Cir Bras. 2019;34(7):e201900708. doi:10.1590/s0102-865020190070000008
49. Du J, Liu J, Zhen J, Yang ST, Zheng EL, Leng JY. Astragaloside IV protects cardiomyocytes from hypoxia-induced injury by down-regulation of lncRNA GAS5. Biomed Pharmacother. 2019;116:109028. doi:10.1016/j.biopha.2019.109028
50. Gong L, Chang H, Zhang J, Guo G, Shi J, Xu H. Astragaloside IV protects rat cardiomyocytes from hypoxia-induced injury by down-regulation of miR-23a and miR-92a. Cell Physiol Biochem. 2018;49(6):2240–2253. doi:10.1159/000493827
51. Yu J, Zhang X, Zhang Y. Astragaloside attenuates myocardial injury in a rat model of acute myocardial infarction by upregulating hypoxia inducible factor1alpha and Notch1/Jagged1 signaling. Mol Med Rep. 2017;15(6):4015–4020. doi:10.3892/mmr.2017.6522
52. Lu M, Tang F, Zhang J, et al. Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-kappaB signaling pathway. Phytother Res. 2015;29(4):599–606. doi:10.1002/ptr.5297
53. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387–407. doi:10.1038/s41569-018-0007-y
54. Deng KQ, Zhao GN, Wang Z, et al. Targeting transmembrane BAX inhibitor motif containing 1 alleviates pathological cardiac hypertrophy. Circulation. 2018;137(14):1486–1504. doi:10.1161/CIRCULATIONAHA.117.031659
55. Yuan J, Liu H, Gao W, et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics. 2018;8(9):2565–2582. doi:10.7150/thno.22878
56. Tang B, Zhang JG, Tan HY, Wei XQ. Astragaloside IV inhibits ventricular remodeling and improves fatty acid utilization in rats with chronic heart failure. Biosci Rep. 2018;38(3):BSR20171036. doi:10.1042/BSR20171036
57. Lu M, Leng B, He X, Zhang Z, Wang H, Tang F. Calcium sensing receptor-related pathway contributes to cardiac injury and the mechanism of Astragaloside IV on cardioprotection. Front Pharmacol. 2018;9:1163. doi:10.3389/fphar.2018.01163
58. Liu ZH, Liu HB, Wang J. Astragaloside IV protects against the pathological cardiac hypertrophy in mice. Biomed Pharmacother. 2018;97:1468–1478. doi:10.1016/j.biopha.2017.09.092
59. Chen Y, Chen J, Gao J, Chai YH, Li W, Qin Z. Effect of astragaloside on vitamin D-receptor expression after endothelin-1-induced cardiomyocyte injury. Afr J Tradit Complement Altern Med. 2017;14(4):278–288. doi:10.21010/ajtcam.v14i4.31
60. Zhang S, Tang F, Yang Y, et al. Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-kappaB/PGC-1alpha signaling mediated energy biosynthesis. PLoS One. 2015;10(3):e0118759. doi:10.1371/journal.pone.0118759
61. Nie P, Meng F, Zhang J, Wei X, Shen C. Astragaloside IV exerts a myocardial protective effect against cardiac hypertrophy in rats, partially via activating the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2019;2019:4625912. doi:10.1155/2019/4625912
62. Mei M, Tang F, Lu M, et al. Astragaloside IV attenuates apoptosis of hypertrophic cardiomyocyte through inhibiting oxidative stress and calpain-1 activation. Environ Toxicol Pharmacol. 2015;40(3):764–773. doi:10.1016/j.etap.2015.09.007
63. Lu M, Wang H, Wang J, et al. Astragaloside IV protects against cardiac hypertrophy via inhibiting the Ca2+/CaN signaling pathway. Planta Med. 2014;80(1):63–69. doi:10.1055/s-0033-1360129
64. Yang J, Wang HX, Zhang YJ, et al. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-κB signaling pathway in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol. 2013;150(3):1062–1070. doi:10.1016/j.jep.2013.10.017
65. Liu T, Yang F, Liu J, et al. Astragaloside IV reduces cardiomyocyte apoptosis in a murine model of coxsackievirus B3-induced viral myocarditis. Exp Anim. 2019;68(4):549–558. doi:10.1538/expanim.19-0037
66. Chen P, Xie Y, Shen E, et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-beta1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol. 2011;658(23):168–174. doi:10.1016/j.ejphar.2011.02.040
67. Zhang ZC, Li SJ, Yang YZ. Effect of astragaloside on myocardial fibrosis in chronic myocarditis. Chin J Integrat Chin West Med. 2007;27(8):728–731.
68. Wan Y, Xu L, Wang Y, Tuerdi N, Ye M, Qi R. Preventive effects of Astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome. Eur J Pharmacol. 2018;833:545–554. doi:10.1016/j.ejphar.2018.06.016
69. Lu J, Wang QY, Zhou Y, et al. Astragaloside IV against cardiac fibrosis by inhibiting TRPM7 channel. Phytomedicine. 2017;30:10–17. doi:10.1016/j.phymed.2017.04.002
70. Wei Y, Wu Y, Feng K, et al. Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-β/Smads pathway. J Ethnopharmacol. 2020;249:112404. doi:10.1016/j.jep.2019.112404
71. Jia G, Leng B, Wang H, Dai H. Inhibition of cardiotrophin1 overexpression is involved in the antifibrotic effect of Astragaloside IV. Mol Med Rep. 2017;16(6):8365–8370. doi:10.3892/mmr.2017.7676
72. Dai H, Jia G, Lu M, Liang C, Wang Y, Wang H. Astragaloside IV inhibits isoprenaline-induced cardiac fibrosis by targeting the reactive oxygen species/mitogen-activated protein kinase signaling axis. Mol Med Rep. 2017;15(4):1765–1770. doi:10.3892/mmr.2017.6220
73. Li JS, Zhu XY, Lu ML, Gao JH, Wang HX, Yu XC. Effect of combined intervention of electroacupuncture and Astragaloside IV on myocardial hypertrophy and TGF-beta 1/Smad signaling in rats with myocardial fibrosis. Acupunct Res. 2017;42(6):477–481. doi:10.13702/j.1000-0607.2017.06.002
74. Zhao M, Sun X, Lian W, et al. Effects of Astragaloside IV on L-type calcium current and intracellular calcium concentration in cardiac myocytes. Chin Pharm Bull. 2013;29(10):1373–1377. doi:10.3969/j.issn.1001-1978.2013.10.011
75. Zhang C, Liu R, Li H, Wang J, Chen H, Zhang WD. Effect of Astragaloside IV on gene expression profile of rat myocardium. Chin J Chin Mater Med. 2008;33(02):172–175.
76. Ji Y, Wang T, Zhang X, et al. Astragalosides increase the cardiac diastolic function and regulate the “Calcium sensing receptor-protein kinase C-protein phosphatase 1” pathway in rats with heart failure. Biomed Pharmacother. 2018;103:838–843. doi:10.1016/j.biopha.2018.04.111
77. Zhao MM, Lian WW, Li Z, et al. Astragaloside IV inhibits membrane Ca2+ current but enhances sarcoplasmic reticulum Ca2+ release. Am J Chin Med. 2017;45(4):863–877. doi:10.1142/s0192415x1750046x
78. Luo W, Chen X, Hao C, Wang WT, Zhao ZY. Mechanisms of Astragaloside IV derivative on chronic heart failure. Drug Eval Res. 2011;34(06):416–420.
79. Lin X, Wang Q, Sun S, et al. Astragaloside IV promotes the eNOS/NO/cGMP pathway and improves left ventricular diastolic function in rats with metabolic syndrome. J Int Med Res. 2020;48(1):300060519826848. doi:10.1177/0300060519826848
80. Zhong F, Zhou WH, Zhang J, et al. Effects of Astragaloside on adriamycin-induced myocardium injury and the Daxx expression in rats. J Hunan Univ Chin Med. 2013;33(03):27–30.
81. Wang Y, Ma Y, Gao JH, et al. Effects of Astragalus extract mixture and its effective components on the cardiac function in the rats with experimental heart failure induced by adriamycin. Chin J Exper Tradit Med Form. 2012;18(07):208–212. doi:10.13422/j.cnki.syfjx.2012.07.068
82. Li ZP, Cao Q. Effects of Astragaloside IV on myocardial calcium transport and cardiac function in ischemic rats. Acta Pharmacol Sin. 2002;23(10):898–904.
83. Xu C, Tang F, Lu M, et al. Astragaloside IV improves the isoproterenol-induced vascular dysfunction via attenuating eNOS uncoupling-mediated oxidative stress and inhibiting ROS-NF-kappaB pathways. Int Immunopharmacol. 2016;33:119–127. doi:10.1016/j.intimp.2016.02.009
84. Nie Q, Zhu L, Zhang L, Leng B, Wang H. Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and Calpain-1 activation. Life Sci. 2019;232:116662. doi:10.1016/j.lfs.2019.116662
85. Leng B, Tang F, Lu M, Zhang Z, Wang H, Zhang Y. Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sci. 2018;209:111–121. doi:10.1016/j.lfs.2018.07.053
86. Zhang N, Wang XH, Mao SL, Zhao F. Astragaloside IV improves metabolic syndrome and endothelium dysfunction in fructose-fed rats. Molecules. 2011;16(5):3896–3907. doi:10.3390/molecules16053896
87. Qiu LH, Xie XJ, Zhang BQ. Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation. Biol Pharm Bull. 2010;33(4):641–646. doi:10.1248/bpb.33.641
88. Sui YB, Wang Y, Liu L, Liu F, Zhang YQ. Astragaloside IV alleviates heart failure by promoting angiogenesis through the JAK-STAT3 pathway. Pharm Biol. 2019;57(1):48–54. doi:10.1080/13880209.2019.1569697
89. Yu JM, Zhang XB, Jiang W, Wang HD, Zhang YN. Astragalosides promote angiogenesis via vascular endothelial growth factor and basic fibroblast growth factor in a rat model of myocardial infarction. Mol Med Rep. 2015;12(5):6718–6726. doi:10.3892/mmr.2015.4307
90. Cheng S, Zhang X, Feng Q, et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 2019;227:82–93. doi:10.1016/j.lfs.2019.04.040
91. Yang L, Liu N, Zhao W, et al. Angiogenic function of Astragaloside IV in rats with myocardial infarction occurs via the PKD1-HDAC5-VEGF pathway. Exp Ther Med. 2019;17(4):2511–2518. doi:10.3892/etm.2019.7273
92. Li Z, Zhang S, Cao L, et al. Tanshinone IIA and Astragaloside IV promote the angiogenesis of mesenchymal stem cell-derived endothelial cell-like cells via upregulation of Cx37, Cx40 and Cx43. Exp Ther Med. 2018;15(2):1847–1854. doi:10.3892/etm.2017.5636
93. Wang H, Zhang Y, Xia T, et al. Synergistic promotion of blood vessel regeneration by Astragaloside IV and ferulic acid from electrospun fibrous mats. Mol Pharm. 2013;10(6):2394–2403. doi:10.1021/mp400031y
94. Yu Y, Li Z, Guo R, et al. Ononin, sec-O-β-d-glucosylhamaudol and Astragaloside I: antiviral lead compounds identified via high throughput screening and biological validation from traditional Chinese medicine Zhongjing formulary. Pharmacol Res. 2019;145:104248. doi:10.1016/j.phrs.2019.04.032
95. Zhang Y, Zhu H, Huang C, et al. Astragaloside IV exerts antiviral effects against coxsackievirus B3 by upregulating interferon-gamma. J Cardiovasc Pharmacol. 2006;47(2):190–195. doi:10.1097/01.fjc.0000199683.43448.64
96. Zhuang Z, Wang ZH, Deng LH, Zheng Q, Zheng GQ, Wang Y. Astragaloside IV exerts cardioprotection in animal models of viral myocarditis: a preclinical systematic review and meta-analysis. Front Pharmacol. 2019;10:1388. doi:10.3389/fphar.2019.01388
97. Shang L, Qu Z, Sun L, et al. Astragaloside IV inhibits adenovirus replication and apoptosis in A549 cells in vitro. J Pharm Pharmacol. 2011;63(5):688–694. doi:10.1111/j.2042-7158.2011.01258.x
98. Qi FH, Wang ZX, Cai PP, et al. Traditional Chinese medicine and related active compounds: a review of their role on hepatitis B virus infection. Drug Discov Ther. 2013;7(6):212–224. doi:10.5582/ddt.2013.v7.6.212
99. Miao M, Liu J, Wang T, Liang X, Bai M. The effect of different proportions of astragaloside and curcumin on DM model of mice. Saudi Pharm J. 2017;25(4):477–481. doi:10.1016/j.jsps.2017.04.009
100. Zhang R, Zhang X, Xing B, et al. Astragaloside IV attenuates gestational diabetes mellitus via targeting NLRP3 inflammasome in genetic mice. Reprod Biol Endocrinol. 2019;17(1):77. doi:10.1186/s12958-019-0522-7
101. Chen J, Chen Y, Luo Y, Gui D, Huang J, He D. Astragaloside IV ameliorates diabetic nephropathy involving protection of podocytes in streptozotocin induced diabetic rats. Eur J Pharmacol. 2014;736:86–94. doi:10.1016/j.ejphar.2014.04.037
102. Zhang Z, Wang J, Zhu Y, Zhang H, Wang H. Astragaloside IV alleviates myocardial damage induced by type 2 diabetes via improving energy metabolism. Mol Med Rep. 2019;20(5):4612–4622. doi:10.3892/mmr.2019.10716
103. Zhu Y, Qian X, Li J, et al. Astragaloside-IV protects H9C2 (2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):4172–4181. doi:10.1080/21691401.2019.1687492
104. Wang Z, Zhu Y, Zhang Y, et al. Protective effects of AS-IV on diabetic cardiomyopathy by improving myocardial lipid metabolism in rat models of T2DM. Biomed Pharmacother. 2020;127:110081. doi:10.1016/j.biopha.2020.110081
105. Qin H, Liu P, Lin S. Effects of Astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of apoE (-/-) mice induced by hyperlipaemia. Evid Based Complement Alternat Med. 2015;2015:385154. doi:10.1155/2015/385154
106. Shahzad M, Shabbir A, Wojcikowski K, Wohlmuth H, Gobe GC. The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr Drug Targets. 2016;17(12):1331–1340. doi:10.2174/1389450116666150907104742
107. Li S, Sun Y, Huang J, et al. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: status and prospect. J Ethnopharmacol. 2020;258:112797. doi:10.1016/j.jep.2020.112797
108. Fu J, Wang Z, Huang L, et al. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother Res. 2014;28(9):1275–1283. doi:10.1002/ptr.5188
109. Yi Z, Li H. Effects of Astragalus injection on hemorheology, immune function and blood lipid metabolism in patients with angina pectoris of coronary heart disease. Lab Med Clin. 2020;17(12):
110. Niu F, Zhu L, Yang H. Effects of Astragalus injection combined with taurine on immune function of patients with acute viral myocarditis. World Chin Med. 2019;14(05):
111. Qi G, Gao J. Effect of Huangqi injection on miR in patients with viral myocarditis and the influence of Treg/Th17 cytokines. J Chin Med Mater. 2019;42(04):924–927. doi:10.13863/j.issn1001-4454.2019.04.046
112. Liang T, Zhang Y, Yin S, et al. Cardio-protecteffect of Qiliqiangxin capsule on left ventricular remodeling, dysfunction and apoptosis in heart failure rats after chronic myocardial infarction. Am J Transl Res. 2016;8(5):2047–2058.
113. Dai Q, Shi Z, Hu J, et al. Meta-analysis of effect of Qishen Yiqi Dripping Pills combined with Western medicine on adverse cardiac events and quality of life after percutaneous coronary intervention. Chin J Chin Mater Med. 2020. doi:10.19540/j.cnki.cjcmm.20200618.501
114. Wang J, Zhao T, Li J, et al. Overview on the clinical efficacy and action mechanism of Naoxintong capsules in the treatment of coronary heart disease. J Tradit Chin Med. 2020;61(09):814–817. doi:10.13288/j.11-2166/r.2020.09.019
115. Liu H. Interpretation of consensus of experts on clinical application of Yangxinshi tablets in treatment of coronary heart disease. World Chin Med. 2020;15(04):637–642. doi:10.3969/j.issn.1673-7202.2020.04.034
116. Sun X. Clinical study on Buxinqi oral liquid combined with nicorandil in treatment of angina pectoris of coronary heart disease. Drugs Clin. 2019;34(03):640–643. doi:10.7501/j.issn.1674-5515.2019.03.013
117. Zhang Y. Analysis of the effect of Xintong oral liquid on unstable angina pectoris of coronary heart disease. Modern J Integr Tradit Chin West Med. 2015;24(31):3473–3475. doi:10.3969/j.issn.1008-8849.2015.31.019
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.